Abstract
Over the past few decades, the number of available spawning grounds for anadromous pikes, Esox lucius, on the Baltic Sea coast has been significantly reduced resulting in a drastic pike population decline in those areas. It is therefore a question if saline Baltic waters may support the spawning of anadromous pike. In the present experimental study, salinity had a harmful impact on the development of eggs from pikes of freshwater origin. The hatching success rates were 0% at 7PSU and only 16.7% at 4PSU, while hatching success at 0PSU was 89.7%. However, when the eggs were fertilized in fresh water and transferred to the tested salinities after 1 h, salinity had a positive effect on egg development and hatching success (0PSU: 80%; 4PSU: 83.3%; and 7PSU: 93.7%). We concluded that the spawning of the freshwater pike population in the saline water of the Baltic Sea is most likely not successful. Simultaneously, inflows of coastal marine waters into near-shore spawning grounds do not have a harmful effect on egg development if the eggs are already fertilized. The main management measures that can be suggested are to rebuild the anadromous population by spawning ground reconstruction. Sea-spawning population introduction can also be considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pike (Esox lucius Linnaeus, 1758) is an economically valuable species (Pierce et al., 1995), and as a top predator, it also plays an important role in ecosystems by regulating fish population abundance (Craig, 1996, 2008; Eriksson et al., 2011; Crane et al., 2015; Persson et al., 2018). Pikes occur in both the fresh waters of lakes and rivers and in the coastal zones of brackish waters, for example, in the Baltic Sea (Ojaveer, 1981; Nilsson, 2006; Jacobsen et al., 2017; Möller et al., 2020). The pike spawning period is from February to May in waters with temperatures from 5 to 14°C (Raat, 1988; Horbowa & Fey, 2013). Anadromous Baltic pike populations spawn in fresh waters in seasonal wetlands (Müller, 1986; Nilsson, 2006; Engstedt et al., 2010; Nilsson et al., 2014), while resident brackish pike populations that do not require fresh water for successful reproduction spawn directly in shallow saline waters in coastal bays (Andersson et al., 2000; Westin & Limburg, 2002; Lappalainen et al., 2008; Lehtonen et al., 2009; Engstedt et al., 2010; Jørgensen et al., 2010; Rohtla et al., 2012; Jacobsen et al., 2017; Möller et al., 2019, 2020). After spawning, the two populations mix during the feeding season in coastal waters (Engstedt et al., 2014). Eggs are deposited on vegetation (Bry, 1996; Raat, 1988; Craig, 2008; Jacobsen & Engstrom-Öst, 2018) and develop, depending on water temperature, within eight to 21 days (Frost & Kipling, 1967; Jørgensen et al., 2010; Cooper, 2016; Fey et al., 2018, 2019). Exogenous feeding starts a few days before yolk absorption, for example, 9 days post-hatching at 12°C (Pospisilova et al., 2019). Immediately after yolk-sac absorption, which occurs at 12°C within 16–22 days post-hatching (Pospisilova et al., 2019), cannibalistic behavior starts (Greszkiewicz & Fey, 2020). The growth of larvae and early juveniles during the first weeks may be higher than 1 mm d−1 if the temperature is above 20°C (Fey & Greszkiewicz, 2020). For a comprehensive and recent review on pike biology and ecology, see Skov & Nilsson (2018).
Resident brackish pike populations occur in the coastal zones of Denmark (Jørgensen et al., 2010; Jacobsen et al., 2017), Finland (Lappalainen et al., 2008; Lehtonen et al., 2009), Sweden (Andersson et al., 2000; Nilsson, 2006; Engstedt et al., 2010), Germany (Möller et al., 2019), and Estonia (Rohtla et al., 2012). The proportion of brackish populations in different areas varies; for example, it can be as high as 94% in Rugen, Germany (Möller et al., 2019) and 56% on the southern Sweden coast (Engstedt et al., 2010) or as low as 18% in Estonia (Rohtla et al., 2012), while there is no information on the occurrence of resident brackish populations in the coastal waters of Poland. Maps from the 1920s indicate that there was a pike protection area in Puck Lagoon that could be an indication of pike spawning in the bay (Fig. 1). However, it could also be an anadromous population under protection. Resident brackish pike populations have no problems with access to spawning grounds located directly in bays, but other factors could reduce spawning success, such as increased eutrophication and turbidity (Sandström & Karås, 2002; Lehtonen et al., 2009; Olsson, 2019), changes in communities of fish that prey on pike eggs, such as stickleback (Nilsson, 2006; Donadi et al., 2020), or inflows of saltwater through the Danish Straits that can cause significant mortality in both early developmental stages and adults (Dahl, 1961; Jacobsen et al., 2007, 2008; Jørgensen et al., 2010). In contrast, the reproduction of anadromous pike populations inhabiting brackish waters is frequently limited as a result of human activities, including the construction of barriers that restrict access to the upper reaches of rivers and the wetlands located there (Casselman & Lewis, 1996), as well as river regulation and land drainage that drastically decrease wetlands (Hoffman et al., 2000). As a consequence of these factors that affect both anadromous and resident brackish pike populations, many Baltic Sea populations are close to extinction, as indicated by pike catches of commercial and recreational fisheries (Andersson et al., 2000; Nilsson, 2006; Lehtonen et al., 2009; Nilsson et al., 2014; Larsson et al., 2015; Olsson, 2019).
