Abstract
A controlled experiment mimicking Atlantic salmon (Salmo salar) pre-smolt farming conditions showed that fish exposed to 150 and 175% super oxygenated water produced higher levels of carbon dioxide with the subsequent decrease in water pH compared to control fish exposed to 100% O2. At the 7th day of exposure the hyperoxic fish showed larger individual variation in swimming activity compared to the controls. The individual variance in activity, tail beat frequency and scattering in the tanks among super oxygenated fish decreased from the 7th to the 21st day of exposure. The behavioural effects of hyperoxia were seen in relation to altered feed consumption halfway through the experiment, lower body weight, and altered haematological variables at day 21 of exposure. Plasma chloride was reduced in the exposed fish and haemoglobin decreased with increasing oxygen saturations. Plasma cortisol was elevated only in the 150% oxygenated group at day 21, while no effect on osmolality was recorded. The alterations in physiology and behaviour from day 7 to day 21 may be explained in terms of acclimation to increased oxygen saturations. This study shows that behaviour may be used as an indication of impaired water quality that may influence animal welfare negatively and eventually prevent an efficient production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In intensive farming of pre-smolt Atlantic salmon (Salmo salar) adding of oxygen to the inlet water is often required to compensate for oxygen deficiency due to reduced water supply and increased fish density. Oxygenation may also be necessary during transportation of fish, while in the salmon farming industry adding of oxygen has been done as an attempt to increase growth and induce disease resistance (Edsall and Smith 1991; Caldwell and Hinshaw 1995). The efficiency of this practice is, however, doubtful (Caldwell and Hinshaw 1994), and supplemental oxygen in excess of saturation may even result in harmful effects among the fish (Caldwell and Hinshaw 1995; Ritola et al. 2002).
Adding oxygen to the inlet water may result in water quality effects, such as increased water carbon dioxide caused by fish production of CO2, increased total ammonia concentration and reduced pH (Fivelstad and Binde 1994). Among reported physiological effects caused by hyperoxia is imbalance in gill ion concentrations induced by respiratory acidosis (Gilmour and Perry 1994; Larsen and Jensen 1997; Brauner et al. 2000) and reduced branchial chloride cell fractional surface area (Goss et al. 1994). Also, a decrease in breathing observed as lowered ventilatory frequencies has been shown (Dejours et al. 1977). Other commonly observed disturbances include different stress indicators measured in blood or plasma, such as effects on cortisol, osmolality, lactate, glucose, haematocrit and haemoglobin (Caldwell and Hinshaw 1994), and plasma chloride (Brauner et al. 2000). Furthermore, exposure to hyperoxic water may cause induction of antioxidants as a defence mechanism against reactive oxygen species (Lygren et al. 2000; Ritola et al. 2002), cause DNA strand breaks (Liepelt et al. 1995) and in extreme cases even cause gas bubble disease and eventually increased mortality (Lygren et al. 2000).
Behaviour may serve as an early warning indicator towards environmental changes in addition to providing relevant information about welfare (Beitnger 1990; Wibe 2003; Dawkins 2004; Huntingford et al. 2006). Behavioural indicators are general indicators in that different stressors may give the same response (Depledge 1994). In aquaculture, establishment of easily quantifiable behavioural indicators will enable the fish farmer to obtain a fast evaluation of fish condition and welfare. Among relevant and easily quantifiable behavioural variables expected to be affected by environmental stressors are swimming (Little and Finger 1990; Steffensen and Farrell 1998), schooling (Shelton and Johnstone 1995; Wibe et al. 2002), vertical and horizontal distribution (Fernö et al. 1995; Juell 1995; Wibe et al. 2002), avoidance or attraction (e.g. Priede et al. 1988; Wannamaker and Rice 2000), and activity (Kramer 1987). We were unable to find relevant literature of behavioural effects of hyperoxia, but behavioural effects of hypoxia have been previously described (Kramer 1987; Beitnger 1990; Steffensen and Farrell 1998; Wannamaker and Rice 2000).
The aims of this study were to investigate the behavioural and physiological effects of hyperoxia in juvenile Atlantic salmon, and to define behavioural variables that may be used to easily evaluate changes in water quality and hence impaired welfare. It was also of interest to see whether the behavioural effects caused by oxygen supply changed with time, possibly as a result of acclimation and/or stress. Physiological indicators were of interest since behaviour is the ultimate consequence of several physiological and biochemical processes and therefore will enable an evaluation of whether the behavioural changes represent impaired welfare.
