Abstract
Many tropical freshwaters experience persistent cyanobacterial blooms throughout the year. However, to date, most of the cyanobacteria–zooplankton interaction studies focused on temperate regions using large generalist grazers, which rarely coexist with cyanobacteria. Here a short-term (3 h) laboratory experiment was conducted to examine the grazing rate of Thermocyclops decipiens exposed to different combinations of phytoplankton (T1 = 100% Nitzschia-0% Cylindrospermopsis, T2 = 75% Nitzschia-25% Cylindrospermopsis, T3 = 50% Nitzschia-50% Cylindrospermopsis, T4 = 25% Nitzschia-75% Cylindrospermopsis and T5 = 0% Nitzschia-100% Cylindrospermopsis). In addition, an 8-day experiment was performed to determine the effect of the diet treatments on Thermocyclops survivorship and growth. The total biomass of phytoplankton in treatments with higher proportion of Nitzschia (i.e., T1 and T2) showed abrupt decline (i.e., down to 61% and 71%, respectively) over time when compared to other treatments. While Thermocyclops largely excluded Cylindrospermopsis when Nitzschia is abundant, they were capable of shortening the longer filaments of Cylindrospermopsis into significantly smaller fragments and ingesting them when provided as a sole food. This, however, negatively influenced their survivorship and growth. Our results generally suggest that dominantly occurring selective grazers in tropical and warmer-temperate lakes may shift the phytoplankton community composition to favor cyanobacteria by grazing on the alternative food source, at least before the cyanobacteria becomes dominant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many inland freshwater systems, eutrophication and climate change have stimulated the proliferation of cyanobacteria (O’Neil et al., 2012). Cyanobacteria often dominate the phytoplankton community composition especially in the tropics and form blooms, which may persist for a long period (Saker, 2000; Paerl & Otten, 2013). The ecological success and blooms of cyanobacteria in eutrophic waters (O’Neil et al., 2012) are attributed to a wide range of traits such as (1) superior nutrient acquisition and storage mechanisms (Reynolds, 1984; Reynolds et al., 2000); (2) thermal and shade tolerance (O’Neil et al., 2012; Sinha et al., 2012); and (3) resistance to zooplankton grazing (Ger et al., 2014, 2016c).
Cyanobacteria have three major attributes that can constrain zooplankton grazing, survival, and growth and reproduction. Firstly, their morphological features, such as mucous layers and large size, reduce zooplankton feeding rates. In particular, the filaments can inhibit ingestion by large daphnids through clogging the filtration apparatus (Gliwicz, 1990; Gliwicz & Lampert, 1990) but not always (Panosso & Lürling, 2010). This ultimately reduces the growth rate and fecundity of zooplankton (Bednarska et al., 2014). Secondly, several cyanobacteria can produce potent toxic metabolites, which may inhibit feeding activity and cause (sub)lethal effects when ingested by zooplankton. These metabolites could be hepatotoxins, cyanotoxins, neurotoxins, and protease inhibitors (Sadler & von Elert, 2014; Ger et al., 2016a, c). The third trait of cyanobacteria that negatively affects zooplankton grazing, survival, and growth and reproduction is associated with their nutritional deficiency (Mülller-Navarra et al., 2000; Mülller-Navarra, 2008).
Cyanobacteria may, thus, cause major disruptions of aquatic ecosystem and lead to trophic uncoupling through negative effects on filter feeder zooplankton, such as Daphnia (Mülller-Navarra et al., 2000), especially in the temperate zone. Nonetheless, most of the studies investigating cyanobacteria and zooplankton interactions have focused on generalist grazers (especially Daphnia) that rarely coexist with cyanobacterial blooms (Soares et al., 2009; Panosso & Lürling, 2010). The focus on a few large cladoceran species, however, hinders the prediction of plankton structure and interactions in systems experiencing cyanobacterial blooms and harboring zooplankton assemblages that are dominated by better adapted species, such as copepods.
Copepods, in the presence of alternative food, are shown to selectively graze against cyanobacteria (Fulton et al., 1988; Burns & Xu, 1990; Sellner et al., 1993). Selective grazing is an important attribute of copepods to withstand the blooming biomass of cyanobacteria by avoiding the ingestion of toxic cyanobacteria via grazing on a better quality prey while eluding poor-quality food items. When they encounter cyanobacteria, copepods use detection cues to avoid ingestion (Ger et al., 2011). However, consumption of cyanobacteria could be anticipated at lower proportions of alternative food sources (DeMott, 1989; DeMott & Moxter, 1991). Indeed, this is revealed to be dependent on the toxin production and filament length of the cyanobacterial species (Ger et al., 2016b; Rangel et al., 2016). On the other hand, while some studies showed the impairment of copepod fitness by cyanobacteria (Engstrom-Ost et al., 2015), others contend that cyanobacteria do not significantly affect copepods (Ka et al., 2012) or may contribute to their feeding and reproduction (Hogfors et al., 2014). However, it has to be understood that investigations on copepod–cyanobacteria interactions mainly employed calanoid copepods as case studies (Hong et al., 2013; Engstrom-Ost et al., 2015; Hong et al., 2015).
Unlike the lakes in the temperate region, which support blooms for relatively short periods, many tropical water bodies experience persistent cyanobacterial blooms (Bouvy et al., 2001). Moreover, contrary to the temperate aquatic systems, which are characterized by large generalist zooplankton (i.e., cladocerans), tropical freshwaters are dominated by smaller size and generally selective grazers (e.g., cyclopoids) (Bouvy et al., 2001; Iglesias et al., 2010). The interactions between cyclopoid copepods and cyanobacteria, however, remain largely unexplored, especially in cyclopoid-dominated tropical systems. This has limited our understanding of the effects of cyanobacteria on the coexisting zooplankton and the responses of zooplankton to cyanobacterial blooms in tropical freshwaters. The phytoplankton and crustacean zooplankton community in Lake Ziway, Ethiopia, for instance, are dominated by cyanobacteria (mainly Cylindrospermopsis) and cyclopoid copepods (mainly Thermocyclops), respectively (Gebrehiwot et al., 2017a, b). In this lake, the highest cyanobacterial biomass (i.e., 60 µg l−1 ± 5 µg l−1, n = 3) was recorded when the density of cyclopoids was the lowest (i.e., 15 ind l−1 ± 3 ind l−1, n = 3). On the other hand, the lowest cyanobacterial biomass (i.e., 15 µg l−1 ± 3 µg l−1, n = 3) was noted during the period of high cyclopoids density (i.e., 1662 ind l−1 ± 150 ind l−1, n = 3) (Gebrehiwot et al., 2017b). This observation tempted us to study the interactions between cyanobacteria and cyclopoid copepods.
