Abstract
The cost of nutrient media is the major challenge for biomass production of Spirulina. Although much effort has been made to use enriched seawater for the cultivation of this microalga, little attention has been given to the potential of water of soda lakes. In this study, growth (μ, day−1) and biomass production (B) of Arthrospira fusiformis cultivated using waters of the soda lakes Chitu and Shala with or without supplementation were evaluated. Comparable μ and B values were achieved in both Lake Chitu water-based media (CBM) and Lake Shala water-based media (SBM), with slightly higher values in the latter. Both CBM and SBM supplemented with the standard Spirulina medium (SM) by 25 % and 50 % supported considerably higher μ and B. The pH and salinity of the cultures showed significant variations (P < 0.05) among the media and had considerable effect on μ and B. The observed higher μ and B were probably associated with the reduction in pH and salinity of the supplemented media due to addition of bicarbonate–carbonates and dilution, and provision of the limiting nutrient nitrogen. The higher μ and B in SBM may have resulted from some of their aggregate chemical parameters, which were closer to those in the SM, and abundant PO4-P. This seems to suggest that Lake Shala water is more conducive to Arthrospira. We contend that 25 % and 50 % supplemented Lake Shala water can be preferably used to produce Arthrospira biomass, thereby reducing the cost of nutrients by 75 % and 50 %, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mass production of Arthrospira (commercially known as Spirulina), an edible photosynthetic cyanobacterium, has gained worldwide attention due to its invaluable nutritional and health benefits. It has received worldwide acceptance as a natural food and source of several pharmaceuticals (Belay and Ota 1993; Belay et al. 1996; Chen et al. 1996). Arthrospira is used as a source of protein (50–70 % protein by dry weight, surpassing all known standard plant proteins), rare fatty acids (gamma-linolenic acid), vitamins (e.g., β-carotene and B12), minerals (e.g., iron) and essential amino acids. It has also therapeutic effects on various health problems including hyperlipidemia, nephrotoxicity, diabetes, obesity, hypertension, cancer and even HIV (Belay and Ota 1993; Belay et al. 1996; Belay 2002). Arthrospira is one of the microalgae that reproduce fast and has high productivity (Kilic et al. 2006). It can be produced in small or large scale using different types of production systems and growth media. Large commercial scale production of Arthrospira is currently used by several big companies around the world including Earthrise Nutritionals and Cyanotech in the USA and Hainan DIC Microalgae in China (Ayala et al. 1988; Jourdan 1993; Belay 1997). Arthrospira is also produced in small scale pond cultures for local consumption to fight malnutrition in various countries including India.
Growth and biomass production of Arthrospira depends on many environmental factors, especially carbonate–bicarbonate alkalinity, pH, nutrient availability, temperature and light (Vonshak 1997; Habib et al. 2008). High biomass of the alga can be obtained when these environmental factors are optimized. It preferably dominates in tropical and sub-tropical water bodies characterized by high carbonate–bicarbonate alkalinity and pH (Ciferri 1983; Cogne et al. 2001). However, optimization of growth conditions is the major challenge to biomass production of Arthrospira in cultivation systems and incurs high production cost. Out of the total production cost of Arthrospira biomass, 15–25 % is accounted for by nutrients, which form the second major cost item (Belay 1997; Vonshak 1997; Habib et al. 2008). As a result, production of Arthrospira biomass using standard carbonate medium is unaffordable.
Much effort has been made to investigate alternative methods of cultivation through biotechnological research and innovation in order to cut down the cost of production without compromising biomass productivity (Vonshak 1997). Promising results have been found in experiments using seawater enriched with phosphate and urea. In such experiments, Materassi et al. (1984) and Tredici et al. (1986), in laboratory and outdoor mass cultivations, respectively, obtained biomass yields which are slightly less than those obtained with the standard medium. Studies in Brazil by Costa et al. (2003) have also shown that comparable Arthrospira biomass could be obtained using lagoon water supplemented with bicarbonate and urea. Use of different organic sources of carbon, nitrogen and phosphorus has also resulted in increased biomass yield of Arthrospira (Neilson and Larsson 1980; Baldia et al. 1991; Habib et al. 2008). However, the potential of water of soda lakes for biomass production of Arthrospira under laboratory or outdoor conditions has received little attention to date though these lakes are conducive for Arthrospira, with some of them supporting its abundant populations.