Materials and methods
Fish maintenance
The experiment was conducted at AKVAFORSK, Institute of Aquaculture Research AS research station in Sunndalsøra, Norway, during October and November 2002. Atlantic salmon parr (Aqua Gen strain; n = 600, body weight = 30.50 ± 0.50 g) (Table 1) were randomly divided into three equivalent groups with two replicates and placed in six cylindrical tanks (170 l), 100 fish per tank. The fish were fed commercial feed (Skretting) continuously and in excess. The tanks were cleaned daily. In order to standardise the behavioural observations, cleaning of the tanks was avoided during the days of behavioural recordings, and on these days fish were fed only directly after termination of the recordings. Bulk weights of each tank were measured before exposure began. Individual length and weight were measured, and condition factor was calculated for 20 fish from each tank at the end of the exposure period (Table 1).
Oxygen exposure
Fish were acclimatised for 7 days in the tanks before start of oxygen exposure. The exposure continued for 25 days; long enough to allow recordings of possibly effects on growth. Three different levels of oxygen were used in the experiment (Table 2), and henceforward the different groups will be named 100% (control), 150%, and 175% dissolved oxygen groups, where the 100% oxygenated fish received no extra oxygen while the other two groups received extra oxygen. About 150% oxygen is suggested to be mild and is not expected to result in severe stress (Ritola et al. 1999), while 175% oxygen was considered high enough to induce stress. In order to avoid acute oxidative stress the increases in oxygen saturation from normoxic to 150% O2 and from normoxic to 175% O2 were conducted stepwise over 2 days.
The dissolved oxygen was pumped into the inlet water with equipment from AGA, Norway. The oxygen in the 100% O2 group was maintained by water supply only. The water flow was 6.0 l/min in all tanks. As a part of standardising, the behavioural observations maintaining the same amount of fish in the tanks was important. As the biomass in the tanks increased, the water supply to the control tanks increased at the end of the experiment to approximately 7.0 l/min in order to maintain normoxic conditions. However, in order not to need to increase the water flow too much, the oxygen levels in the control tanks were kept at 84.9 ± 0.4%. For a water temperature of 12°C, this saturation results in an oxygen concentration of 7.5 mg/l that is within the limits for acceptable conditions. In the tanks receiving 150 and 175% dissolved oxygen, the saturation was maintained by increasing the supply of gas from the pumps, thus maintaining stable water supply.
Behavioural measurements
The behaviour of the fish was recorded from above the tanks with a video camera mounted on a tripod. As the recordings proceeded, the camera was quietly moved from one tank to the next avoiding disturbance to the fish. All tanks were recorded during two periods; 7 days after exposure start and then repeated 21 days after exposure start. For both periods, each tank was recorded twice per day (morning and afternoon), 30 min every time, for 2 days. From the video recordings, measurements were done both on the fish group level and on individual level.
On the fish group level, horizontal distribution of the fish was measured once every 5 min. The tank was divided into three imaginary locations: 1 = the fish were located close to the centre of the tank; 2 = the fish were distributed from the centre of the tank and half way out in the tank; and 3 = the fish were horizontally distributed in the whole tank (from the centre of the tank and to the tank walls). The vertical distribution of the fish was measured once every 5 min. Because the angle of vision made it difficult to observe vertical distribution from the video recordings, these quantifications were additionally done by daily observations directly of the tanks, but outside the period of video recordings. The tank was divided into three imaginary vertical sections: 1 = the fish were located close to the bottom (25% up from the bottom, not above the bottleneck that constitutes the bottom of the tank); 2 = the fish were distributed from the bottom and half way up in the tank; and 3 = the fish were distributed from the bottom of the tank and up to the water surface.
In individual fish, tail beat frequency (Hz) and amount of time spent on swimming (seconds per minute) were measured. Individuals measured for tail beat frequency and amount of time spent swimming were randomly selected from the videotape, though by putting attention to visual individual differences no effort was made to measure the same individual more than once. Effort was also made to avoid selecting the easily detected fish (e.g. fish in the edges, fish pointed out to be especially small or big). For every 30 min video sequence the behaviour of six randomly picked individuals were quantified.