Therefore, a short-term grazing experiment was conducted to examine the grazing rate of Thermocyclops exposed to different combinations of phytoplankton (cyanobacteria and diatom). In addition, an eight-day feeding experiment was performed to determine the extent to which different combinations of Cylindrospermopsis raciborskii (Woloszyńska) Seenayya & Subba Raju and diatom influence the survivorship and growth of cyclopoids. We tested the hypotheses that (1) Thermocyclops show selection against C. raciborskii; and (2) Thermocyclops survivorship and growth depend on the proportion of Cylindrospermopsis in the diet.
Materials and methods
Phytoplankton collection, isolation and culturing
Phytoplankton samples were collected from Lake Ziway, Ethiopia and transported immediately to Addis Ababa University. In the laboratory, two different groups of phytoplankton (i.e., straight filament forms of C. raciborskii and Nitzschia sp.) were identified and isolated using an inverted microscope (Krüss, A. Krüss Optronic, Germany). C. raciborskii was isolated using a serial dilution technique (Andersen, 2005). An aliquot of a lake water sample was diluted with modified WC (MWC) medium (Soares et al., 2009) without vitamins, in a small test tube, from which drops were transferred to multi-well plates using Pasteur micropipette and concentration of individuals was checked under an inverted microscope. After a series of similar dilutions and microscopic observations, some individuals were picked up with the micropipette and introduced into multi-well plates containing MWC medium.
For Nitzschia, an enrichment culture was prepared by adding MWC medium to the natural sample and allowed to stand for 2 days. Nitzschia was carefully picked up from the enrichment culture and placed in a sterile well plate. It was then transferred to the second sterile well plate using a clean micropipette. The procedure was repeated as described in Andersen (2005) until only the required species, free of all other protists, could be obtained.
The cultures were exposed to an artificial light source from three fluorescent lamps (36 W each), providing a total photon flux density of about 50 μmol photons m−2 s−1 measured on the surface of the culture, and temperatures of 22–24°C (Ogato et al., 2014) in 12-h/12-h light/dark cycle. All these cultures, which served as sources of inoculum, were mixed manually four to five times a day.
Zooplankton collection, isolation, and culturing
Cyclopoid copepods were collected from the same lake using 50-µm mesh size plankton net. On return to the laboratory, cyclopoids were identified to the species level as Thermocyclops decipiens (Kiefer, 1929) and Mesocyclops aequatorialis Kiefer, 1929 (Sandercock & Scudder, 1994; Fernando, 2002; Witty, 2004). Since Thermocyclops decipiens (hereafter Thermocyclops) accounted for about 80% of the relative numerical abundance of cyclopoids in the lake, it was selected for the present experiments and hence isolated using a wide bore plastic pipette under a dissecting microscope. Individuals of Thermocyclops were then rinsed at least three times to remove other prey items (Liu et al., 2016). Thermocyclops cultures were maintained under identical culture conditions as those of the phytoplankton in 1 l gently aerated jars (Nogueira et al., 2006) containing equal biomass concentration of Nitzschia and Cylindrospermopsis (i.e., 50% Nitzschia-50% Cylindrospermopsis). The total concentration of food in the jar was equivalent to 101,800 µm3 ml−1 ~ 0.03 mg C l−1 (Rocha & Duncan, 1985), which was comparable to the natural C. raciborskii and Nitzschia concentration in Lake Ziway (Gebrehiwot, 2018). In the process, the copepods were transferred to a fresh medium at 2-day intervals and maintained under the culture conditions for 2 weeks.
Short-term grazing experiment
To test the hypothesis that Thermocyclops would clear non-filamentous algae (Nitzschia) more readily than filamentous Cylindrospermopsis and that the clearance of Cylindrospermopsis would be dependent on its relative biomass, a short-term (3 h) grazing experiment was performed in a dark at a temperature of 22°C. This short-term incubation is expected to avoid the undesirable effects of food limitation (Panosso & Lürling, 2010) and algal growth owing to darkness (Ka et al., 2012).
Prior to this experiment, non-egg bearing and similar sized adult Thermocyclops were isolated and starved for about 2 h. The zooplankton used in the experiment had a mean body length of about 828 µm (± 21 µm, standard deviation, hereafter indicated by ±, n = 340). Their body length was defined as the distance from the most anterior part of the head to the base of the junction of the tail spine with the caudal rami.
The Thermocyclops were then fed with different proportions of Cylindrospermopsis and Nitzschia. For this experiment, five treatments with different proportions of the phytoplankton taxa were prepared in 12-welled culture plates by diluting exponentially growing batch cultures in the experimental medium. The treatments were T1 = 100% Nitzschia-0% Cylindrospermopsis, T2 = 75% Nitzschia-25% Cylindrospermopsis, T3 = 50% Nitzschia–50% Cylindrospermopsis, T4 = 25% Nitzschia–75% Cylindrospermopsis and T5 = 0% Nitzschia-100% Cylindrospermopsis. Each treatment had four replicates, and the well plates were filled with 2.5 ml MWC medium containing equal food concentrations of 101,800 µm3 ml−1, which is equivalent to 0.03 mg C l−1 (Rocha & Duncan, 1985). The aliquot of Cylindrospermopsis and Nitzschia consisted of filaments with an initial mean length of about 157 µm (± 30 µm, n = 100) and 35 µm (± 5 µm, n = 100), respectively.
The volume of water in the well plates was determined assuming an addition of a droplet of water (~ 0.1 ml) while introducing a Thermocyclops. Initially, the well plates that were anticipated to receive 1, 2, 4, and 10 Thermocyclops (described below) were filled with 2.4, 2.3, 2.1, and 1.5 ml water, respectively. The remaining volume of water was added together with the copepods.
To determine the effect of Thermocyclops density on their grazing rate, the experiments were conducted using different densities of Thermocyclops. The densities of Thermocyclops were 0.4, 0.8, 1.6, and 4 individuals per mL, which correspond to 1, 2, 4, and 10 individuals per well plate, respectively. The experimental densities were chosen taking the environmental cyclopoid and total zooplankton densities into account, which were indicated to be as high as 2000 ind l−1 and 4000 ind l−1, respectively (Gebrehiwot et al., 2017b). There were also four replicates of control with no animals.