Ethiopia has alkaline soda lakes such as lakes Chitu and Shala, which have several interesting features. Lake Chitu is a small shallow lake best known for its natural monoculture of Arthrospira (Talling et al. 1973; Kebede 1996). Harvesting Arthrospira biomass from the natural ecosystem of the small lakes like Chitu will not be sustainable ecologically and economically. Lake Shala is a large and deep soda lake whose gross water chemistry is very similar to that of the adjacent Lake Chitu and which is suitable for Arthrospira production (Wood and Talling 1988; Kebede 1996). The lakes are situated in areas with high temperature and irradiance and nearly constant photoperiod, which seem to be ideal tropical climatic conditions favoring the high productivity of Arthrospira (Richmond and Grobbelaar 1986; Vonshak 1997; Talling and Lemoalle 1998). These conditions presumably indicate potential of the large Lake Shala water for Arthrospira production. However, natural populations of Arthrospira have not been reported for Lake Shala for reasons not known to date. This calls for an in-depth experimental testing of the suitability of the lake water for Arthrospira production under laboratory and outdoor conditions. The purpose of this work was, therefore, to evaluate growth and biomass production of Arthrospira in waters of the soda lakes Chitu and Shala with or without supplementation with standard Spirulina medium (SM) at a constant light and temperature condition in the laboratory. The results that emanate from such investigations are crucial to efforts being made to develop suitable low-cost media for Arthrospira cultivation.
Materials and methods
Description of the soda lakes
Lakes Chitu and Shalla are tropical crater lakes located in the Ethiopian rift some 285 km south of Addis Ababa. These lakes are typical examples of African soda lakes characterized by highly saline–alkaline waters in which the concentrations of the major cations are in the order Na>K>Ca>Mg in contrast to that of most temperate lakes in which ionic dominance is in the order Ca>Mg>Na>K (Tudorancea et al. 1999). The lakes are closed and evaporative concentration is the prime factor for their saline–alkaline nature (Wood and Talling 1988; Zinabu 2002). The abundance of alkali elements in the lakes is attributed to their large concentration in the trachytic and rhyolitic rocks of the Ethiopian rift (Klemper and Cash 2007). The lakes’ region has semi-arid to sub-humid type of climate with mean annual precipitation and temperature of 600 mm and 25 °C, respectively (Legesse et al. 2002). The region has higher rate of evaporation than precipitation, which causes rainfall deficit.
Lake Chitu is a small closed and highly productive soda lake with ideal environmental conditions for the formation of monoalgal population of A. fusiformis (Kebede et al. 1994; Kebede 1996). The lake is characterized by high salinity, pH and HCO3 − + CO3 2− alkalinity. The water surface temperature of the lake ranges from 21 to 24 °C. Lake Shala, the deepest (maximum depth 266 m) soda lake of the Ethiopian rift lakes, is also characterized by high salinity and alkalinity, which are less than those of Lake Chitu. The water surface temperature of this lake ranges from 22 to 26 °C. The lake is unproductive and devoid of Arthrospira although some features of its gross water chemistry are supposed to be conducive for this alga. These highly saline–alkaline conditions of the lakes could be suitable for the production of Arthrospira.
Measurement of some physicochemical parameters of the soda lakes
Before using for experimental culture, some chemical parameters of the lake waters were determined in situ or in the laboratory to see their variation under the two conditions. pH and conductivity were measured in situ and in laboratory using digital pH meter (model HI 9024, Hanna Instruments) and conductivity meter (model CC-505, Elmetrron). Salinity (g L−1) was calculated from conductivity measurements according to UNESCO (1983). Surface water samples, collected with 10-L plastic containers, were used for the analysis of chemical features in laboratory. Alkalinity of unaltered water sample was determined by titration with 1 N HCl to pH 4.5 using a mixed indicator (Bromocresol green-methyl red). Carbonate–bicarbonate alkalinities as CaCO3 and their ions were calculated from total alkalinity and pH according to APHA et al. (1999). NO3 −, NH3, PO4-P and SO4 2− were determined, using samples filtered through Whatman GF/F, by sodium salicylate (APHA 1995), phenate, ascorbic acid and turbidimetric methods, respectively (APHA et al. 1999). Samples acidified to a pH of 2 with HNO3, were used for the analysis of major ions and some micronutrients according to the standard analytical methods outlined in APHA et al. (1999): Na+ and K+ by flame photometric method, Ca2+ by direct nitrous oxide-acetylene flame method, Cl− by argentometric method, Mg2+, Fe, Zn, Mn, Cu, and Co by direct air-acetylene flame method. Boron (B) was determined by azomethine H-colorimetric method (FAO 2008).
Isolation of Arthospira and scaling-up of its seed cultures
Trichomes of A. fusiformis (Fig. 1) were isolated from Lake Chitu by the serial dilution technique (Andersen and Kawachi 2005). An aliquot from a sample of the lake water was diluted with liquid SM, Zarrouk medium as modified by Aiba and Ogawa (1977), in a small test tube from which drops were transferred to multi-well plate using Pasteur micropipette and concentration of trichomes was checked under an inverted microscope. After a series of similar dilutions and observations, some trichomes were picked up with the micropipette and introduced into two small test tubes of 15 mL capacity containing about 4 mL of SM. The trichomes in the test tubes were allowed to grow at a photon flux density of about 20 μmol photons m−2 s−1 (produced by two fluorescent lamps, 36 W each) and temperatures of 22–24 °C. Dense cultures of the microalga were diluted by the addition of the SM and scaled up to a large volume (125 mL). All these cultures, which served as sources of inocula, were mixed manually four to five times a day.