Map of Puck Lagoon and Puck Bay with the Płutnica and Reda Rivers marked, which no longer provided appropriate freshwater pike spawning habitats because of the drainage of surrounding lands and the location of hydrotechnical installations (triangles) constructed in the 1970s. The pike protection area designated in the 1920s was located in the saline waters of the bay (marked with green translucent area), which suggests that presence of a resident brackish pike population in this geographical area could not be excluded
Puck Lagoon is one example of a brackish bay with restricted access to freshwater spawning grounds in the Baltic Sea. Data from fisheries catches indicate that the pike population in this reservoir has declined drastically over the past few decades. From the 1960s to the mid-1970s, pike catches in Puck Lagoon and Puck Bay were 40 to 50 tons annually (Jackowski, 2002). Since this period, catches have decreased steadily, and since the 1990s, annual catches have been in the range of 50–100 kg (Jackowski, 2002) (Fig. 2). Catches from Puck Lagoon constitute approximately 95% of the total catches presented.
Pike catches in Puck Lagoon and Puck Bay in 1964–2004 (Jackowski, 2002) and 2005–2019 (data from the Fisheries Monitoring Center in Gdynia, Poland). The gray area indicates 2010–2013 when pike fry stocking was performed, which corresponded to notable increases in catches
As is the case in other Baltic regions, one possible explanation for the drop in pike abundance in Puck Lagoon is the absence of appropriate spawning habitats resulting from vanishing wetlands from river regulation and hydrological constructions blocking access to local rivers, which is a current problem (see Fig. 1). Because of their considerably limited reproductive success, the continued existence of pike populations currently depends on stocking (data in this study), which is conducted in Puck Lagoon with fry obtained from freshwater spawners and is only performed during research projects such as ZOSTERA (2010–2013) and PIKE (2020–2023). Although the Polish Angling Association regularly stocks small feeding fry, this is only done in rivers that have very restricted connections with Puck Lagoon waters. Since freshwater fry are used for stocking, the pike population in Puck Lagoon is not a typical self-reproducing anadromous population but rather one of freshwater specimens.
In spawning grounds with restricted access to fresh water, the question arises of whether it is possible for freshwater populations to reproduce directly in brackish bay waters. Is there a salinity level that permits reproduction directly in bay waters at river mouths where salinity is relatively low? There are many reports of successful pike spawning in saline waters, but these refer to resident brackish populations (Westin & Limburg, 2002; Lappalainen et al., 2008; Lehtonen et al., 2009; Engstedt et al., 2010; Jørgensen et al., 2010; Jacobsen et al., 2017; Möller et al., 2020), and there is no such population in Puck Lagoon. Although information on the phenomenon of freshwater populations adapting to saline conditions is available (Sunde et al., 2018), no data of this kind are available for Puck Lagoon. It is also worth considering how spawning efficiency is affected if eggs deposited in freshwater wetlands located directly in coastal zones are flooded with brackish saline waters; however, no data of this kind are available.
The aim of this study was to determine under experimental conditions the impact of water salinity (0, 4, and 7 PSU) on the success of egg development and larval hatching of pikes from a freshwater population. The current Puck Lagoon freshwater pike population originates from stocking with fishes from a freshwater population, while the original brackish population that undertook spawning migrations into rivers no longer exists. The option of fertilizing eggs in freshwater and transferring them to higher salinities of 4 and 7 PSU was considered in light of possible flooding in spawning grounds located in wetlands in coastal saline waters (natural or restored). The water salinity range used in the experiments reflected that of fresh water (control group), transitional waters in the lower reaches of rivers flowing into the Baltic Sea, and the waters of the coastal Puck Lagoon. This work also describes the current state of the pike population in Puck Lagoon, includes data on pike catches from 1964 to 2019, and discusses possible management strategies.
Materials and methods
Study area
Although the present study is based on data obtained under experimental conditions, the concept of the experiment is strongly linked to the drastic decline in the pike population in Puck Lagoon, Baltic Sea (Fig. 1), which has been noted since the 1970s (Fig. 2). Even if these data are not catch per unit effort (CPUE) data, they do indicate a decline. According to the Polish Fisheries Monitoring Center, which has collected electronic catch data since 2004, pikes are caught with the use of fyke nets and gill nets in Puck Lagoon. Because the same type of nets were the main fishing tools in 60s–80s catches (National Marine Fisheries Research Institute, unpublished data), the catch data presented in Fig. 2 for different years should be considered comparable. Figure 1 presents a map of the local Płutnica and Reda Rivers that were regulated in the 1970s and are currently more akin to channels with limited vegetation than to natural rivers. Pump stations in their lower segments contributed to the disappearance of wetlands along these rivers and restricted access to shallower streams that branch off and flow away from the main river channels. The pike protection area designated in the 1920s is also noted on the map (redrawn from historical map from year 1920), which could suggest that a resident brackish pike population lived in Puck Lagoon at this time.
Experimental system set-up
The experimental system set-up comprised three separate recirculating systems (Fig. 3). Each of them included three experimental mini Weiss jars of 0.5 l each connected to glass tanks that served as hatched larva containers. Each system included a 70 l PVC filtration-sedimentation tank that was filled with 20 l of biological media (Bioceramax Pro 600, 600 m2 l−1, Aquael). A UV lamp was installed at the water flow. Each recirculating system permitted conducting the experiment at three different salinities (0, 4, and 7 PSU) and in three replicates for each salinity.