During the experiment, all food suspensions were gently bubbled by a sterile-plastic pipette once every hour to minimize settling of cells (Ger et al., 2016a). For each treatment and zooplankton density, algal counts, filament length of Cylindrospermopsis, and biomass of the two phytoplankton were monitored hourly. This was performed by transferring a certain volume of fully homogenized subsamples from the well plates into the Sedgwick-Rafter cell. The phytoplankton were dispersed randomly across the counting area and not localized to one particular region. The phytoplankton were enumerated using an inverted microscope (Krüss, A. Krüss Optronic, Germany). The density of each taxon from the Sedgwick–Rafter counts was calculated according to relevant literature (Wetzel & Likens, 2000; Edward & David, 2010). The volume of each phytoplankton was calculated according to the corresponding geometric configuration after measurement of appropriate dimensions (Hillebrand et al., 1999; Sun & Liu, 2003) using a digital imager (A. Krüss Optronic GmbH, Video-Okular für PC, VOP). The algal biovolume (µm3 ml−1) was then estimated by multiplying the density of each taxon by their volume. The biovolume is indicated to be equivalent to fresh weight biomass, hereafter biomass (Wetzel & Likens, 2000).
Differences in the mean biomass and filament length of Cylindrospermopsis during the 3-h grazing time were evaluated using repeated measures ANOVA, while one-way ANOVA was used to compare differences of the mean filament length among treatments. The clearance rate (CR) was estimated from the decrease in biomass concentration between controls and treatments according to the following equation (Peters, 1984; Panosso & Lürling 2010):
where CR is the clearance rate (ml ind−1 h−1), Vcontrol and Vtreatment are the biomass concentration, hereafter biomass (µm3 ml−1) in the controls and treatments, respectively, after an incubation period t(h) and m is the volume of medium per individual Thermocyclops (ml ind−1).
In order to test whether food composition, zooplankton density or their interaction affects the clearance rate of Thermocyclops on Cylindrospermopsis and Nitzschia, a two-way ANOVA, followed by Tukey’s post hoc comparison test was computed. The factor food type consisted five levels as T1–T5, while zooplankton density comprised four (1, 2, 4, and 10) levels.
To assess prey selection of Thermocyclops, the Chesson’s selectivity index (α) was calculated (Chesson, 1983):
where \(i\) and j are prey categories, \(d\) is the proportion of prey in the diet and e is the proportion of prey in the environment.
Zooplankton growth experiment
In order to study the effect of different combinations of Nitzschia and Cylindrospermopsis on Thermocyclops survivorship and growth, the non-egg bearing copepodite (C5) Thermocyclops were cultured with different proportions of phytoplankton identical to the grazing experiment (i.e., T1–T5) for eight consecutive days. Another treatment without the food organisms in MWC medium was included as a control (T6). All treatments had the same initial phytoplankton food biomass concentration (101,800 µm3 ml−1). All treatments and the control had four replicates each.
This experiment was also conducted in 12-well plates containing 2.5 mL MWC medium and different densities of Thermocyclops (described above). Each treatment received similar sized Thermocyclops with mean body length of about 728 µm (± 20 µm, n = 340). The experiment was conducted under identical conditions as those of the culturing. The animals were transferred daily to clean well plates filled with freshly prepared food suspensions. Survival/mortality rate and growth of cyclopoids were monitored daily using an inverted microscope connected to a digital imager (A. Krüss Optronic GmbH, Video-Okular für PC, VOP). The variations in the growth of Thermocyclops were analyzed by means of two-way ANOVA for all treatments over the first 4 days and over the entire experimental period for T1-T4. Significant differences between mean growth were distinguished by Tukey’s post hoc test (P < 0.05). All the statistical analyses were run using STATISTICA 13.0 (StatSoft company, Hamburg, Germany) (StatSoft, 2015). Differences at P < 0.05 level were considered as significant.
Results
Grazing experiment
Grazing and phytoplankton biomass
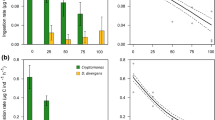
Two-way ANOVA run per Thermocyclops densities indicated significant effects of time (P < 0.0001), food type (P < 0.0001), and time and food type interaction (P < 0.0001) on the biomass of total phytoplankton (Nitzschia + Cylindrospermopsis). The results of the grazing experiment indicated a significant decline in total phytoplankton biomass over the course of the experimental period in all treatments (repeated measures ANOVA, P < 0.0001; Fig. 1). The total biomass of phytoplankton in treatments with a higher proportion of Nitzschia (i.e., T1 and T2) showed an abrupt decline over time when compared to other treatments (T3–T5).
Total phytoplankton biomass (mean ± SD) of different treatments (T1–T5) fed to 1, 2, 4, and 10 individuals of Thermocyclops during the 3-h experimental period; T1 = 100% Nitzschia-0% Cylindrospermopsis, T2 = 75% Nitzschia-25% Cylindrospermopsis, T3 = 50% Nitzschia-50% Cylindrospermopsis, T4 = 25% 34 Nitzschia-75% Cylindrospermopsis and T5 = 0% Nitzschia-100% Cylindrospermopsis
Zooplankton density also significantly influenced the biomass of phytoplankton (P < 0.0001). Higher densities of zooplankton per well plate expectedly led to a higher decline in phytoplankton biomass. For example, the biomass was reduced by about 40% and 25% in T1 and T2, respectively, when fed to 10 individuals of Thermocyclops. These figures were as low as 15% and 10% for T1 and T2, respectively, when fed to 1 individual of Thermocyclops (Fig. 1).
When we look at the biomass of Cylindrospermopsis separately, this trend is not clearly noticeable. The biomass showed a slight decline over time especially when Thermocyclops density was lower. Significant biomass decline of Cylindrospermopsis was observed only in treatments without Nitzschia (T5), while treatments with 25% or more of diatoms did not show a significant biomass decline (Table 1).