Preparation of growth media for experimental cultures
Soda lake water was first filtered through a plankton net (64 μm pore size) to remove large microorganisms and particulate materials, within a few hours of its collection. The filtered water was then sterilized by a chemical sterilization technique (bleaching) and neutralized with sodium thiosulfate solution following the procedure outlined in Kawachi and Noel (2005). To evaluate growth responses of Arthrospira, with the aim to reduce the cost of growth media by substituting standard medium with soda lake waters (by 50 %, 75 % or 100 %), nine different types of media were prepared as indicated in Table 1. These media include SM, Lake Chitu water-based media (CBM) and Lake Shala water-based media (SBM). CBM and SBM consist of unsupplemented lake waters and lake waters supplemented in different proportions with SM and SM− (SM lacking carbonate–bicarbonate components — Na2CO3, NaHCO3 and NaCl). The idea of using SM− was to determine if these chemicals (Na2CO3, NaHCO3 and NaCl), which are required in large quantities in the standard SM, are replaceable by the carbonate- and bicarbonate-rich lake waters.
Experimental design and growth conditions
The experiment was designed to test growth and biomass production of Arthrospira in a laboratory using media produced from soda lake waters. The independent variable to be optimized was the growth medium, keeping such growth conditions as temperature, light and mixing constant. The response variables analyzed were specific growth rate (μ), biomass production (B) and doubling time (dt).
The experiment was carried out in 3000 mL Erlenmeyer flasks holding 150 mL culture medium without replication. The culture flasks were inoculated with 15-mL aliquots, constituting 10 % of the final culture volume, removed from exponentially growing cultures. The cultures were exposed to an artificial light source from fluorescent lamps (three, 36 W each), providing a photon flux density of about 50 μmol photons m−2 s−1 on the surface of the culture. The experimental cultures were grown in a temperature-regulated water bath (model DKZ series) at 35 °C in a light–dark cycle of 10:14 for 18 days. These light and temperature conditions were reported in several research articles as the optimal levels for Arthrospira growth in the laboratory (Vonshak 1997; Oliveira et al. 1999; Andersen and Kawachi 2005). Mixing of the cultures was achieved manually by gentle shaking of the culture flasks four times a day.
Analytical methods
Measurement of pH and conductivity and estimation of chlorophyll-a and growth parameters
Before inoculation, initial value of the biomass index — chlorophyll-a (chl-a) of the inocula and the initial pH and conductivity of all culture media were measured. During the experimental period, similar measurements were made every 2 days except for the first set of measurements, which were taken after 24 h to check the occurrence of a lag phase. The measurement and analyses were not replicated for the variables.
Chl-a was determined spectrophotometrically from 5-mL samples filtered onto GF/F and extracted in 90 % acetone. The absorbance of pigment extracts was measured at 665 and 750 nm with a UV–VIS Spectrophotometer (model 6405, Jenway) and chl-a concentration was estimated using the equation in Vonshak (1997). Biomass production (B, mg L−1) was calculated as the change in biomass per volume of sample filtered (Colla et al. 2007). Specific growth rate (μ) and doubling time (dt) were calculated using the following equations used for batch culture of microalgae in the exponential growth phase (Guillard 1973; Vonshak 1997).
where N 2 and N 1 are concentrations of the indicator of biomass (chl-a, mg L−1) at the end and beginning of the time intervals, t 2 and t 1, respectively.
Statistical analysis
The differences in growth parameters and some growth conditions of the various media were analyzed by one-way ANOVA, and Tukey’s LSD post-hoc test was used for multiple comparisons. The variables, which contributed to the observed variations in growth and biomass production of Arthrospira in various media, were determined by multiple regression analysis. All statistical analyses were done using SPSS statistical program (Version 20).
Results and discussion
Some chemical features of the study lakes
Certain physicochemical features of Lakes Chitu and Shala including those chemical features, which seem to be of overriding importance to the growth of Arthrospira are presented in Table 2. In laboratory and in situ measurement of some variables such as pH, conductivity and salinity did not show perceptible differences, and those physicochemical data measured in the laboratory were used in this study. The observed high levels of carbonate–bicarbonate alkalinity (as mg L−1 CaCO3), pH, salinity, conductivity, Na+, Cl− and PO4-P in both lakes are characteristic of soda lakes. Most of the recorded chemical parameters were considerably higher in Lake Chitu than in Lake Shala and SM, with the levels in Lake Shala approaching those measured in the SM (Table 2). In both lakes, these parameters did not show considerable seasonal variations although the variation was slightly higher in Lake Chitu due to its productivity and small size. Na+ and HCO3 − + CO3 2− and Cl− ions accounted for large proportions of the total cations and anions, respectively, in both lakes. The main mechanisms for the formation of these ions are leaching of rock materials rich in Na from volcanic rocks of the catchment areas and evaporative concentration enhanced by the dry and warm climate (Klemper and Cash 2007). In most saline lakes of arid regions, these dominant ions are responsible for the high pH, alkalinity, conductivity and salinity of their waters (Wood and Talling 1988; Kebede et al. 1994). The concentrations of the divalent cations, Ca2+ and Mg2+, are low compared to those of the dominant ions. The low level of these divalent cations is a salient feature of tropical saline–alkaline lakes (Kebede et al. 1994; Zinabu 2002; Klemper and Cash 2007) as these cations are precipitated from solution as their respective carbonates at high alkaline pH.