Experimental conditions
Two experiments were conducted to study the effect of salinity on pike egg development. Eggs for the studies were obtained from four females (66–73 cm TL), and milt was obtained from two males (52–59 cm TL) caught in mid-April with fyke nets from freshwater populations in Ostrzyckie Lake (geographical position: 54° 15′ 13.1″ N; 18° 06′ 15.8″ E). The pikes were stripped directly after they were caught, and eggs were mixed with milt. Approximately 4.5 ml of milt was used. Fertilization (adding water) was performed after the eggs were transported to the laboratory. The transport took one hour. In the laboratory, the eggs were divided into two groups. In Experiment I, the eggs from one group were fertilized in fresh water (nonchlorine tap water). After one hour, the eggs were transferred to incubation jars with different salinities (salinity during the incubation time, mean ± SD): 0.18 ± 0.04; 4.0 ± 0.21; and 6.9 ± 0.17 PSU (Fig. 3). In Experiment II, the eggs from the other group were fertilized directly at different salinities and then transferred to incubation jars (salinity during the incubation time, mean ± SD: 0.17 ± 0.05; 4.1 ± 0.26; and 7.0 ± 0.15 PSU) (Fig. 3). Water pumped from Puck Bay (7 PSU) was used to obtain different salinities. The quantity of eggs placed in each incubation jar was 200 ml in both experiments, which corresponds, as estimated by volumetric method, to ca. 10 000 eggs. Although some variation in the salinities in the three groups was noted, we referred throughout the publication to salinity Groups 0, 4, and 7 PSU. The light regime for simulating natural conditions in spring was set to 12 h light/12 h dark. The light period was from 08:00 to 20:00 and was regulated automatically. The temperature was set to 11 ± 0.4°C (mean ± SD) and measured twice daily at the same time as salinity. This temperature was within the optimum for pike reproduction (8–15°C, Raat, 1988) that occurs during spawning periods in shallow, sun-heated coastal waters (8.9–13.8°C, Nilsson, 2006). During the period from fertilization to larval hatching, egg samples (approximately 10 ml) were collected from each incubation jar daily to assess egg development.
This experimental set-up based on using pikes from a freshwater environment was intentional since pikes inhabiting Puck Lagoon originate from stocking with freshwater fry, and self-reproducing anadromous populations of a number of generations are absent from this area even if it cannot be excluded that occasionally some specimens come from natural spawning.
Analysis of egg development
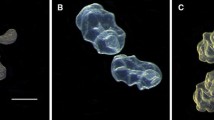
The eggs collected daily from individual jars were mixed among the three replicates from each of the three salinities. Then, 50 eggs were chosen at random daily from each of the three salinity variants. The eggs were analyzed with an image analysis system (NIS Elements, Nikon) coupled with a Nikon SMZ-18 binocular microscope (Nikon Corp., Japan). The analyses included (1) determining the developmental stage of individual eggs according to the six-stage scale in Fig. 4 (blastula formation, gastrulation, embryo formation, embryos with optic cups, eyed embryos, and hatched larvae), which was based on descriptions of pike egg development by Raat (1988) and Cooper (2016), and (2) determining the hatching rate. The fertilization rate was not determined. Egg swelling, which is an indicator of successful fertilization, could not be detected retrospectively.
Data analysis
Hatching success (%) was estimated by counting the unhatched eggs and subtracting them from the number of fertilized eggs. Differences in hatching success among salinities in the experiments were evaluated with Kruskal–Wallis test for independent samples and the post hoc Dunn’s test with Bonferroni correction for multiple tests. The statistical significance of the effect of salinity (0, 4, and 7 PSU) on egg development was evaluated separately for the two experiments with GLM repeated measure model (from the mixed-effects models category) with egg development stage as the categorical response variable with 6 levels, salinity as the between-subject variable (fixed effects factor), and degree-days as the within-subject variable (a random effects factor) with 13 (experiment I) and 10 (Experiment II) levels. The degree-days as a continuous variable has been added to the model as a random slope. In addition to the main effects, the interaction of between-subjects term with within-subjects term is included in the model as well. The assumption of the sphericity of the covariance matrix was verified with Mauchly’s test. If this assumption was violated, a Greenhouse–Geisser correction adjustment to the degrees of freedom was applied for the within-subject analysis. Model evaluation showed that the model residuals were normally distributed. Pairwise comparisons of salinity effects were evaluated with the post hoc Tukey HSD test. Pairwise comparisons among salinity groups within degree-days were based on estimated marginal means with a Bonferroni adjustment for repeated comparisons. Additionally, the rate of egg development was evaluated among the salinities by comparing the slopes of the line regressions fitted to the relationships between egg development stage and degree-days. The egg developmental stage is the same categorical response variable with 6 levels as in the GLM repeated measure analysis. The pairwise differences between slopes for salinity groups were assessed with Tukey’s HSD post hoc test. Statistica ver. 12.0 (TIBCO Software, Inc., Palo Alto, CA) and IBM SPSS Statistics ver. 26 (IBM Corp.) were used for the data analysis. Differences were considered statistically significant at P < 0.05 (α = 0.05).