Clearance rate (CR) and selective grazing
Two-way ANOVA indicated a significant effect of food composition and Thermocyclops density on total clearance rate (TCR) of Thermocyclops (P < 0.001). The TCR on diets with a higher share of Cylindrospermopsis was significantly lower (P < 0.001) than that on mixtures with a lower share of Cylindrospermopsis. For all zooplankton densities, there were two homogenous groups of food types that were not statistically different from each other in terms of clearance rate. The first group comprised treatments with higher or equal proportion of Nitzschia (i.e., T1 = 100% Nitzschia-0% Cylindrospermopsis, T2 = 75% Nitzschia-25% Cylindrospermopsis and T3 = 50% Nitzschia-50% Cylindrospermopsis), which resulted in higher TCR. The second group consisted of the other two treatments (i.e., T4 and T5), which resulted in lower TCR. Despite being very low, the CR of Thermocyclops on Cylindrospermopsis (CR cyl) was detectable in treatments with higher proportions of Cylindrospermopsis (T4 and T5) (Fig. 2).
Clearance rate (mean ± SD) of Thermocyclops for total phytoplankton (TCR), for Cylindrospermopsis only (CR Cyl) and for Nitzschia only (CR diatom) in different proportions of food items (T1–T5); T1 = 100% Nitzschia-0% Cylindrospermopsis, T2 = 75% Nitzschia-25% Cylindrospermopsis, T3 = 50% Nitzschia-50% Cylindrospermopsis, T4 = 25% 34 Nitzschia-75% Cylindrospermopsis and T5 = 0% Nitzschia-100% Cylindrospermopsis
Comparison of the total clearance rate (TCR) at different densities of Thermocyclops showed that fewer individuals of zooplankton (1 or 2 Thermocyclops) cleared phytoplankton more readily (2–4 fold) than when the zooplankton was at higher densities. For instance, the total clearance rate of 1 Thermocyclops and 10 Thermocyclops were about 0.15 and 0.04 ml ind−1 h−1, respectively, in T1. Differences in clearance rate among treatments with 1, 2, 4 or 10 individuals of Thermocyclops increased significantly (P < 0.001) with increasing proportion of Nitzschia in the treatments. When diets were composed of 100% Nitzschia (T1), differences in clearance rate among treatments were the highest (1 Thermocyclops = 0.15 mL ind−1 h−1, 10 Thermocyclops = 0.04 ml ind−1 h−1; hence difference = 0.11 ml ind−1 h−1). However, this difference declined in treatment 5 (T5) (i.e., 1 Thermocyclops = 0.04 ml ind−1 h−1, 10 Thermocyclops = 0.02 ml ind−1 h−1; difference = 0.02 ml ind−1 h−1) (Fig. 2).
Selection coefficient
Thermocyclops showed a clear selection preference for Nitzschia in all treatments with mixed diets (Table 2). The selection coefficients for Cylindrospermopsis in all food items combinations were less than 0.5, which revealed feeding preference against Cylindrospermopsis raciborskii. But the selection coefficient for Cylindrospermopsis was slightly higher when the proportion of this cyanobacterium exceeded 75% of the total phytoplankton biomass (T4) (Table 2).
Filament length of Cylindrospermopsis
The results of experiments designed to test whether Thermocyclops could shorten the filament length of Cylindrospermopsis indicated that when it is the sole food source (T5), Thermocyclops could cut the longer filaments into significantly smaller pieces (repeated measures ANOVA, P < 0.0001). The initial experimental Cylindrospermopsis, which had a mean length of 157 µm (± 30 µm, n = 100), were shortened down to 120 µm (± 30 µm, n = 100) (Table 3) equivalent to 23% size reduction. This was especially noticeable at high number of grazers (Thermocyclops, e.g., 10). Taking T5 as an example, two-way ANOVA showed that zooplankton density (P < 0.0001), duration of exposure/experiment (P < 0.0001), and zooplankton density and time interaction (P < 0.0001) significantly influenced the filament length of Cylindrospermopsis.
Conversely, when the diet comprised a certain proportion of diatoms, the shortening of the filament of Cylindrospermopsis by Thermocyclops was not significant (repeated measures ANOVA, P > 0.05). However, in the mixed diet (T4), the filament length of Cylindrospermopsis was significantly shortened at the highest Thermocyclops density and longest duration of exposure (P < 0.0001, Table 3).
Thermocyclops growth experiment
Survivorship (%) of Thermocyclops
All the Thermocyclops cultured in 100% (T1) and 75% (T2) Nitzschia survived during the whole experimental period except those of the treatment with 10 individuals of Thermocyclops, in which 7.5% died since the 6th day in T2. At least 87.5% of the Thermocyclops also survived during the whole experimental period when grown in T1, T2, T3, and T4. However, at the highest zooplankton density (10 Thermocyclops per well plate), less zooplankton survived for the whole period (Fig. 3). For instance, at the end of the experiment, the survivorship declined to 45% per well plate in T4. Thermocyclops also survived for slightly longer period in sole-Cylindrospermopsis suspension (T5) than in MWC medium with no food (T6) (Fig. 3). All the Thermocyclops died within four days when kept without food, while they survived for two more days (Figs. 3, 4) when fed with sole-Cylindrospermopsis suspension (T5).
Percent survivorship of Thermocyclops (mean ± SD) cultured with different combinations of the phytoplankton (T1–T5) and without food (T6) during the eight-day experiment; T1 = 100% Nitzschia-0% Cylindrospermopsis, T2 = 75% Nitzschia-25% Cylindrospermopsis, T3 = 50% Nitzschia-50% Cylindrospermopsis, T4 = 25% 34 Nitzschia-75% Cylindrospermopsis and T5 = 0% Nitzschia-100% Cylindrospermopsis
Body length of Thermocyclops (mean ± SD) cultured with different combinations of the phytoplankton (T1–T5) and without food (T6) during the eight-day experiment; T1 = 100% Nitzschia-0% Cylindrospermopsis, T2 = 75% Nitzschia-25% Cylindrospermopsis, T3 = 50% Nitzschia-50% Cylindrospermopsis, T4 = 25% 34 Nitzschia-75% Cylindrospermopsis, T5 = 0% Nitzschia-100% Cylindrospermopsis
Growth of Thermocyclops
The growth of Thermocyclops was affected by the biomass of Cylindrospermopsis in the diet and duration of the experiment. A two-way ANOVA of treatments with 1 Thermocyclops indicated significant effects of time (P < 0.0001), food type (P < 0.0001), and time and food type interaction (P < 0.0001) on the growth of Thermocyclops during the first 4 days. The same effect was also observed for treatments with two, four and ten individuals of Thermocyclops. The interaction effect was reflected in the progressive deviations of the body length of Thermocyclops among different food treatments (Fig. 4). Tukey’s post hoc test indicated that the growth of the zooplankton in T5 (0% Nitzschia) and T6 (no food) was significantly lower when compared to other treatments (T1–T4).