PO4-P, whose concentration in Lake Shala was about two times that in Lake Chitu, was quite high in both lakes considering its levels commonly recorded for freshwater lakes. In contrast, the concentration of nitrogen compounds NO3 − and NH3, which were often low or undetectable, were rather higher in Lake Chitu than in Lake Shala. In both lakes, the concentration of these macronutrients was considerably low compared to that of SM.
High pH and salinity and a large reserve of inorganic carbon sources and PO4-P are characteristic of some of the East African soda lakes, which favor dense populations of microalgae (Talling et al. 1973; Melack et al. 1982; Wood and Talling 1988; Kebede et al. 1994; Jones and Grant 1999). The pH and salinity of a medium affect different physiological processes such as growth, photosynthesis and chemical production of Arthrospira (Guterman et al. 1989; Vonshak et al. 1996; Kebede 1997). The high level of carbonate–bicarbonate is important as a buffer system to maintain optimum alkaline pH and provides carbon source for aquatic autotrophs such as alkaliphilic cyanobacteria (Richmond 1990; Vonshak 1997). It has been suggested that high level of CO3 2− to HCO3 − ratio at pH >10 may indicate a low level of HCO3 −, which is the principal and preferred carbon source for Arthrospira (Binaghi et al. 2003) and buffer against a pH rise. The low CO3 2 to HCO3 − ratio (or high HCO3 −) in Lake Shala compared to Lake Chitu may indicate that its water is well buffered against a pH rise and presence of surplus carbon source for growth of Arthrospira.
Growth and biomass production of algae are enhanced when nitrogen and phosphate sources are sufficiently available (Melack et al. 1982; Larned 1998; Miller et al. 1999). In some African freshwaters, inorganic sources of nitrogen and phosphorus were often suggested to be limiting to algal production (Talling and Talling 1965; Melack et al. 1982). Very high concentration of PO4-P was, however, recorded in the present study lakes. This is consistent with the results of earlier studies, which also recorded similarly high concentration of PO4-P in Lakes Chitu, Shala and Arenguade, with concentrations sometimes exceeding 2 mg L−1 in Lake Chitu despite the large algal standing crop it supported (224 μg L−1 chl-a) (Wood and Talling 1988; Kebede et al. 1994). Thus, PO4-P may not be limiting algal growth in these soda lakes but its concentration may not be adequate to support optimal growth of Arthrospira from the production point of view.
Nitrogen sources were low or undetectable in both lakes in the present study, corroborating the contention that nitrogen limitation of algal production is more likely in these soda lakes (Wood and Talling 1988; Kebede et al. 1994; Talling and Lemoalle 1998). In productive soda lakes like Chitu, over 90 % of the total inorganic nitrogen is in the algal biomass (Wood and Talling 1988) making nitrogen sources less available in the water body but which can be regenerated by recycling from the anoxic layer during destratification (Kebede et al. 1994). It is also argued that the high denitrification activities by abundant populations of some bacteria (e.g., Halomonas spp.) associated with tropical temperature in productive soda lakes may contribute to low nitrogen levels (Jones and Grant 1999).The unusually low level of nitrogen sources in Lake Shala may be attributed to the low concentration of decomposable organic matter and reduced rate of recycling owing to the great depth of the lake.
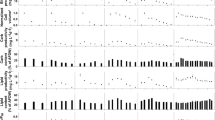
Salinity and pH of the experimental culture
In all media, pH increased while salinity decreased progressively with time during the cultivation period (Fig. 2). The decrease in salinity and increase in pH was gradual during early phase of the growth period, but became abrupt towards the end starting from days 11 to 12 in all media. These changes in pH can result from the chemical reactions among the carbonate systems and photosynthetic activities by dense algal biomass. According to Talling et al. (1973), dense algal biomass and vigorous photosynthesis in Lake Arenguade caused a rise in pH more than one pH unit despite the high buffering capacity associated with the lake’s large carbonate–bicarbonate alkalinity. During growth, Arthrospira preferably consumes bicarbonate and releases carbonate into the medium and this carbonate/bicarbonate imbalance causes a progressive pH rise. The association of carbonate with proton (H+) to produce bicarbonate, which is rapidly consumed by the alga, and dissolution of bicarbonate to CO2 causes a rapid increase in pH (Richmond and Grobbelaar 1986; Richmond 2002). pH increased more slowly in lake waters-based media than in the SM, which may be attributable to the high buffering capacity of the lake waters owing to their high carbonate–bicarbonates contents. The rapid decline of salinity observed after exponential phase may have resulted from the chemical transformation of carbonate–bicarbonate species, the major salinity components, and dilution of the culture through growth.