Results
Experiment I
During Experiment I (eggs fertilized in fresh water and transferred after 1 h to incubate at 0, 4, and 7 PSU), all the eggs in the subsamples analyzed from the three salinities were fertilized. The effect of the degree-day (DD, temperature x day) on the egg developmental stage was statistically significant (GLM repeated measure, P < 0.01), but the effect of salinity was not (GLM repeated measure, P = 0.138) (Table 1). However, the statistically significant interaction term between degree-days and salinity indicated that the magnitude of the within-subject differences was salinity dependent. Until 85 DD, egg development in the three salinity variants was very similar (Fig. 5 and S1), and no significant differences were observed, except for 55 DD (GLM repeated measure, salinity comparisons within DD based on estimated marginal means) (Table 2). The acceleration of egg development at 7 PSU from 98 DD resulted in significant differences at 115 DD when all the larvae hatched at 7 PSU. At 0 and 4 PSU, most of the eggs were still in the eyed embryo stage at 115 DD, and mass hatching occurred at 135 DD.
The overall rate of egg development (i.e., slopes of linear regression lines fitted to the egg development stage at 0 DD data; Fig. 6) was significantly different among salinities (ANCOVA, P < 0.001) (Table 2); however, the differences were only significant between 0 and 7 PSU and between 4 and 7 PSU (post hoc Tukey HSD, P < 0.001). There were no differences between 0 and 4 PSU (post hoc Tukey HSD, P = 0.655). The differences in slopes indicated faster egg development rates at 7 PSU than at 0 and 4 PSU.
Differences in the number of eggs at individual stages of development (scale from 1 to 6, according to Fig. 1) on given sampling days (DD) at salinities of 0 (A), 4 (B), and 7 (C) after fertilization in fresh water (Experiment I). The size of the circles corresponds to the number of eggs (only live eggs were analyzed). Linear regression functions were fitted to describe egg development rates at given salinities
Low egg mortality at all three salinities resulted in high rates of hatching success (80.0%—salinity 0 PSU; 83.3%—salinity 4 PSU; and 93.7%—salinity 7 PSU) (Table 3). The differences were statistically significant overall (Kruskal–Wallis, P < 0.05) and between 0 and 7 PSU (post hoc Dunn-Bonferroni test, P < 0.05). The differences between 0 and 4 PSU and between 4 and 7 PSU were not statistically significant (post hoc Dunn-Bonferroni test, P = 0.89 and P = 0.41).
Experiment II
During Experiment II (eggs fertilized at 0, 4, and 7 PSU), at the beginning of the experiment, there were no signs of egg development in the eggs fertilized at 7 PSU (Figs. 7 and S2), while at 4 PSU, 16.7% of the eggs, and at 0 PSU, 5% of eggs, did not develop. The effects of both salinity and degree-days on the differences in the egg developmental stage among the salinity groups (0, 4, and 7 PSU) were statistically significant (GLM repeated measure, P < 0.01) (Table 1). Pairwise comparison showed significant differences among all the salinity pairs (0–4, 0–7, and 4–7 PSU) (post hoc Tukey HSD, all P < 0.05). The interaction term between the effect of degree-days and salinity was also significant (GLM repeated measure, P < 0.01), which indicated that the magnitude of within-subject differences was dependent on salinity. At 0 PSU, egg development proceeded normally, and after gastrulation, most of the eggs reached stage 5 (embryos with optic cups) at 54 DD, followed by the eyed embryo stage at 77 DD. At 4 PSU, egg development proceeded similarly to that at 0 PSU until 47 DD, but significant differences were noted from 54 DD (GLM repeated measure, salinity comparisons within DD based on estimated marginal means) (Table 1), and the development of most eggs at 4 PSU stopped at the gastrulation stage.
The effect of salinity on the rate of pike egg development (i.e., slopes of linear regression lines fitted to the egg development stage at 0 DD data) (Fig. 8) was statistically significant (ANCOVA, P < 0.001) (Table 2). The differences in slopes reflect the faster egg development rate at 0 PSU than at 4 PSU.
Difference in the number of eggs at individual stages of development (scale from 1 to 6, according to Fig. 1) on given sampling days (DD) at salinities of 0 (A) and 4 (B) after fertilization in saline water (Experiment II). Circle size corresponds to the number of eggs (only live eggs were analyzed)
Pronounced statistically significant differences among the three salinities were observed in successful hatches (89.7%—at 0 PSU; 19.7%—at 4 PSU; and 0.0%—at 7 PSU; Table 3) (Kruskal–Wallis, P < 0.05). The differences among salinity (Kruskal–Wallis, P < 0.05) and between 0 and 7 PSU (post hoc Dunn-Bonferroni test, P < 0.05) were statistically significant. The differences between 0 and 4 PSU and between 4 and 7 PSU were not statistically significant (post hoc Dunn-Bonferroni test, P = 0.54).