A two-way ANOVA of the entire experimental period, excluding treatments 5 (T5) and 6 (T6), also indicated significant effect of time on the growth of animals (P < 0.0001). Indeed, the growth of Thermocyclops cultured in treatments T1–T4 did not show significant variation among each other. However, they showed a relatively slower growth and shorter body length (0.82 mm ± 0.01 mm, n = 40) at its higher density than at its lower density (0.94 mm ± 0.01 mm, n = 12).
Discussion
Grazing experiment
Clearance rate and selective grazing of Thermocyclops
Copepods are indicated to coexist with blooms of cyanobacteria (Bouvy et al., 2001). However, the interactions between copepods and cyanobacteria are usually complex and controversial. Some investigators argue that copepods could negatively affect cyanobacteria (Urrutia-Cordero et al., 2015), while others produced results that showed its positive effect (Wang et al., 2010; Leitão et al., 2018).
The present study generally revealed the low clearance and strong avoidance of Cylindrospermopsis raciborskii by Thermocyclops. Specifically, in treatments with mixed diets, Thermocyclops cleared Nitzschia 2–7 times faster than the filamentous cyanobacteria. The clearance rates for the total phytoplankton (TCR) and Nitzschia (CR diatom) declined with increased proportion of Cylindrospermopsis in the cultures, which implies that the cyanobacteria caused grazing inhibition.
Our present results are in agreement with the optimal diet theory, which predicts that consumers select against lower-value prey as the abundance of higher-value prey increases (DeMott, 1989). As cyanobacteria such as C. raciborskii lack important fatty acids and can potentially produce toxic compounds (Nogueira et al., 2004), other groups of phytoplankton are the more preferred food source than cyanobacteria for copepods. The results of stable isotope analysis by Major et al. (2017) and of the feeding experiment by Pagano (2008) also indicated the general preference of tropical zooplankton species in Ethiopia and Ivory Coast, respectively, for small seston particles (< 20 μm) (mainly green algae, flagellates and diatoms). Similarly, other researchers have noted higher selective grazing of the calanoid copepods, Boeckella (Hong et al., 2013) and Diaptomus (DeMott & Moxter, 1991) against cyanobacteria than that of other algae. Tropocyclops and diaptomid copepods were also found to exhibit relatively high clearance rate when feeding on low concentrations of large algae (DeMott & Watson, 1991).
Though copepods are commonly known to feed on motile planktonic cells (Bouley & Kimmerer, 2006), they can also graze on colonial and filamentous cyanobacteria when the phytoplankton community is dominated by them (Moriarty et al., 1973; Burns & Xu, 1990; Urrutia-Cordero et al., 2015). Work & Havens (2003) also showed that copepods can graze on cyanobacteria, although other groups of phytoplankton are the preferred ones. In the present study, the greatest selection for C. raciborskii by Thermocyclops occurred when Nitzschia biomass was limiting/nil, which indicates the possible consumption of cyanobacteria in the absence of alternative food source. Our experiments also revealed that when C. raciborskii is the only food source available, Thermocyclops could shorten filaments of C. raciborskii, as illustrated by the observed reduction in the average length of its filaments. This behavior of breaking down food particles was previously noted as a feeding mechanism for calanoid copepods (Bouvy et al., 2001; Ka et al., 2012). Therefore, it can be deduced that Thermocyclops changes its feeding behavior depending on prey availability, being a selective consumer when the suitable food is abundant and less-selective under the limiting condition of the preferred food.
Besides the proportion of food mixtures, the density of zooplankton seems to have an important role in determining the filament length and biomass of C. raciborskii. Increased density of Thermocyclops and the consequent grazing pressure, especially when alternative prey is low, resulted in shortened filaments and reduced biomass of Cylindrospermopsis, which were not noted at low zooplankton density. This may be attributable to increased encounter rates at higher Thermocyclops density and depletion of biomass of Nitzschia during which Thermocyclops will be forced to cut and use the cyanobacterium. Ekvall et al. (2014) and Urrutia-Cordero et al. (2015) have also indicated that high density of zooplankton is expected to influence the grazing intensity and, thus, biomass of Cylindrospermopsis. However, it has to be understood that the copepod density in this experimental work was higher especially while considering the natural phytoplankton–zooplankton ratio in the field (Gebrehiwot et al., 2017a, b). Hence, although our experimental results provided good insight into zooplankton density-cyanobacteria filament length and biomass relationships, it may not directly imply the natural scenario. Future researches, therefore, could focus on the effect of more environmentally relevant grazer densities on cyanobacterial filament shortening and biomass.
Generally, our results seem to suggest that selective grazing on other better quality algae may help Thermocyclops coexist with cyanobacteria. This is consistent with the contention that selective feeding behavior promotes grazer coexistence with cyanobacteria (Hong et al., 2013). The results also further lead us to suggest that selectively grazing Thermocyclops may shift the phytoplankton community composition to favor cyanobacteria by grazing on the alternative food source, at least before the cyanobacteria becomes dominant. Hence, the inverse relationship observed between the cyclopoids density and cyanobacteria biomass in the previous studies in Lake Ziway (Gebrehiwot et al., 2017b) is not directly due to grazing. In a nutshell, our results could be extended to explain that as selective grazers are the dominant zooplankton in tropical and warmer-temperate lakes, they may facilitate cyanobacterial blooms and thus influence plankton dynamics in such systems.
Growth experiments
Survivorship of Thermocyclops
While zooplankton influence phytoplankton community, phytoplankton diets could also play a significant role in determining zooplankton community structure. Blooms of filamentous cyanobacteria, for instance, are commonly considered to impair survival and growth of grazers (Wiegand & Pflugmacher, 2005), although some investigators have reported neutral or positive effects on zooplankton (Kozlowsky-Suzuki et al., 2003).
In the present study, Thermocyclops was found to survive longer when fed with mixed phytoplankton. This is particularly true at lower proportions of Cylindrospermopsis biomass in the diet. However, significant reduction of the percent survivorship of Thermocyclops was observed at high Cylindrospermopsis proportions, especially at 100%. This might be related to the poor manageability and nutritional deficiency of the cyanobacterial species (Panosso & Lürling, 2010; Engstrom-Ost et al., 2015).