Physiological and water quality measurements
To quantify feed consumption feed in excess was collected from the outlet water during 7 days in the middle of the experiment, not at the same time as the behavioural observations were done. During this week, the feed waste was daily collected from a wire mesh strainer under the effluent water. The collected feed waste was rinsed in water to remove left-over faeces. Only the faeces dissolved in water while feed did not. After cleaning the feed, waste was weighed and stored at −20°C before analysis of dry matter was done by drying the samples at 105°C for 3 h. Feed consumption was calculated by subtracting uneaten feed from fed feed based on dry matter. Recovery of uneaten feed was calculated as described by Helland et al. (1996). The recorded uneaten feed was corrected for dry matter losses during feeding and collection.
At day 21, after termination of the behavioural studies, fish were sampled for physiological measurements. Ten random fish from each of the six tanks were gently netted and killed with a blow to the head, measured for weight and length, and blood samples were immediately taken from the caudal vein (needle 22G × 1; 0.7 × 25) and put in heparinised vacutainers. For measurements of haemoglobin, one drop of blood from 10 fish per tank were pooled in one tube and analysed immediately. Pooling of blood for haemoglobin analyses was done out of logistic reasons. For measurements of cortisol, chloride and osmolality blood samples from each of the 10 fish were immediately centrifuged. The plasma was divided in two and stored in Eppendorf tubes at −20°C for further analysis (1st set for analysis of cortisol; 2nd set for analysis of chloride and osmolality).
Total haemoglobin was measured with spectrophotometer (Shimadzu UV 260), reading at 540 nm; drabkins was used as standard. Analysis of plasma chloride was conducted by titration (Radiometer CMT10 Chloride Titrator) while osmolality was measured with Knauer osmometer (Semi-micro-osmometer (M no. 21.20) after the samples were mixed with a Coulter Mixer. Serum cortisol levels were measured with cortisol radioimmunoassay (RIA, Hormone Laboratory, Aker University Hospital, Oslo, Norway). The RIA had a sensitivity range of 46–1381 nmol/l, and the intra- and inter-assay coefficients of variation were 6 and 8%, respectively.
Water temperature (12.0 ± 0.5°C) was logged daily with data loggers (Ebro data logger EBI-85A). Oxygen was measured daily in inlet and outlet water using equipment from Oxy Guard (Oxy Guard Handy MK II). pH and CO2 were measured three times per week in inlet and outlet water using equipment typed Royce (Model 5300 pH/CO2 analyser) (Table 2).
Statistics
Physiological data were analysed with one-way analysis of variance and all post hoc multiple comparisons were done with Tukey post hoc test. Differences in frequency distribution were tested with chi-square analysis. To analyse for differences in variances between two samples, an F-test was used, while differences in variances between three samples were tested with Bartlett’s test for homogeneity of variance (Zar 1984). Differences in tail beat frequencies between oxygen groups at day 7 and day 21 were tested with ANOVA one-way analysis, while differences between day 7 and day 21 for each group was tested with a pairwise t-test. The software program Unistat (version 5.0.10) was used as statistical tool. Means were given with standard errors, and significant differences were defined as when P < 0.05. Since no differences between the respective replicates were found replicates were combined.
This study was approved by the Norwegian Animal Research Authority and was thus performed in accordance with laws and regulations concerning experiments with live animals.
Results
Within the 100% and 150% oxygen groups mortality was absent, while in the tanks receiving 175% dissolved O2 six individuals (3%) died. The fish in the different groups did not differ in weight before exposure start, while at the end of the exposure period the controls were significantly heavier than the fish exposed to 150% and 175% dissolved oxygen (P < 0.001) (Table 1). No differences in condition factor were found between the groups (Table 1).
Behavioural measurements
The behavioural observations of the fish groups showed large differences in distribution in the tanks between the 100, 150, and 175% oxygen groups in both vertical (χ 2 = 132.3, df = 10, P < 0.001) and horizontal (χ 2 = 230, df = 10, P < 0.001) distribution (Fig. 1a, b). Besides showing horizontal and vertical preferences, the fish were not prone to locate close to the inlet water.