The mean pH values of all CBM and SBM were higher than that of SM (9.52). Compared to CBM, SBM had relatively low pH and supplementation with SM reduced it further to values approaching the SM. Statistically significant differences in pH (F(8, 81) = 2.45, P < 0.05) were observed among all media, with significantly (P < 0.05) higher values in all CBM except 50CM. In contrast, the pH of all SBM (SHM, 25SHM, 50SHM and 50SHM−) were not significantly different from that of SM despite the decreased pH levels in 25SHM and 50SHM. Mean salinity also showed significant differences (F(8, 63) = 40.61, P < 0.01) among the media, with significantly (P < 0.01) higher salinity being in all CBM than those of other media. The salinities of 25CM, 50CM and 50CM− were significantly lower (P < 0.05) than that of CM although they were still higher than that of SM. Among SBM, those supplemented with SM showed some insignificant reduction in salinity (SHM > 25SHM > 50SHM), particularly that of 25SHM and 50SHM was closely approaching the salinity of SM (12.88 g L−1), but that supplemented with SM− (50SHM−) was significantly reduced (P < 0.05).
The pH of Lake Chitu water was close to the upper limit of the optimal range for the growth of Arthrospira (8–10) (Richmond 1990; De Oliveira et al. 1999). Supplementation of the lake water with SM, however, led to significant pH reduction, which was pronounced in 50CM. The pH of Lake Shala water was lower than that of Lake Chitu water and supplementation caused its further reduction, though not significant, close to that of SM. The reduction in pH of the lake waters shortly after supplementation with SM (pH: 8.9) may be due to addition of carbonate–bicarbonates from SM, as the dissociation of bicarbonate to carbonate releases protons (H+) in the system causing a short term pH decline. The change in pH due to supplementation was not large in Lake Shala because it had low ratio of CO3 2− to HCO3 −, which probably made it more resistant to pH change. On the other hand, supplementation with SM− (pH: 9.87) did not cause changes in pH (almost similar to the lake water), which may be due to the absence of the carbonate–bicarbonates in the supplement.
Lake Chitu water had relatively high salinity but supplementation with SM (salinity: 13.53 g L−1) and SM− (salinity: 5.23 g L−1) significantly reduced its salinity closer to that of SM. The salinity of Lake Shala water was relatively low and supplementation reduced it further very close to that in the SM. The reduction in salinity following supplementation could result from the dilution effect of the SM and SM−, which had low salinity compared to both CBM and SBM. The large reduction in salinity of CBM and SBM when supplemented with SM− seems to have resulted from the greater dilution effect of SM− as it was more dilute than SM due to the lack of the major ions (Na+, Cl− and CO3 2− + HCO3 −). In general, supplementation of the lake waters with SM lowered pH and salinity to values approaching that of the standard SM while supplementation with SM− resulted in a large decline in salinity but not pH. Supplementation of the soda lake waters, by 25 % and 50 % with SM is, therefore, necessary to adjust pH and salinity to the optimal levels and also to provide deficient nutrients including nitrogen and phosphorus.
Growth and biomass production
Figure 3 shows growth curves of Arthrospira cultured in different complex media in comparison with that in SM. The growth curves show the typical pattern of exponential growth of microlagae without distinct lag phase. High growth of Arthrospira was observed in the SM, 25CM and 50CM of CBM and 25SHM and 50SHM of SBM although better growth was exhibited by the cultures in the SBM. Low growth was observed in the CM, SHM, 50CM− and 50SHM−.
Responses of Arthrospira reflected in such growth parameters as specific growth rate (μ), biomass production (B) and doubling time (dt) determined from chl-a measurements are given in Table 3. Higher μ and B and the shortest dt were achieved in SM, 25CM and 50CM of CBM and 25SHM and 50SHM of SBM, with higher values comparable to that of SM in the SBM. The least values of μ and B and the largest dt were observed in CM and 50CM−. The μ and B in both 50CM− and 50SHM− showed a slight increase compared to SHM and CM. Analysis of variance (ANOVA) of the growth parameters μ and B did not, however, show significant differences among the various media, implying that comparable growth rate and biomass production can be achieved using the soda lake waters with or without supplementation.
The results of multiple regression analysis of the causal relationship between the response variable (B) and the chemical factors — pH and salinity indicated the statistically significant (F(2,69) = 21.52, P < 0.01) effect the two factors had on the biomass production of Arthrospira. pH was the main factor accounting for more than 52 % of the variations in biomass. The relationships of μ and B with mean pH and salinity in different media of the cultures (Fig. 4) show that the increased μ and B in SM, 25CM and 50CM of CBM and 25SHM and 50SHM of SBM coincided with decreased mean pH and salinity while the lowest values of μ and B in CM and 50CM− corresponded to the highest pH.