Discussion
Egg development: comparison to freshwater population
The results of the present study on pikes from a freshwater population showed that egg development in the saline waters of the coastal Baltic Sea (7 PSU) would be unsuccessful. Although the salinity of 4 PSU, which might occur in river mouths, provided some likelihood of pike egg survival, the number of hatched larvae was low (20%). The number of publications providing results on egg development at different salinities for strictly freshwater populations, not anadromous populations, is limited. Although a slight increase in water salinities of 1–2 PSU might have positive effects on egg development and hatching success, further increases in salinity are disadvantageous for pike eggs (Bonisławska, 2014; Kuznetsov et al., 2016). In a synopsis on northern pike biological data, Raat (1988) reported that freshwater pikes are unable to produce offspring at salinities above 7 PSU. Bonisławska (2014) presented results from an experimental study on significantly increased freshwater pike embryo mortality and decreased hatching success at salinities higher than 2 PSU (hatching success at 3, 4, and 5 PSU was 29.2, 17.4, and 3.5%, respectively). At the same time, the number of deformed larvae at the hatching stage increased from 4.8% at 2 PSU to 24.6 at 3 PSU and 40% at 5 PSU. Thus, our results confirm previously reported information on significantly increased freshwater pike egg mortality starting from salinities of approximately 3 PSU and on the upper limit for their development at salinities of approximately 5 PSU.
Egg development: comparison to anadromous population
Our results can also be compared to other studies on the freshwater spawning of anadromous pike populations in the Baltic Sea. For pikes collected in freshwater streams and coastal waters of Kalmar Sound and Gotland Island, Westin & Limburg (2002) reported that a salinity of 6 PSU was the barrier for anadromous populations but that egg development was successful at 5 PSU. Although these authors did not provide detailed information on the proportion of hatching eggs, their results were generally consistent with ours. A significant difference in the number of developing eggs in the anadromous pike population from Kalmar Sound was noted between salinities of 0 and 3 PSU, while differences were higher between salinities of 0 and 5, 7, and 9 PSU (Sunde et al., 2018). Interestingly, an approximately 6% hatching success was observed even at 7 and 9 PSU. As mentioned previously, our results showed no egg development at a salinity of 7 PSU. In comparison with the present study, the higher resistance of pike eggs to increased salinity that Sunde et al. (2018) reported could have resulted from different adaptations between anadromous and freshwater specimens. In particular, they reported that the subpopulation they studied was exposed in its natural spawning habitat to occasional brackish water inflows. Moreover, the subpopulation occasionally exposed to increased salinities exhibited a significantly greater tolerance to high salinity with hatching success at 7–9 PSU of approximately 35% than did the subpopulation that spawned naturally in a stable, freshwater habitat. Sunde et al. (2018) indicated that some differences in egg development success in saline water could exist among anadromous populations in different areas of the Baltic Sea. Analogically, differences between anadromous and strictly freshwater populations, such as the one studied in this work, can also be expected. Vetemaa & Saat (1996) provided support for this theory in their study, which indicated that the eggs of ruffe, Gymnocephalus cernuus (Linnaeus, 1758), which is primarily a freshwater species, from a brackish population hatched at higher salinities (10 PSU) than did eggs from a freshwater population (below 8 PSU).
The mechanisms of the negative impact of salinity on embryonic development vary depending on fish species and salinity levels, and they can impact different periods of embryogenesis. For example, increased salinity can affect sperm mobility, eggshell permeability, or the development of the perivitelline space (Bonisławska, 2014). In the present experiment, normal egg development at 4 PSU was observed for up to 54 DD, after which most eggs ceased development at the gastrulation stage. Similar salinity results affecting egg development before the embryonic phase were presented by Jørgensen et al. (2010) for pike and by Vetemaa & Saat (1996) for ruffe. The most likely explanation in the case of pikes seems to be the potential effect of saline water on eggshell permeability resulting in disturbed ion transfer inside the eggs (Bonisławska, 2014).
Egg development: comparison to resident brackish population
Although salinity above approximately 2–3 PSU significantly affects egg development and the hatching success of both anadromous and freshwater pike populations, this is not the case for resident brackish populations that successfully reproduce in waters with salinities above 6 PSU. Jørgensen et al. (2010) reported on a resident brackish pike population from Stege Nor (Denmark), and their eggs developed normally at salinities of 6 and 8.5 PSU, while at the lower salinities of 0 and 3, PSU development was abnormal and led to zero hatching success. Similar results for resident brackish populations were reported by Westin & Limburg (2002), who observed normal embryonic development at 6.6 and 6.9 PSU. Although the occurrence of resident pike populations has been described in numerous Baltic coastal areas, there is no sign of them in Polish coastal waters, including Puck Lagoon. However, historical information about the location of a pike protection area in Puck Lagoon (redrawn from historical map from year 1920) (see Fig. 1) suggests that a sea-spawning population might have inhabited this location in the past. Unfortunately, it is impossible to verify this theory.
Egg development: inflow of saline waters into freshwater wetlands
In the present study, embryonic development was also observed when eggs were fertilized in fresh water and transferred to saltwater after 1 h. The eggs developed normally not only in a salinity of 0 but also in salinities of 4 and 7 PSU. In fact, hatching success was the highest at a salinity of 7 PSU and was higher at 4 PSU than at 0 PSU. Thus, it can be concluded that as soon as fertilization is successful and embryonic development is initiated, higher salinities are not only not harmful but might even have a positive effect on freshwater pike egg development and hatching success. This situation could occur under natural conditions, for example, when freshwater marshes (both natural and restored) are flooded during wind-driven backflows of brackish water into the mouths of rivers or streams. This conclusion is, of course, limited to geographical areas in which the salinity of coastal waters is not higher than 7 PSU. In the western Baltic, for example, on the Danish coast, inflows of highly saline water of 10 PSU and greater are natural phenomena (Jørgensen et al., 2010). No other data on egg development in such an ecological scenario are available for comparison.