Distribution in the tanks among Atlantic salmon (Salmo salar) parr subjected to 100%, 150%, and 175% oxygen at day 7 and at day 21 of the experiment. For each oxygen group, there are 48 observations at day 7 and 48 observations at day 21. (a) Vertical distribution. Fish were either located on the bottom (□); between the bottom and the centre ( ); or between the bottom and the surface of the tank (■). The three groups differed in vertical distribution (chi-square; P < 0.001). (b) Horizontal distribution. Fish were either located close to the centre of the tank (□); from centre of the tank and halfway out from the centre (
); or between the bottom and the surface of the tank (■). The three groups differed in vertical distribution (chi-square; P < 0.001). (b) Horizontal distribution. Fish were either located close to the centre of the tank (□); from centre of the tank and halfway out from the centre ( ); or the fish were horizontally evenly distributed in the whole tank (■). The three groups differed in horizontal distribution (chi-square; P < 0.001)
); or the fish were horizontally evenly distributed in the whole tank (■). The three groups differed in horizontal distribution (chi-square; P < 0.001)
The vertical position of the controls changed from the bottom and closer up to the centre from day 7 to day 21 while the 150% oxygenated fish tended to move in the opposite direction. The 175% oxygenated fish were spread in the tank at day 7 but were mostly found at the bottom or up to the centre at day 21 (Fig. 1a).
The horizontal distribution showed only minor alterations from day 7 to day 21 (Fig. 1b). The controls were aggregated compared to the exposed fish and only in approximately 1% of the observations were the controls observed horizontally evenly distributed in the whole tank. The 150% oxygenated fish were never observed in the centre of the tank; on the contrary, the majority of these fish were horizontally distributed in the whole tank. Also, the 175% oxygenated fish were most often distributed in the whole tank, and only sporadically were they observed aggregated to the tank centre (Fig. 1b).
Tail beat frequency was quantified for individual fish and was calculated as the number of tail beats per second. At day 7, there were no differences between the groups regarding tail beat frequency (Fig. 2a), but at day 21, the 175% oxygen exposed fish performed fewer tail beats than the other two group (F = 16.71, df = 2, P < 0.001). The alteration in performed tail beats from day 7 to day 21 showed that all groups performed fewer tail beats at day 21 compared to day 7 (100% O2: t = 4.18, df = 1, P = 0.0001; 150% O2: t = 2.47, df = 1, P = 0.02; 175% O2: t = 7.05, df = 1, P < 0.001) (Fig. 2a).
Behaviour in tanks among fish exposed to 100% (□), 150% ( ), and 175% (■) dissolved oxygen at day 7 and at day 21 of the experiment. Different letters indicate differences between oxygen groups within the same day (inter group differences), while asterisks indicate differences within the same oxygen group from day 7 to day 21 (intra group differences). For each oxygen group, there are 48 observations at day 7 and 48 observations at day 21. (a) Tail beat frequency in numbers per second (Hz). Inter group differences are tested with ANOVA one-way, P < 0.05; intra group differences are tested with paired t-test, ** P < 0.01; *** P < 0.001. (b) Individual differences in swimming activity measured as variance. Differences in variance between two groups are tested with F-test while differences in variance between three groups are tested with Bartlett’s test for homogeneity of variance
), and 175% (■) dissolved oxygen at day 7 and at day 21 of the experiment. Different letters indicate differences between oxygen groups within the same day (inter group differences), while asterisks indicate differences within the same oxygen group from day 7 to day 21 (intra group differences). For each oxygen group, there are 48 observations at day 7 and 48 observations at day 21. (a) Tail beat frequency in numbers per second (Hz). Inter group differences are tested with ANOVA one-way, P < 0.05; intra group differences are tested with paired t-test, ** P < 0.01; *** P < 0.001. (b) Individual differences in swimming activity measured as variance. Differences in variance between two groups are tested with F-test while differences in variance between three groups are tested with Bartlett’s test for homogeneity of variance
Regarding the amount of time spent on swimming (seconds per minute), there were no differences between the oxygen exposure groups either at day 7 (100% O2 = 4.92 ± 0.83 s; 150% O2 = 7.27 ± 1.62 s; 175% O2 = 7.10 ± 1.44 s) or at day 21 (100% O2 = 5.49 ± 0.85 s; 150% O2 = 4.31 ± 0.71 s; 175% O2 = 5.44 ± 0.90 s). None of the oxygen groups showed any alteration in swimming time between day 7 and day 21. However, the individual differences, defined as variance were considerable (Fig. 2b). At day 7 the individual variance in swimming activity was larger for exposed fish than for control fish (χ 2 = 20.09, df = 2, P < 0.0001). There were no differences in variance between the groups at day 21 (Fig. 2b). The 150 and 175% oxygen groups showed a reduction in variance from day 7 to day 21 (150% O2: F = 5.24, df = 1, P < 0.001; 175% O2: F = 2.52, df = 1, P = 0.002), whereas the controls showed no change in variance between the 2 days (Fig. 2b).