However, even though the cyanobacteria was harmful to the Thermocyclops, animals fed with C. raciborskii alone could survive for 6 days, which is slightly longer than that observed in treatments without food. This indicates that the cyanobacterial species is not acutely lethal to Thermocyclops, and/or it may offer some nutrition relative to the no-food treatment (Mülller-Navarra, 2008; Pandey & Pandey, 2009). This finding is in concordance with the observations made by Soares et al. (2010) for Daphnia magna. Bouvy et al. (2001) also indicated that copepods could tolerate exposure to cyanobacterial bloom and show peak abundance in the presence of toxic cells in situ.
Growth of Thermocyclops
The growth and body length of copepods are determined partly by the quality of food (Ahlgren et al., 2005; Ger et al., 2014), which suggests that Thermocyclops’s growth may have also been dependent on the composition of Cylindrospermopsis and Nitzschia in this study. Accordingly, our experiments showed that as long as the food mixtures consisted of diatoms, the growth of Thermocyclops was not negatively affected. Even though diatoms are not as good food source as green algae owing to their frustules, they provide energy for copepod larval growth (Ianora et al., 2003). This is also possible as many copepods are able to reach high biomass in systems dominated by cyanobacteria through selective grazing of their preferred prey by size, taste and toxicity (Burns & Xu, 1990; Rangel et al., 2016).
Conversely, slower growth of Thermocyclops was noted in treatments consisted of sole Cylindrospermopsis. The results seem to suggest that harmful effects on the growth of Thermocyclops are expected to occur at only blooming conditions when the phytoplankton community is considerably dominated by Cylindrospermopsis. This is possibly because cyanobacteria, as a monospecific food, could cause oxidative stress by increasing the formation of reactive oxygen species, thereby decreasing the antioxidant capacity (Smith et al., 2008).
However, as also discussed in relation to the survivorship of copepods, filamentous cyanobacteria could have positive effect on juvenile development (Vehmaa et al., 2013). Cyanobacteria, for instance, may contain complementary nutrients and microelements (e.g., vitamin, protein, antioxidant, p and N) which are important for copepod growth (Mülller-Navarra, 2008; Pandey & Pandey, 2009; Hogfors et al., 2014). Therefore, a cyanobacterium may not always be deleterious to grazers, and its ecological effects may vary depending on the presence/absence of alternative food (Jiang et al., 2010). Similar results were also recorded for a larger cladoceran, Daphnia magna, which was fed with Scenedesmus and Cylindrospermopsis (Soares et al., 2009).
Even though similar trends in Thermocyclops’s growth were noted for different densities of zooplankton, the growth was slightly slower when Thermocyclops’s density was increased by tenfold. This might be due to food limitation (in this experimental setup) and associated low clearance rate. Furthermore, at the highest Thermocyclops density, the animals could fragment and ingest filaments of non-edible species (as suggested by a relatively higher selection coefficient), which in turn could hamper their body growth and even survival if not acute.
Conclusions
The results of the present study suggested that, within the complex interactions brought about by trophic cascades, cyclopoid copepods can ingest filamentous cyanobacteria, but select alternative food when available. However, as a sole food source, Cylindrospermopsis negatively influenced the survivorship and growth of Thermocyclops. The growth experiments conducted with either Cylindrospermopsis or Nitzschia alone, and mixture of both taxa as food organisms is believed to provide useful information on the potential ecological effect of Cylindrospermopsis. Given the predicted climate warming and the expected persistence of cyanobacterial blooms in the temperate region (O’Neil et al., 2012), the results of the present study on a tropical system are also believed to provide good insights into phytoplankton–zooplankton interactions in temperate zones. However, performing in situ experiments investigating cyanobacteria–cyclopoid interactions using the natural phytoplankton samples is recommended.
References
Ahlgren, G., L. V. Nieuwerburgh, I. Wanstrand, M. Pedersen, M. Boberg & P. Snoeijs, 2005. Imbalance of fatty acids in the base of the Baltic Sea food web a mesocosm study. Canadian Journal of Fisheries and Aquatic Sciences 62: 2240–2253.
Andersen, R. A. K. & M. Kawachi, 2005. Traditional microalgae isolation techniques. In Andersen, R. A. (ed.), Algal Culturing Techniques. Elsevier, London: 83–100.
Bednarska, A., B. Pietrzak & J. Pijanowska, 2014. Effect of poor manageability and low nutritional value of cyanobacteria on Daphnia magna life history performance. Journal of Plankton Research 36: 838–847.
Bouley, P. & W. Kimmerer, 2006. Ecology of a highly abundant, introduced cyclopoid copepod in a temperate estuary. Marine Ecology Progress Series 324: 219–228.
Bouvy, M., M. Pagano & M. Troussellier, 2001. Effects of a cyanobacterial bloom (Cylindrospermopsis raciborskii) on bacteria and zooplankton communities in Ingazeira reservoir (northeast Brazil). Aquatic Microbial Ecology 25: 215–227.
Burns, C. W. & Z. Xu, 1990. Calanoid copepods feeding on algae and filamentous cyanobacteria: rates of ingestion, defaecation and effects on trichome length. Journal of Plankton Research 12: 201–213.
Chesson, J., 1983. The estimation and analysis of preference and its relationship to foraging models. Ecology 64: 1297–1304.
DeMott, W. R., 1989. Optimal foraging theory as a predictor of chemically mediated food selection by suspension-feeding copepods. Limnology and Oceanography 34: 140–154.
DeMott, W. R. & F. Moxter, 1991. Foraging cyanobacteria by copepods: responses to chemical defense and resource abundance. Ecology 72: 1820–1834.
DeMott, W. R. & M. D. Watson, 1991. Remote detection of algae by copepods: responses to algal size, odors and motility. Journal of Plankton Research 13: 1203–1222.
Edward, G. B. & C. S. David, 2010. Freshwater Algae Identification and Use as Bioindicators. Wiley, The Atrium.
Ekvall, M. K., P. Urrutia-Cordero & L.-A. Hansson, 2014. Linking cascading effects of fish predation and zooplankton grazing to reduced cyanobacterial biomass and toxin levels following biomanipulation. PLoS ONE 9: e112956.