Using freshwater population in the current study referring to coastal waters
To some readers, it may be confusing that the current study of the environmental impact of Puck Lagoon (i.e., Baltic Sea coastal waters) on pike spawning was conducted using spawners from freshwater populations. The reason for this was the lack of any established anadromous populations in this geographical area. Natural reproduction has been limited since the 1970s because of restricted access to suitable freshwater spawning habitats. River regulation eliminated wetlands, and hydrological constructions such as pumping stations blocked access to shallow parts of two local rivers (see map in Fig. 1). Even if some individuals occasionally find their way to the main corridors of local rivers, these no longer provide appropriate habitats for pike spawning since they lack shallow, sheltered areas with slow currents and vegetation suitable for egg deposition. The magnitude of such natural spawning is extremely limited, which is evidenced by the pike catch data presented in this study. The existence of the Puck Lagoon pike population is possible mostly due to occasional stocking performed with fry obtained from freshwater populations. This is why the question of whether pike reproduction is successful in the saltwater environment of Puck Lagoon must be answered with experiments on eggs obtained from freshwater spawners.
Conclusion and suggested management strategies
The extremely low pike population level in Puck Lagoon raises questions about which management strategies should be undertaken. As the results of our study revealed, the ability of the local population, which is more freshwater in nature than anadromous, to spawn in the saline waters of the bay is negligible. At the same time, natural pike spawning in fresh water is currently almost impossible because of the lack of suitable spawning grounds. Stocking might be a way to improve the situation, but only in the short-term (see Fig. 2) unless a long-term stocking program is developed. However, a long-term stocking program conducted in Danish waters was unsuccessful, probably because of the low survival of the freshwater fry that were released (Larsen et al., 2005; Jacobsen et al., 2008) into waters that had a much higher salinity (approximately 8–10 PSU) than that in Puck Lagoon (approximately 6–7 PSU). Thus, presumably a long-term stocking program in Puck Lagoon could be successful. However, with the Danish experience in mind, acclimatizing fry to saline waters during rearing before their release as stocking material must also be considered.
The most promising solution, however, seems to be restoring spawning grounds by introducing so-called pike factories, as has been done in Sweden and Denmark (Nilsson et al., 2014; Engstedt et al., 2017). Engstedt et al. (2017) provided an extended review of pike spawning habitat restoration. In general, pike factories are a more natural way of restoring spawning grounds compared to the popular North American rearing marshes into which adult pikes are stocked to spawn (Fago, 1977). The present study revealed that the survival of eggs was good in saline waters if they were fertilized and embryonic development began in fresh water; thus, introducing pike factories could be a successful solution. The results of the current study indicate that it would be possible even to locate pike factories in areas directly adjacent to lagoons and bays despite the risk of flooding with saline waters. In fact, an increased salinity of up to 7 PSU just one hour after fertilization was beneficial for hatching success.
One more management possibility would be to introduce a resident brackish pike population by releasing this type of fry stocking material into Puck Lagoon and other similar habitats in Baltic Sea coastal areas. There are indications that such a population inhabited Puck Lagoon in the past. This strategy of introducing a resident brackish pike population to restore pikes to Baltic Sea habitats has already been suggested by Lappalainen et al. (2008) as a parallel solution to the creation of pike factories.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Andersson, J., J. Dahl, A. Johansson, P. Karas, J. Nilsson, O. Sandstrom & A. Svensson, 2000. Utslagen fiskrekrytering och sviktande fiskbestånd i Kalmar läns kustvatten. Fiskeriverket Rapport 1998–2000: 3–42.
Bonisławska, M., 2014. Przebieg wczesnej ontogenezy wybranych gatunków ryb w wodach o zróżnicowanym zasoleniu [The course of early ontogenesis of selected fish species in waters characterized by different salinities]. Zachodniopomorski Uniwersytet Technologiczny w Szczecinie: 84 pp.
Bry, C., 1996. Role of vegetation in the life cycle of pike. In Craig, J. F. (ed), Pike Fish and Fisheries Series, Vol. 19. Springer, Dordrecht.
Casselman, J. & C. A. Lewis, 1996. Habitat requirements of northern pike (Esox lucius). Canadian Journal of Fisheries and Aquatic Sciences 53: 161–174.
Cooper, J.E., 2016. Development, growth, and food of early life stages of Northern Pike (Esox lucius) and Muskellunge (Esox masquinongy) in the Upper St. Lawrence River. http://cooperenvironmentalresearch.com.
Craig, J. F., 1996. Pike: Biology and Exploitation, Chapman and Hall, London.
Craig, J. F., 2008. A short review of pike ecology. Hydrobiologia 601: 5–16.
Crane, D. P., L. M. Miller, J. S. Diana, J. M. Casselman, J. M. Farrell, K. L. Kapuscinski & J. K. Nohner, 2015. Muskellunge and Northern Pike ecology and management: important issues and research needs. Fisheries 40: 258–267.
Dahl, J., 1961. Alder og vækst hos danske og svenske brakvandsgedder. Ferskvandsfiskeribladet 59: 34–38 ((in Danish)).
Donadi, S., L. Bergström, J. M. B. Berglund, B. Anette, R. Mikkola, A. Saarinen & U. Bergström, 2020. Perch and pike recruitment in coastal bays limited by stickleback predation and environmental forcing. Estuarine, Coastal and Shelf Science 246: 107052.