Physiological and water quality measurements
Water quality measurements revealed that the fish exposed to dissolved oxygen produced significantly more CO2 than the control fish (P < 0.001), while pH measured in the outlet water decreased significantly with increasing oxygen levels (P < 0.001) (Table 2).
The feed consumption among the 150% oxygen exposed fish was significantly higher than for the 175% oxygen exposed fish (P < 0.001), but there were no differences between the 150% and 100% oxygen groups. Neither did the controls differ from the 175% oxygen exposed fish regarding consumed feed (Table 1).
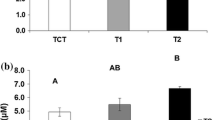
The haematological measurements at day 21 showed that the levels of haemoglobin decreased with increasing oxygen saturation (P < 0.001) (Table 3). Additionally, exposed fish had significantly lower levels of chloride than the controls (P < 0.001). The measurements of plasma cortisol revealed significantly higher levels among 150% superoxygenated individuals compared to the other two groups (P = 0.002) (Table 3). Regarding osmolality, no differences between the oxygen groups were found (Table 3).
Discussion
This study shows that pre-smolt salmon behaviour is affected by hyperoxia. The oxygen saturations used in the present study do not occur under normal farming conditions; however, since the saturation in a tank depends on many factors, such as fish biomass and distribution, optimal adding of extra oxygen may be difficult and may result in too high saturations. The distribution in the tanks increased with increasing oxygen saturation, and 7 days after the exposure started the individual variation in activity was larger for the hyperoxygenated fish compared to the controls. However, at day 21 the hyperoxic fish had moved closer to the bottom while the horizontal distribution remained the same, and the individual variation in activity decreased. Also, tail beat frequencies decreased at day 21 compared to day 7 even though the water flow in the hyperoxic tanks was kept constant. The oxygen saturation was measured in the inlet and outlet water, but not in the different sections in the tanks. The fish were never observed aggregated close to the inlet water. During the experiment, the hyperoxic fish produced more CO2 with a consequent reduction in water pH compared to the controls, but the CO2 levels always stayed within the range that are not supposed to negatively affect the fish (Portz et al. 2006). At day 21, both the hyperoxic fish groups had grown less than the controls despite there being no differences in feed consumption between the controls and the two hyperoxic groups. The haematological analyses revealed that the haemoglobin levels decreased with increasing oxygen saturation and the plasma chloride levels decreased in both hyperoxic groups. The given treatment caused elevated plasma cortisol levels only in the 150% group. The changes in CO2 and pH, and many of the physiological parameters as a result of hyperoxia are in accordance with previous findings (Fivelstad and Binde 1994; Ritola et al. 1999; Brauner et al. 2000; Ruyet Person-Le et al. 2002).
In commercial aquaculture, pre-smolt in tanks are normally standing still against the water current, with minimal swimming. Single fish are attached to one place in the tank and may push away intruders, even though they normally do not attack. The fish may occasionally swim away but return to their place within seconds (personal observations). Analyses of individual variance in activity were done since large individual differences (Huntingford and Adams 2005) may have prevented detection of differences between groups, as previously suggested (Schurmann and Steffensen 1994; Wibe 2003). Inter-individual variation may also be used as an indicator of stress and environmental changes (e.g. Kolok et al. 1998; Øverli et al. 2006). Observations of the fish suggest that the exposed fish were more active than the controls, even though the data failed to prove this statement. However, at day 7, the individual variation in swimming activity among the exposed fish was larger compared to the controls, though not at day 21. The individual variation may be dependent on the fish condition and level of stress (Schurmann and Steffensen 1994).