Saline–alkaline conditions and availability of nutrients in the soda lakes are ideal conditions for the growth and production of Arthrospira. Better growth and biomass production, comparable to those observed in the SM, occurred in both CBM and SBM supplemented with SM by 25 % and 50 %, which had relatively low pH and salinity. This seems to have resulted from the reduction in pH and salinity by supplementation to levels, which were closer to those in the standard SM, due to dilution and addition of bicarbonate–carbonates. In contrast, the relatively low growth rates and biomass production observed in the CM and 50CM− may be attributable to the high pH of the media. The pH determines the formation of carbonate–bicarbonate species and the solubility of minerals in the lake waters thereby influencing the metabolism of algae (Guterman et al. 1989). Supplementation with SM adds more carbonate–bicarbonates that maintain alkaline pH and prevents carbon depletion, which is an important condition for the optimal growth of Arthrospira (Richmond 1990; Vonshak 1997). In an experiment carried out using lagoon water supplemented with sodium bicarbonate and urea, Costa et al. (2003) also obtained the highest biomass production with the addition of 2.88 g L−1 sodium bicarbonate. Salinity of a medium affects different physiological processes such as growth, photosynthesis and chemical production of Arthrospira (Vonshak et al. 1996; Kebede 1997). Several studies made on the growth and production of A. fusiformis in media with different salinity levels have demonstrated the association of higher growth rate and production of the alga with media of relatively low salinity (Kebede 1997; Vonshak 1997; Mussagy 2006). Exposure of Arthrospira culture to high salinity was found to result in the reduction and eventual cessation of growth (Vonshak 1997; Mussagy 2006), which was associated with substantial decrease in photosystem II activities due to osmotic shock (Zeng and Vonshak 1998). In addition, availability of limiting nutrients such as nitrogen and phosphorus enhances growth and biomass production of algae (Larned 1998; Miller et al. 1999). Supplementation may have provided these nutrients, particularly nitrogen, which is limiting in these soda lakes. The results of experiments carried out using seawater enriched with phosphate and urea, in both laboratory and outdoor mass cultivation of Arthrospira, showed high biomass yield, which was slightly less than that in the standard medium (Tredici et al. 1986; Materassi et al. 1984).
The higher growth rate and biomass production with shorter doubling time observed in SBM seems to be associated with some chemical features of Lake Shala. Primarily, pH, salinity, conductivity, CO3 2− + HCO3 − and Na+ of this lake were closer to those of the SM, indicating suitable conditions and huge carbon source (HCO3 −) for the alga. Secondly, the abundant PO4-P level in Lake Shala, which was about twice higher than that in Lake Chitu, could favor better growth and higher biomass production of the species under consideration. Furthermore, Lake Shala was deficient in nitrogen sources compared to that of Lake Chitu, and hence supplementation would provide this nutrient thereby enhancing the production of the alga.
The large reserve of inorganic carbon sources, which prevails even at high pH values, is a salient feature of soda lakes that favor dense populations of A. fusiformis (Talling et al. 1973; Wood and Talling 1988). In the natural ecosystem, Lake Chitu supports superabundant populations of Arthrospira while the exceptionally deep adjacent Lake Shala, which has broadly similar gross chemical features, is devoid of Arthrospira and much less productive. On the basis of the high light extinction coefficient and optical depth, and undetectable concentration of nitrogen compounds in Lake Shala, light and nitrogen were previously identified as the major factors associated with its low phytoplankton productivity (Wood and Talling 1988; Kebede et al. 1994; Zinabu 2002). As the results of the present study show, Lake Shala water supported better growth of Arthrospira with provision of light and nutrients, and its aggregate chemical features except nitrogen were in the range required for Arthrospira growth. Light and nitrogen nutrients together with other hydrographic and morphometric variables, therefore, probably have contributed to the low productivity and absence of Arthrospira in the natural ecosystem of Lake Shala. Pilot-scale experimental testing of Arthrospira production in shallow ponds using Lake Shala water may enable one to provide further plausible explanations for the low productivity of the lake.
Conclusion
Comparable growth and biomass production of Arthrospira was achieved using water of the soda lakes Chitu and Shala. Supplementation of the waters of the soda lakes with SM by 25 and 50 % further enhanced the growth and biomass production of Arthrospira. This appears to be attributed to the adjustment of the pH and salinity close to the optimal range by dilution and addition of carbonate–bicarbonates and provision of some deficient nutrients such as nitrogen by supplementation. pH and salinity differed significantly among the media and caused considerable effect on the growth and biomass production of Arthrospira in this study. Supplementation with SM reduced pH and supported better growth and more production than SM−, indicating the importance of addition of carbonate–bicarbonates even to the lake waters rich in these compounds. Lake Shala-based media supported better growth and higher biomass of Arthrospira than Lake Chitu-based media, indicating that some of the chemical features of Lake Shala are more conducive for Arthrospira production. This was interesting and desired result as the main goal was to show the use of the large volume of Lake Shala water for mass production. In general, Arthrospira can be produced using 25 and 50 % supplemented soda lake waters, preferably the large lake Shala water, thereby reducing the cost of nutrient media by 75 % and 50 %, respectively, which is a rough estimation based on the proportion of lakes water that could be combined with SM to produce a culture medium. In order to make use of soda lake water as a low cost medium for Arthrospira production, further optimization studies and nutrients (nitrogen) enrichment of the lake waters are deemed essential.