Engstrom-Ost, J., A. Brutemark, A. Vehmaa, N. H. Motwani & T. Katajisto, 2015. Consequences of a cyanobacteria bloom for copepod reproduction, mortality and sex ratio. Journal of Plankton Research 37: 388–398.
Fernando, C. H., 2002. Guide to tropical freshwater zooplankton: identification, ecology and impact on fisheries. Backhuys, Leiden.
Fulton, I., S. Rolland & H. W. Paerl, 1988. Zooplankton feeding selectivity for unicellular and colonial Microcystis aeruginosa. Bulletin of Marine Science 43: 500–508.
Gebrehiwot, M., 2018. Biomonitoring in Tropical Freshwater Lakes: Plankton dynamics, interactions and ecological determinants. PhD thesis, VUB Press, Brussels.
Gebrehiwot, M., D. Kifle, I. Stiers & L. Triest, 2017a. Phytoplankton functional dynamics in a shallow polymictic tropical lake: the influence of emergent macrophytes. Hydrobiologia 797: 69–86.
Gebrehiwot, M., D. Kifle & L. Triest, 2017b. Emergent macrophytes support zooplankton in a shallow tropical lake: a basis for wetland conservation. Environmental Management 60: 1127–1138.
Ger, K. A., R. Panosso & M. Lürling, 2011. Consequences of acclimation to Microcystis on the selective feeding behavior of the calanoid copepod Eudiaptomus gracilis. Limnology and Oceanography 56: 2103–2114.
Ger, K. A., L. A. Hansson & M. Lürling, 2014. Understanding cyanobacteria–zooplankton interactions in a more eutrophic world. Freshwater Biology 59: 1783–1798.
Ger, K. A., E. J. Faassen, M. G. Pennino & M. Lürling, 2016a. Effect of the toxin (microcystin) content of Microcystis on copepod grazing. Harmful Algae 52: 34–45.
Ger, K. A., E. Leitão & R. Panosso, 2016b. Potential mechanisms for the tropical copepod Notodiaptomus to tolerate Microcystis toxicity. Journal of Plankton Research 38: 843–854.
Ger, K. A., P. Urrutia-Cordero, P. C. Frost, L.-A. Hansson, O. Sarnelle, A. E. Wilson & M. Lürling, 2016c. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful algae 54: 128–144.
Gliwicz, Z. M., 1990. Why do Cladocerans Fail to Control Algal Blooms? Biomanipulation Tool for Water Management. Springer, New York: 83–97.
Gliwicz, Z. M. & W. Lampert, 1990. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 71: 691–702.
Hillebrand, H., C. D. Durselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Hogfors, H., N. H. Motwani, S. Hajdu, R. El-Shehawy, T. Holmborn, A. Vehmaa, J. Engstrom-OST, A. Brutemark & E. Gorokhova, 2014. Bloom-forming cyanobacteria support copepod reproduction and development in the Baltic Sea. PLoS ONE 9: e112692.
Hong, Y., M. A. Burford, P. J. Ralph, J. W. Udy & M. A. Doblin, 2013. The cyanobacterium Cylindrospermopsis raciborskii is facilitated by copepod selective grazing. Harmful Algae 29: 14–21.
Hong, Y., M. A. Burford, P. J. Ralph & M. A. Doblin, 2015. Subtropical zooplankton assemblage promotes the harmful cyanobacterium Cylindrospermopsis raciborskii in a mesocosm experiment. Journal of Plankton Research 37: 90–101.
Ianora, A., S. A. Poulet & A. Miralto, 2003. The effects of diatoms on copepod reproduction: a review. Phycologia 42: 351–363.
Iglesias, C., N. Mazzeo, M. Meerhoff, G. Lacerot, J. M. Clemente, F. Scasso, C. Kruk, G. Goyenola, J. García-Alonso & S. L. Amsinck, 2010. High predation is of key importance for dominance of small-bodied zooplankton in warm shallow lakes: evidence from lakes, fish exclosures and surface sediments. Hydrobiologia 667: 133–147.
Jiang, X., D. J. Lonsdale & C. J. Gobler, 2010. Density-dependent nutritional value of the dinoflagellate Cochlodinium polykrikoides to the copepod Acartia tonsa. Limnology and Oceanography 55: 1643–1652.
Ka, S., J. M. Mendoza-Vera, M. Bouvy, G. Champalbert, R. N’Gom-Ka & M. Pagano, 2012. Can tropical freshwater zooplankton graze efficiently on cyanobacteria? Hydrobiologia 679: 119–138.
Kozlowsky-Suzuki, B., M. Karjalainen, M. Lehtiniemi, J. Engstrom-Ost, M. Koski & P. Carlsson, 2003. Feeding, reproduction and toxin accumulation by the copepods Acartia bifilosa and Eurytemora affinis in the presence of the toxic cyanobacterium Nodularia spumigena. Marine Ecology Progress Series 249: 237–249.
Leitão, E., K. A. Ger & R. Panosso, 2018. Selective Grazing by a Tropical Copepod (Notodiaptomus iheringi) Facilitates Microcystis Dominance. Frontiers in Microbiology 9: 301.
Liu, H., M. Chen, F. Zhu & P. J. Harrison, 2016. Effect of diatom silica content on copepod grazing, growth and reproduction. Frontiers in Marine Science 3: 89.
Major, Y., D. Kifle, G. H. Niedrist & R. Sommaruga, 2017. An isotopic analysis of the phytoplankton–zooplankton link in a highly eutrophic tropical reservoir dominated by cyanobacteria. Journal of Plankton Research 39: 220–231.
Moriarty, D., J. P. Darlington, I. Dunn, C. M. Moriarty & M. Tevlin, 1973. Feeding and grazing in Lake George, Uganda. Proceedings of the Royal Society of London. Series B, Biological Sciences 184: 299–319.
Mülller-Navarra, D. C., 2008. Food web paradigms: the biochemical view on trophic interactions. International Review of Hydrobiology 93: 489–505.
Mülller-Navarra, D. C., M. T. Brett, A. M. Liston & C. R. Goldman, 2000. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403: 74–77.
Nogueira, I. C., M. L. Saker, S. Pflugmacher, C. Wiegand & V. M. Vasconcelos, 2004. Toxicity of the cyanobacterium Cylindrospermopsis raciborskii to Daphnia magna. Environmental Toxicology 19: 453–459.