Even though behavioural effects of hyperoxia are not previously documented, there are studies where behaviour has been used to indicate environmental changes (Beitnger 1990; Little and Finger 1990; Claireaux et al. 1995; Wibe 2003). In a review by Kramer (1987), it is suggested that suboptimal oxygen saturations might be detected by studying animal behaviour, such as the positive correlations between oxygen concentration and horizontal and vertical distribution. It is also well known that environmental changes may cause stress among fish (Pickering 1998). Previous studies have suggested that schooling fish, i.e. aggregated fish, are less stressed (Juell 1995). In the present study, the control fish were aggregated compared to the hyperoxic fish, and since the hyperoxic groups experienced lower growth, increased CO2 production, lower levels of haemoglobin and chloride compared to the controls, and the cortisol levels were highest in the 150% oxygen group, it may be suggested that the exposed fish experienced suboptimal conditions and stress compared to the controls. It is thus likely that the observed behavioural changes among the exposed fish in the present study are a consequence of oxidative stress shown by the haematological results.
The decrease in chloride among hyperoxic fish may be explained as compensation against respiratory acidosis caused by hyperoxia (Brauner et al. 2000; Ruyet Person-Le et al. 2002) indicating that the fish in the present study have developed an acclimation towards the hyperoxic conditions. In the study by Ruyet Person-Le et al. (2002), the lack of effects in haematological variables, such as haemoglobin, cortisol and osmolality, was explained by the fish ability to adapt to hyperoxia. We experienced a decrease in haemoglobin with increasing oxygen saturation, explained by the decreased need of oxygen binding proteins. It is suggested that this phenomena is because fish exposed to oxygen over a period of time experience anaemia (Edsall and Smith 1991; Caldwell and Hinshaw 1994). The fish may show acclimation towards high oxygen levels and at the same time being stressed in terms of affected primary and secondary stress responses (Caldwell and Hinshaw 1994). In the present study, the decreased body weight among exposed fish at day 21 may suggest that the fish experienced the conditions as suboptimal, even though feed consumption did not differ from the controls. Weight loss and loss of appetite have previously been shown in fish exposed to oxidative stress (Fivelstad and Binde 1994; Wilhelm et al. 2005). The behaviour of the fish further indicated an acclimation to the hyper oxygenation. In addition to the decreased vertical spreading from day 7 to day 21 for the exposed fish, the reduced tail beat frequency and individual variation in swimming during the experimental progress indicated an acclimation. However, acclimation does not explain the stable horizontal distribution between day 7 and day 21, and super oxygenation does not explain the reduced tail beat frequency from day 7 to day 21 for the controls.
This experiment started with low fish density compared to that in most commercial facilities (13 kg/m3), but ended with a density of 44 kg/m3. According to the industry, fish densities of 30–50 kg/m3 are normal for salmon of comparable size to the present study, and when oxygenation of the tanks are applied higher densities may be used. However, it is suggested that a density of 44 kg/m3 may be high from a welfare point of view (Tornbull et al. 2005). The reported behavioural and physiological findings are therefore representative for the industry where super oxygenation of inlet water is common.
Pre-smolt Atlantic salmon change their behaviour under hyperoxic conditions, even though it is difficult to pinpoint the mechanisms behind the observed changes. It is, however, documented that, in the long term, hyperoxia is harmful for the fish (Gilmour and Perry 1994; Brauner et al. 2000). The results obtained in the present study may be useful for fish farmers and people that handle fish on daily basis, since by observing the behaviour they might be able to obtain an evaluation and warning that the water quality is sub-optimal. This may have large positive effects on fish welfare as well as helping the farmers to maintain a stable and efficient production.