Engstedt, O., P. Stenroth, P. Larsson, L. Ljunggren & M. Elfman, 2010. Assessment of natal origin of pike (Esox lucius) in the Baltic Sea using Sr: Ca in otoliths. Environmental Biology of Fishes 89: 547–555.
Engstedt, O., R. Engkvist & P. Larsson, 2014. Elemental fingerprinting in otoliths reveals natal homing of anadromous Baltic Sea pike (Esox lucius L.). Ecology of Freshwater Fish 23: 313–321.
Engstedt, O., J. Nilsson & P. Larsson, 2017. Habitat restoration – A sustainable key to management. In Skov, C. & A. Nilsson (eds), Biology and ecology of pike CRC Press, Taylor & Francis Group, Boca Ratón, FL: 248–268.
Eriksson, B. K., K. Sieben, J. Eklöf, L. Ljunggren, J. Olsson, M. Casini & U. Bergström, 2011. Effects of altered offshore food webs on coastal ecosystems emphasize the need for cross-ecosystem management. AMBIO 40: 786.
Fago, D. M., 1977. Northern pike production in managed spawning and rearing marshes. Technical Bulletin: Wisconsin Department of Natural Resources 96: 1–30.
Fey, D. P. & M. Greszkiewicz, 2020. Effects of temperature on somatic growth, otolith growth, and uncoupling in the otolith to fish size relationship of larval northern pike, Esox lucius L. Fisheries Research 236: 105843.
Fey, D. P., A. M. Lejk & M. Greszkiewicz, 2018. Daily deposition of growth increments in sagittae and lapilli of laboratory-reared larval northern pike (Esox lucius). Fisheries Bulletin 116: 302–309.
Fey, D. P., M. Greszkiewicz, Z. Otremba & E. Andrulewicz, 2019. Effect of static magnetic field on the hatching success, growth, mortality, and yolk-sac absorption of larval Northern pike Esox lucius. Science of the Total Environment 647: 1239–1244.
Frost, W. & C. Kipling, 1967. A study of reproduction, early life, weight-length relationship and growth of Pike, Esox lucius L., in Windermere. Journal of Animal Ecology 36: 651–693.
Greszkiewicz, M. & D. P. Fey, 2020. Positive temperature effects on the initiation and intensity of cannibalistic behavior of larval pike, Esox lucius L. Is cannibalism reflected in otolith fluctuating asymmetry? Hydrobiologia 847: 3139–3152.
Hoffman, M., H. Johnsson, A. Gustafson & A. Grimvall, 2000. Leaching of nitrogen in Swedish agriculture—a historical perspective. Agriculture Ecosystems & Environment 80: 277–290.
Horbowa, K., & Fey, D.P., 2013. Atlas of early developmental stages of fish - 34 species of the southern Baltic Sea. Morski Instytut Rybacki - Państwowy Instytut Badawczy, Gdynia, 152 pp.
Jackowski, E., 2002. Ryby Zatoki Puckiej [Fish of Puck Bay]. Morski Instytut Rybacki - Państwowy Instytut Badawczy, Gdynia (in Polish), 108 pp.
Jacobsen, L. & J. Engström-Öst, 2018. Coping with environments; vegetation, turbidity and abiotics. In Skov, C. & A. Nilsson (eds), Biology and Ecology of Pike CRC Press, Taylor & Francis Group, Boca Ratón, FL: 32–61.
Jacobsen, L., C. Skov, A. Koed & S. Berg, 2007. Short term salinity tolerance of Northern pike (Esox lucius L.) fry, related to temperature and size. Fisheries Management and Ecology 14: 303–308.
Jacobsen, L., Skov, C., Berg, S., Koed, A., & Larsen, P F., 2008. Udsætning af Geddeyngel som Bestandsophjælpning i Danske Brakvandsområder – Effektvurdering og Perspektivering. DTU Aqua-rapport no. 196–08. Institute for Aquatic Resources, Danish Technical University. (In Danish).
Jacobsen, L., D. Bekkevold, S. Berg, N. Jepsen, A. Koed, K. Aarestrup, H. Baktoft & C. Skov, 2017. Pike (Esox lucius L.) on the edge: consistent individual movement patterns in transitional waters of the western Baltic. Hydrobiologia 784: 143–154.
Jørgensen, A. T., B. W. Hansen, B. Vismann, L. Jacobsen, C. Skov, S. Berg & D. Bekkevold, 2010. High salinity tolerance in eggs and fry of a brackish Esox lucius population. Fisheries Management and Ecology 17: 554–560.
Kuznetsov, V. A., S. V. Lukiyanov, Y. A. Lobachyov & A. N. Loginova, 2016. Influence of salinity fluctuations on the embryonic and larval development of pike Esox lucius (Esocidae). Journal of Ichthyology 56: 281–288.
Lappalainen, A., M. Härmä, S. Kuningas & L. Urho, 2008. Reproduction of pike (Esox lucius) in reed belt shores of the SW coast of Finland, Baltic Sea: a new survey approach. Boreal Environment Research 13: 370–380.
Larsen, P. F., M. M. Hansen, E. E. Nielsen, L. F. Jensen & V. Loeschcke, 2005. Stocking impact and temporal stability of genetic composition in a brackish northern pike population (Esox lucius L.), assessed using microsatellite DNA analysis of historical and contemporary samples. Heredity 95: 136–143.