References
Aiba S, Ogawa T (1977) Assessment of growth yield of a blue-green alga Spirulina platensis in axenic and continuous culture. J Gen Microbiol 102:179–182
American Public Health Association (APHA) (1995) Standard methods for the examination of water and wastewater, 19th edn. Washington, DC
American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF) (1999) Standard methods for the examination of water and waste water, 20th edn. Washington, DC
Andersen RA, Kawachi M (2005) Traditional microalgae isolation techniques. In: Andersen RA (ed) Algal culturing techniques. Elsevier, London, pp 83–100
Ayala F, Vargas T, Cardenas A (1988) Chilean experiences on microalgae culture. In: Stadler T, Mollion J, Verdus MC, Karamanos Y, Morvan H, Christiasen D (eds) Algal biotechnology, proceedings of the 4th international meeting of the SAA. Elsevier, London, pp 229–236
Baldia SF, Nishijima T, Hata Y (1991) Effects of physicochemical factors and nutrients on the growth of Spirulina platensis isolated from Lake Kojima Japan. Nippon Suisan Gakkaishi 57:481–490
Belay A (1997) Mass culture of Spirulina outdoors—the Earthrise Farms experience. In: Vonshak A (ed) Spirulina platensis (Arthrospira): physiology cell-biology and biotechnology. Taylor & Francis, London, pp 131–158
Belay A (2002) The potential application of Spirulina (Arthrospira) as a nutritional and therapeutic supplement. JANA 5:27–48
Belay A, Ota Y (1993) Current knowledge on potential health benefits of Spirulina. J Appl Phycol 5:235–241
Belay A, Kato T, Ota Y (1996) Spirulina (Arthrospira): potential application as an animal feed supplement. J Appl Phycol 8:303–311
Binaghi L, Borghi AD, Lodi A, Converti A, Borghi MD (2003) Batch and fed-batch uptake of carbon dioxide by Spirulina platensis. Process Biochem 38:1341–1346
Chen F, Zhang Y, Guo S (1996) Growth and phycocyanin formation of Spirulina platensis in photoheterotrophic culture. Biotechnol Lett 18:603–608
Ciferri O (1983) Spirulina, the edible micro-organism. Microbiol Rev 47:551–578
Cogne G, Lasseur C, Cornet JF, Dussap CG, Gros JB (2001) Growth physiology of microorganism (S. platensis) by pressure measurement. Biotechnol Lett 23:1309–1314
Colla LM, Reinehr CO, Reichert CJ, Costa AV (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour Technol 98:1489–1493
Costa JAV, Colla LM, Filho FD (2003) Spirulina platensis growth in open raceway ponds using freshwater supplemented with carbon nitrogen and metal ions. Z Naturforsch 58c:76–80
Food and Agriculture Organization of the United Nations (FAO) (2008) Guide to laboratory establishment for plant nutrient analysis. FAO Fertil Plant Nutr Bull 19, Rome
Guillard RRL (1973) Culture methods and growth measurements division rates. In: Stein JR (ed) Handbook of phycological methods. Cambridge University Press, Cambridge, pp 289–311
Guterman H, Vonshak A, Ben-Yaakov S (1989) Automatic online growth estimation method for outdoor algal biomass production. Biotechnol Bioeng 34:143–152
Habib MAB, Parvin M, Huntington TC, Hasan MR (2008) A review on culture production and use of Spirulina as food for humans and feeds for domestic animals and fish. FAO Fisheries and Aquaculture Circular No 1034, Rome, pp 33
Jones BE, Grant WD (1999) Microbial diversity and ecology of the soda lakes of East Africa. In: Bell CR, Brylinsky M, Johnson-Green JP (eds) Microbial biosystems: new frontiers, proceedings of the 8th international symposium for microbial ecology. Antic Canada Society for Microbial Ecology, Halifax, pp 681–687
Jourdan JP (1993) Solarium Spirulina farm in the Atacama desert (North Chile) In: Doumenge F, Durand-Chastel H, Toulemont A (eds) Spiruline algue de vie. Bull Inst Oceanogr Monaco Mus Oceanogr 12:191–194
Kawachi M, Noel MH (2005) Sterilization and sterile techniques. In: Andersen RA (ed) Algal culturing techniques. Elsevier, London, pp 65–81
Kebede E (1996) Phytoplankton in a salinity–alkalinity series of lakes in the Ethiopian rift valley. Dissertation, Uppsala University
Kebede E (1997) Response of Spirulina platensis (Arthrospira fusiformis) from Lake Chitu Ethiopia to salinity stress from sodium salts. J Appl Phycol 9:551–558
Kebede E, Zinabu GM, Ahlgren I (1994) The Ethiopian rift valley lakes: chemical characteristics of a salinity–alkalinity series. Hydrobiologia 288:1–12