References
Beitnger TL (1990) Behavioural reactions for the assessment of stress in fishes. J Great Lakes Res 16:495–528
Brauner CJ, Seidelin M, Madsen SS, Jensen FB (2000) Effects of freshwater hyperoxia and hypercapnia and their influences on subsequent seawater transfer in Atlantic salmon Salmo salar smolts. Can J Fish Aquat Sci 57:2054–2064. doi:10.1139/cjfas-57-10-2054
Caldwell AC, Hinshaw J (1994) Physiological and haematological responses in rainbow trout subjected to supplemental dissolved oxygen in fish culture. Aquaculture 126:183–193. doi:10.1016/0044-8486(94)90259-3
Caldwell AC, Hinshaw J (1995) Tolerance of rainbow trout to dissolved oxygen supplementation and a Yersinia ruckeri challenge. J Aquat Anim Health 7:168–171. doi :10.1577/1548-8667(1995)007<0168:CTORTT>2.3.CO;2
Claireaux G, Webber DM, Kerr SR, Boutilier RG (1995) Physiology and behaviour of free-swimming Atlantic cod Gadus morhua facing fluctuating salinity and oxygenation conditions. J Exp Biol 198:61–69
Dawkins MS (2004) Using behaviour to assess animal welfare. Anim Welf 13:s3–s7
Dejours P, Toulmond A, Truchot JP (1977) The effect of hyperoxia on the breathing of marine fishes. Comp Biochem Physiol 58A:409–411. doi:10.1016/0300-9629(77)90164-5
Depledge MH (1994) The rational basis for the use of biomarkers as ecotoxicological tools. In: Fossi MC, Leonzio C (eds) Nondestructive biomarkers in vertebrates. Lewis, Boca Raton, pp 271–295
Edsall DA, Smith CE (1991) Performance of rainbow trout and Snake River cutthroat trout reared in oxygen saturated water. Aquaculture 90:251–259. doi:10.1016/0044-8486(90)90249-M
Fernö A, Huse I, Juell JE, Bjordal Å (1995) Vertical distribution of Atlantic salmon Salmo salar L. in net pens: trade-off between surface light avoidance and food attraction. Aquaculture 132:285–296. doi:10.1016/0044-8486(94)00384-Z
Fivelstad S, Binde M (1994) Effects of reduced waterflow (increased loading) in soft water on Atlantic salmon smolts Salmo salar L. while maintaining oxygen at constant level by oxygenation of the inlet water. Aquac Eng 13:211–218. doi:10.1016/0144-8609(94)90004-3
Gilmour KM, Perry SF (1994) The effects of hypoxia, hyperoxia or hypercapnia on the acid-base disequilibrium in the arterial blood of rainbow trout. J Exp Biol 192:269–284
Goss GG, Wood CM, Laurent P, Perry SF (1994) Morphological responses of the rainbow trout Oncorhynchus mykiss gill to hyperoxia, base (NaHCO3) and acid (HCl) infusions. Fish Physiol Biochem 12:465–477. doi:10.1007/BF00004449
Helland SJ, Grisdale-Helland B, Nerland S (1996) A simple method for the measurement of daily feed intake of groups of fish in tanks. Aquaculture 139:157–163. doi:10.1016/0044-8486(95)01145-5
Huntingford F, Adams C (2005) Behavioural syndromes in farmed fish: implications for production and welfare. Behaviour 142:1213–1227. doi:10.1163/156853905774539382
Huntingford FA, Adams C, Braithwaite VA, Kadri S, Pottinger TG, Sandøe P et al (2006) Current issues in fish welfare. J Fish Biol 68:332–372. doi:10.1111/j.0022-1112.2006.001046.x
Juell JE (1995) The behaviour of Atlantic salmon in relation to efficient cage-rearing. Rev Fish Biol Fish 5:320–335. doi:10.1007/BF00043005
Kolok AS, Plaisance EP, Abdelghani A (1998) Individual variation in the swimming performance of fishes: an overlooked source of variation in toxicity studies. Environ Toxicol Chem 17:282–285. doi :10.1897/1551-5028(1998)017<0282:IVITSP>2.3.CO;2
Kramer DL (1987) Dissolved oxygen and fish behaviour. Environ Biol Fishes 18:81–92. doi:10.1007/BF00002597
Larsen BK, Jensen FB (1997) Influence of ionic composition on acid-base regulation in rainbow trout Oncorhynchus mykiss exposed to environmental hypercapnia. Fish Physiol Biochem 16:157–170. doi:10.1007/BF00004672
Liepelt A, Karbe L, Westendorf J (1995) Induction of DNA strand breaks in rainbow trout Oncorhynchus mykiss under hypoxic and hyperoxic conditions. Aquat Toxicol 33:177–181. doi:10.1016/0166-445X(95)00016-W
Little EE, Finger SE (1990) Swimming behaviour as an indicator of sublethal toxicity in fish. Environ Toxicol Chem 9:15–19
Lygren B, Hamre K, Waagbø R (2000) Effect of induced hyperoxia on the antioxidant status of Atlantic salmon Salmo salar L. fed three different levels of dietary vitamin E. Aquac Res 31:401–407. doi:10.1046/j.1365-2109.2000.00459.x