Larsson, P., P. Tibblin, P. Koch-Schmidt, O. Engstedt, J. Nilsson, O. Nordahl & A. Forsman, 2015. Ecology, evolution and management strategies of Northern pike populations in the Baltic Sea. AMBIO A Journal of the Human Environment 44: 451–461.
Lehtonen, H., E. Leskinen, R. Selen & M. Reinikainen, 2009. Potential reasons for the changes in the abundance of pike, Esox lucius, in the western Gulf of Finland, 1939–2007. Fisheries Management and Ecology 16: 484–491.
Möller, S., H. M. Winkler, A. Klügel & S. Richter, 2019. Using otolith microchemical analysis to investigate the importance of brackish bays for pike (Esox lucius Linnaeus, 1758) reproduction in the southern Baltic Sea. Ecology of Freshwater Fish 28: 602–610.
Möller, S., H. M. Winkler, S. Richter & R. Bastrop, 2020. Genetic population structure of pike (Esox lucius Linnaeus, 1758) in the brackish lagoons of the southern Baltic Sea. Ecology of Freshwater Fish 30: 1–10.
Müller, K., 1986. Seasonal anadromous migration of the pike (Esox lucius L.) in coastal areas of the northern Bothnian Sea. Archiv Fur Hydrobiologie 107: 315–330.
Nilsson, J., 2006. Predation of Northern Pike (Esox lucius L.) eggs: a possible cause of regionally poor recruitment in the Baltic Sea. Hydrobiologia 553: 161–169.
Nilsson, J., O. Engstedt & P. Larsson, 2014. Wetlands for northern pike (Esox lucius L.) recruitment in the Baltic Sea. Hydrobiologia 721: 145–154.
Ojaveer, E., 1981. Fish fauna of the Baltic Sea. In Voipio, A. (ed), The Baltic Sea. Oceanography Series 30. Elsevier, Amsterdam.
Olsson, J., 2019. Past and current trends of coastal predatory fish in the Baltic Sea with a focus on perch, pike, and pikeperch. Fishes 4: 7.
Persson, A., P. A. Nilsson & C. Brönmark, 2018. Trophic interactions. In Skov, C. & A. Nilsson (eds), Biology and Ecology of Pike CRC Press, Taylor & Francis Group, Boca Ratón, FL: 185–211.
Pierce, R. B., C. M. Tomcko & D. H. Scchupp, 1995. Exploitation of nrthern pike in seven small north-central Minnesta lakes. North American Journal of Fisheries Management 15: 601–609.
Pospisilova, A., J. Brejcha, V. Miller, R. Holcman, R. Šandra & J. Stundl, 2019. Embryonic and larval development of the northern pike: an emerging fish model system for evo-devo research. Journal of Morphology 280: 1118–1140.
Raat, A., 1988. Synopsis of biological data on the northern pike: Esox lucius Linnaeus, 1758. FAO Fisheries Synopsis 145: 178 pp.
Rohtla, M., M. Vetemaa, K. Urtson & A. Soesoo, 2012. Early life migration patterns of Baltic Sea pike Esox lucius. Journal of Fish Biology 80: 886–893.
Sandström, A. & P. Karås, 2002. Effects of eutrophication on young-of-the-year freshwater fish communities in coastal areas of the Baltic. Environmental Biology of Fishes 63: 89–101.
Skov, C. & P. A. Nilsson, 2018. Biology and Ecology of Pike, CRC Press, Milton:
Sunde, J., C. Tamario, P. Tibblin, P. Larsson & A. Forsman, 2018. Variation in salinity tolerance between and within anadromous subpopulations of pike (Esox lucius). Scientific Reports 8: 22.
Vetemaa, M. & T. Saat, 1996. Effects of salinity on the development of fresh-water and brkish-water ruffe Gymnocephalus cernuus (L) embryos. Annales Zoologici Fennici 33: 687–691.
Westin, L. & K. E. Limburg, 2002. Newly discovered reproductive isolation reveals sympatric populations of Esox lucius in the Baltic. Journal of Fish Biology 61: 1647–1652.
Funding
This paper is a contribution to Agreement No. 00002-6520.13-OR1100004/19 granted to the National Marine Fisheries Research Institute (Poland) as part of the Rybactwo i Morze Programme (PO RYBY 2014-2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest to declare.
Ethical approval
No animal ethics committee approval and no other specific permissions were required to carry out this study.
Additional information
Handling editor: Grethe Robertsen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10750_2022_4893_MOESM1_ESM.tif
Supplementary file1 (TIF 58177 kb) Figure S1. Photographs of examples of pike eggs in different stages of development (20, 50, 65, 98, and 115 DD) at salinities of 0, 4, and 7 from Experiment I (fertilization in fresh water).
10750_2022_4893_MOESM2_ESM.tif
Supplementary file2 (TIF 57148 kb) Figure S2. Photographs of examples of pike eggs at different stages of development at given hours (17, 38, 54, 77, and 93 DD) in salinities of 0, 4, and 7 from Experiment II (fertilization in one of the three salinities analyzed).
Rights and permissions
About this article
Cite this article
Greszkiewicz, M., Fey, D.P., Lejk, A.M. et al. The effect of salinity on the development of freshwater pike (Esox lucius) eggs in the context of drastic pike population decline in Puck Lagoon, Baltic Sea. Hydrobiologia 849, 2781–2795 (2022). https://doi.org/10.1007/s10750-022-04893-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04893-x