Kilic C, Goksan T, Ak I, Gokpinar S (2006) Iki farkli Spirulina paltensis supunun buyume ozelliklerinin karsilastirilmasi. EU J Fish Aquat Sci 23:189–192
Klemper SL, Cash MD (2007) Temporal geochemical variation in Ethiopian lakes Shala, Arenguade, Awasa and Beseka: possible environmental impacts from underwater and borehole detonations. J Afr Earth Sci 48:174–198
Larned ST (1998) Nitrogen- versus phosphorus-limited growth and sources of nutrients for coral reef algae. Mar Biol 132:409–421
Legesse D, Gasse F, Radakovitch O, Vallet-Coulomb C, Bonnefille R, Verschuren D, Gibert E, Barker P (2002) Environmental changes in a tropical lake (Lake Abiyata; Ethiopia) during recent centuries. Palaeogeogr Palaeoclimatol Palaeoecol 187:233–258
Materassi R, Tredici M, Balloni W (1984) Spirulina culture in seawater. Appl Microbiol Biotechnol 19:384–386
Melack JM, Kilham MP, Fisher TR (1982) Responses of phytoplankton to experimental fertilization with ammonium and phosphate in an African soda lake. Oecologia 55:1–6
Miller MW, Hay ME, Miller SL, Sotka E, Szmant AM (1999) Effects of nutrients versus herbivores on reef algae: a new method for manipulating nutrients on coral reefs. Limnol Oceanogr 44:1847–1861
Mussagy A (2006) The cyanophyte Arthrospira fusiformis in African waters-ecophysiology and potential use in tropical aquaculture. Dissertation, Lund University, Lund, pp 118
Neilson AH, Larsson T (1980) The utilization of organic nitrogen for growth of algae: physiological aspects. Physiol Plant 48:542–553
Oliveira MACL, Monteiro MPC, Robbs PG, Leite SGF (1999) Growth and chemical composition of Spirulina maxima and S. platensis biomass at different temperatures. Aquacult Int 7:261–275
Richmond A (1990) Large scale microalgal culture and applications. Prog Phycol Res 7:269–330
Richmond A (2002) Microalgal biotechnology at the turn of the millennium: a personal view. J Appl Phycol 12:441–451
Richmond A, Grobbelaar JU (1986) Factors affecting the output rate of Spirulina platensis with reference to mass cultivation. Biomass 10:253–264
Talling JF, Lemoalle J (1998) Ecological dynamics of tropical inland waters. Cambridge Univ. Press, Cambridge
Talling JF, Talling IB (1965) The chemical composition of African lake waters. Int Rev Gesamten Hydrobiol 50:421–463
Talling JF, Wood RB, Prosser MV, Baxter RM (1973) The upper limit of photosynthetic productivity by phytoplankton: evidence from Ethiopian soda lakes. Freshw Biol 3:53–76
Tredici M, Papuzzo T, Tomaselli L (1986) Outdoor mass culture of Spirulina maxima in seawater. Appl Microbiol Biotechnol 24:47–50
Tudorancea C, Zinabu GM, Elias D (1999) Limnology in Ethiopia. In: Wetzel RG, Gopal B (eds) Limnology in developing countries, vol. 2. International Association for Limnology (SIL) International Scientific Publications, New Delhi, pp 63–118
UNESCO (1983) Algorithms for computation of fundamental properties of seawater. UNESCO technical papers in marine science 44, 58 pp
Vonshak A (1997) Outdoor mass production of Spirulina: the basic concept. In: Vonshak A (ed) Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. Taylor and Francis, London, pp 79–99
Vonshak A, Torzillo G, Accolla P, Tomaselli L (1996) Light and oxygen stress in Spirulina platensis (cyanobacteria) grown outdoors in tubular reactors. Physiol Plant 97:175–179
Wood RB, Talling JF (1988) Chemical and algal relationships in a salinity series of Ethiopian inland waters. Hydrobiologia 158:29–67
Zeng MT, Vonshak A (1998) Adaptation of Spirulina platensis to salinity-stress. Comp Biochem Physiol A Mol Integr Physiol 120/121:113–118
Zinabu GM (2002) The effect of wet and dry seasons on the concentrations of solutes and phytoplankton biomass in seven Ethiopian rift valley lakes. Limnologica 32:169–179
Acknowledgments
This work was funded by Graduate Studies Program and Thematic Research Project (Ziway–Shala Basin Sub-thematic Research Group) of Addis Ababa University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogato, T., Kifle, D., Fetahi, T. et al. Evaluation of growth and biomass production of Arthrospira (Spirulina) fusiformis in laboratory cultures using waters from the Ethiopian soda lakes Chitu and Shala. J Appl Phycol 26, 2273–2282 (2014). https://doi.org/10.1007/s10811-014-0251-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0251-4