Nogueira, I. C., A. Lobo-da-Cunha & V. T. M. Vasconcelos, 2006. Effects of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum (cyanobacteria) ingestion on Daphnia magna midgut and associated diverticula epithelium. Aquatic Toxicology 80: 194–203.
Ogato, T., D. Kifle, T. Fetahi & B. Sitotaw, 2014. Evaluation of growth and biomass production of Arthrospira (Spirulina) fusiformis in laboratory cultures using waters from the Ethiopian soda lakes Chitu and Shala. Journal of Applied Phycology 26: 2273–2282.
O’Neil, J., T. Davis, M. Burford & C. Gobler, 2012. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14: 313–334.
Paerl, H. W. & T. G. Otten, 2013. Blooms bite the hand that feeds them. Science 342: 433–434.
Pagano, M., 2008. Feeding of tropical cladocerans (Moina micrura, Diaphanosoma excisum) and rotifer (Brachionus calyciflorus) on natural phytoplankton: effect of phytoplankton size-structure. Journal of Plankton Research 30: 401–414.
Pandey, U. & J. Pandey, 2009. Enhanced production of delta-aminolevulinic acid, bilipigments, and antioxidants from tropical algae of India. Biotechnology and Bioprocess Engineering 14: 316–321.
Panosso, R. & M. Lürling, 2010. Daphnia magna feeding on Cylindrospermopsis raciborskii: the role of food composition, filament length and body size. Journal of Plankton Research 32: 1393–1404.
Peters, R. H., 1984. Methods for the study of feeding, grazing and assimilation by zooplankton. In Edmondson, W. T. (ed.), A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters, 2nd ed. IBP Handbook, Oxford: 336–412.
Rangel, L. M., K. A. Ger, L. H. Silva, M. C. S. Soares, E. J. Faassen & M. Lürling, 2016. Toxicity overrides morphology on Cylindrospermopsis raciborskii grazing resistance to the calanoid copepod Eudiaptomus gracilis. Microbial Ecology 71: 835–844.
Reynolds, C. S., 1984. The Ecology of Freshwater Phytoplankton. Cambridge University Press, Cambridge.
Reynolds, C., M. Dokulil & J. Padisak, 2000. Understanding the Assembly of Phytoplankton in Relation to the Trophic Spectrum: Where Are We Now? The Trophic Spectrum Revisited. Springer, New York: 147–152.
Rocha, O. & A. Duncan, 1985. The relationship between cell carbon and cell volume in freshwater algal species used in zooplanktonic studies. Journal of Plankton Research 7: 279–294.
Sadler, T. & E. von Elert, 2014. Physiological interaction of Daphnia and Microcystis with regard to cyanobacterial secondary metabolites. Aquatic Toxicology 156: 96–105.
Saker, M. L., 2000. Cyanobacterial Blooms in Tropical North Queensland Water Bodies. School of Tropical Biology, James Cook University, Douglas.
Sandercock, G., Scudder, G., 1994. An introduction and key to the freshwater calanoid copepods (Crustacea) of British Columbia. Technical report, Resources Inventory Committee Publications, British Columbia, Canada.
Sellner, K., D. Brownlee, M. Bundy, S. Brownlee & K. Braun, 1993. Zooplankton grazing in a Potomac River cyanobacteria bloom. Estuaries 16: 859–872.
Sinha, R., L. A. Pearson, T. W. Davis, M. A. Burford, P. T. Orr & B. A. Neilan, 2012. Increased incidence of Cylindrospermopsis raciborskii in temperate zones-is climate change responsible? Water Research 46: 1408–1419.
Smith, J. L., G. L. Boyer & P. V. Zimba, 2008. A review of cyanobacterial odorous and bioactive metabolites: impacts and management alternatives in aquaculture. Aquaculture 280: 5–20.
Soares, M. C. S., M. Lürling, R. Panosso & V. Huszar, 2009. Effects of the cyanobacterium Cylindrospermopsis raciborskii on feeding and life-history characteristics of the grazer Daphnia magna. Ecotoxicology and environmental safety 72: 1183–1189.
Soares, M. C. S., M. Lürling & V. L. Huszar, 2010. Responses of the rotifer Brachionus calyciflorus to two tropical toxic cyanobacteria (Cylindrospermopsis raciborskii and Microcystis aeruginosa) in pure and mixed diets with green algae. Journal of Plankton Research 32: 999–1008.
StatSoft, I., 2015. STATISTICA (data analysis software system), version 13.0. Tulsa, USA.
Sun, J. & D. Y. Liu, 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331–1346.
Urrutia-Cordero, P., M. K. Ekvall & L.-A. Hansson, 2015. Responses of cyanobacteria to herbivorous zooplankton across predator regimes: who mows the bloom? Freshwater Biology 60: 960–972.
Vehmaa, A., H. Hogfors, E. Gorokhova, A. Brutemark, T. Holmborn & J. Engstro-Ost, 2013. Projected marine climate change: effects on copepod oxidative status and reproduction. Ecology and Evolution 3: 4548–4557.
Wang, X., B. Qin, G. Gao & H. W. Paerl, 2010. Nutrient enrichment and selective predation by zooplankton promote Microcystis (Cyanobacteria) bloom formation. Journal of Plankton Research 32: 457–470.
Wetzel, R. & G. Likens, 2000. Limnolgical Analysis. WB Saunders Co., Philadelphia.
Wiegand, C. & S. Pflugmacher, 2005. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicology and Applied Pharmacology 203: 201–218.
Witty, L. M., 2004. Practical guide to identifying freshwater crustacean zooplankton. Cooperative Freshwater Ecology, Ontario.
Work, K. A. & K. E. Havens, 2003. Zooplankton grazing on bacteria and cyanobacteria in a eutrophic lake. Journal of Plankton Research 25: 1301–1306.
Acknowledgements
The authors would like to acknowledge Vlaamse Interuniversitaire Raad—Universitaire Ontwikkelingssamenwerking (VLIR-UOS), Vrije Universiteit Brussel (VUB-BAS42), and Addis Ababa University for their financial and logistic support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: John M. Melack
Rights and permissions
About this article
Cite this article
Gebrehiwot, M., Kifle, D. & Triest, L. Grazing and growth rate of a cyclopoid copepod fed with a phytoplankton diet constituted by a filamentous cyanobacterium. Hydrobiologia 828, 213–227 (2019). https://doi.org/10.1007/s10750-018-3813-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3813-7