Øverli Ø, Sørensen C, Nilsson GE (2006) Behavioural indicators of stress-coping style in rainbow trout: do males and females react differently to novelty? Physiol Behav 87:506–512. doi:10.1016/j.physbeh.2005.11.012
Pickering AD (1998) Stress responses of farmed fish. In: Black KD, Pickering AD (eds) Biology of farmed fish. Sheffield Academic Press, University of Sheffield, UK, pp 222–255
Portz DE, Woodley CM, Cech JJ (2006) Stress-associated impacts of short-term holding on fishes. Rev Fish Biol Fish 16:125–170. doi:10.1007/s11160-006-9012-z
Priede IG, Solbé JF, Nott JE, O`Grandy KT, Cragg-Hine D (1988) Behaviour of adult Atlantic salmon Salmo salar L., in the estuary of the River Ribble in relation to variations in dissolved oxygen and tidal flow. J Fish Biol 33:133–139. doi:10.1111/j.1095-8649.1988.tb05567.x
Ritola O, Kiuru T, Koponen K, Mölsä H, Hänninen O, Lindström-Seppä P (1999) Rainbow trout Oncorhynchus mykiss exposed to oxygen supersaturation and handling stress: plasma cortisol and hepatic glutathione status. Acta Biol Hung 50:215–227
Ritola O, Livingstone DR, Peters LD, Lindström-Seppä P (2002) Antioxidant processes are affected in juvenile rainbow trout Oncorhynchus mykiss exposed to ozone and oxygen-supersaturated water. Aquaculture 210:1–19. doi:10.1016/S0044-8486(01)00823-7
Ruyet Person-Le J, Pichavant K, Vacher C, Le Bayon N, Sévère A, Boeuf G (2002) Effects of O2 supersaturation on metabolism and growth in juvenile turbot Scophthalmus maximus L. Aquaculture 205:373–383. doi:10.1016/S0044-8486(01)00689-5
Schurmann H, Steffensen JF (1994) Spontaneous swimming activity of Atlantic cod Gadus morhua exposed to graded hypoxia at three temperatures. J Exp Biol 197:129–142
Shelton RGJ, Johnstone ADF (1995) Preliminary aquarium observations of the schooling behaviour of Atlantic salmon Salmo salar L., smolts on transfer to sea water. Fish Res 24:73–78. doi:10.1016/0165-7836(94)00363-2
Steffensen JF, Farrell AP (1998) Swimming performance, venous oxygen tension cardiac performance of coronary-ligated rainbow trout Oncorhynchus mykiss exposed to progressive hypoxia. Comp Biochem Physiol 119A:585–592
Tornbull J, Bel A, Adams C, Bron J, Huntingford F (2005) Stocking density and welfare of cage farmed Atlantic salmon: application of a multivariate analysis. Aquaculture 243:121–132. doi:10.1016/j.aquaculture.2004.09.022
Wannamaker CM, Rice JA (2000) Effects of hypoxia on movements and behaviour of selected estuarine organisms from the south-eastern United States. J Exp Mar Biol Ecol 249:145–163. doi:10.1016/S0022-0981(00)00160-X
Wibe ÅE (2003) Behavioural effects of environmental pollution in threespine stickleback Gasterosteus aculeatus. Doctor Scient thesis, Norwegian University of Science and Technology
Wibe ÅE, Billing A, Rosenqvist G, Jenssen BM (2002) Butyl benzyl phthalate affects shoaling behaviour and bottom-dwelling behaviour in threespine stickleback. Environ Res 89A:180–187. doi:10.1006/enrs.2002.4360
Wilhelm D, Torres MA, Zaniboni E, Pedrosa RC (2005) Effect of different oxygen tensions on weight gain, feed conversion, and antioxidant status in piapara Leporinus elongates (Valenciennes, 1847). Aquaculture 244:349–357. doi:10.1016/j.aquaculture.2004.11.024
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice Hall, New Jersey
Acknowledgements
We would like to thank the technical staff at AKVAFORSK for help during the experimental setup. We are also grateful to the laboratory personnel at AKVAFORSK and Peter Torjessen (Hormone Laboratory, Aker University Hospital) for performing the physiological analyses. Finally, we wish to thank two anonymous reviewers for valuable comments on an earlier draft of this manuscript. This experiment was financially supported by the Research Council of Norway (project no. 133964/120).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Espmark, Å.M., Baeverfjord, G. Effects of hyperoxia on behavioural and physiological variables in farmed Atlantic salmon (Salmo salar) parr. Aquacult Int 17, 341–353 (2009). https://doi.org/10.1007/s10499-008-9206-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-008-9206-6