Abstract
We examined the effects of two types of piers on composition, abundance, and diversity of small epibenthic invertebrates and on several taxa known to be important prey for juveniles of three species of Pacific salmon. Using an epibenthic pump, invertebrates were sampled under and away from piers. Piers located within a dense urban aggregation of overwater structures and ferry piers occurring singly in less urbanized landscapes negatively impacted small invertebrates. Except for polychaetes at ferry piers and the harpacticoid copepods Tisbe species at urban piers, taxa richness and densities of invertebrate groupings and several juvenile salmon prey taxa were significantly decreased underneath both pier types and also near the edge of ferry piers. Assemblage structure was also greatly influenced by piers, with under-pier assemblages dominated by Tisbe species and several other taxa, and assemblages outside piers characterized by many taxa. Many of the negatively impacted taxa are associates of algae and seagrasses that were reduced under the piers. For juvenile salmon and other fish, reducing shade under piers by adding light to the environment may improve habitat access and quality in areas where piers decrease fish feeding opportunities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Piers and other structures in nearshore marine and estuarine environments impact plant and animal assemblages that live around and beneath them. Long-term impacts appear to be driven by shade cast by these overwater structures that limits sunlight and reduces or eliminates seagrasses, algae, and marsh plants (Burdick & Short, 1999; Glasby, 1999; Blockley, 2007; Thom et al., 2008; Vasilas et al., 2011; Gladstone & Courtenay, 2014). Overwater structures also negatively affect invertebrate assemblages, for example, by decreasing estuarine marsh invertebrate densities and diversity under low highway bridges (Struck et al., 2004). However, effects can also be variable, with some invertebrates decreasing and others increasing under structures. For example, in a study on seawalls in Sydney, Australia, algae were largely absent under wharves, and grazers such as chitons and limpets were also less abundant there, but some barnacles and other sessile invertebrates were more abundant under wharves (Blockley, 2007). Lower light levels under overwater structures also affect higher trophic levels. Extensive studies on fish and crabs in the Hudson River estuary (NY, USA) showed that juvenile fish abundance and species richness was significantly lower under piers (Able et al., 1998); caged fish under piers had growth rates similar to those held in the laboratory without food, while those of fish caged in pile fields and open water were significantly higher (Duffy-Anderson & Able, 1999); feeding by juvenile flounders was significantly depressed under a large municipal pier (Duffy-Anderson & Able, 2001); and decapod crustaceans and several fish species that are non-visual feeders were either unaffected or relatively abundant under piers (Able & Duffy-Anderson, 2005). Similarly, in Puget Sound (WA, USA), Munsch et al. (2014) found that rock crabs were more abundant under piers while overall fish densities were reduced there, and juvenile salmon (Oncorhynchus spp.) experienced lower densities and feeding rates in the shaded areas.
Piers may affect the availability of important fish prey. Small non-sessile invertebrates such as gammarid amphipods, harpacticoid copepods, and polychaete worms provide food for many fish occurring around piers (Duffy-Anderson & Able, 2001; Munsch et al., 2015). We found only one study that examined the effects of pier shading on these organisms: in their study on juvenile flounder feeding mainly on harpacticoid copepods, Duffy-Anderson & Able (2001) found that densities of potential prey were consistently higher underneath a pier than at the edge of the pier or in open water but that there were no differences in potential prey biomass. However, these authors identified potential prey only to order level, and thus did not address pier effects on individual prey species or on finer level assemblage structure of the invertebrates. Given the potentially major role of light in affecting invertebrate assemblages, pier effects on fish and their invertebrate prey could be widespread.
The goal of our study was to examine pier effects on assemblage composition, abundance, and diversity of small epibenthic invertebrates and on several taxa important to juveniles of the three species of Pacific salmon that can dominate fish numbers around these overwater structures (Toft et al., 2007; Munsch et al., 2015). In their initial marine residence, small Chinook salmon occupy shallow water during spring and early summer, where much of their prey consists of epibenthic invertebrates such as gammarid amphipods and polychaete worms (Brennan et al., 2004; Toft et al., 2007; Duffy et al., 2010). Similarly, the smaller juvenile pink and chum salmon feed extensively on smaller epibenthic invertebrates such as harpacticoid copepods. In fact, throughout the Pacific rim, juvenile pink and chum salmon appear to feed mainly on a few taxa of harpacticoids including the genera Tisbe and Zaus, and species of Harpacticus related to and including H. uniremis (Healey, 1979; Sibert, 1979; Landingham, 1982; D’Amours, 1987; Webb, 1991; Ivankov & Valentina, 1996; Mayama & Ishida, 2003; Chebanova et al., 2015). Here, we examine the effects of large piers in Puget Sound on small non-sessile invertebrate assemblages and several individual juvenile salmon prey taxa, measured at two types of structures: urban piers located close together within a landscape of dense overwater structures, and ferry terminals that were farther apart, and located in less urbanized landscapes.
Methods
Puget Sound is a fjordal estuary within the Salish Sea in the Pacific Northwest of the United States. In its main basin waters are classed as cold temperate and salinity is above 25. Shoreline development has substantially modified waterfronts of Puget Sound, including the presence of many piers that collectively cover 6.5 km2 of the intertidal area (Simenstad et al., 2011).
In two separate study components, epibenthic invertebrates were collected from intertidal sites modified by piers in Puget Sound. The first component, conducted in 2000, included ferry piers at three locations in Puget Sound relatively distant from each other (tens of kilometers apart); we refer to this as the Ferrry Pier study. The second component, conducted in 2014, included epibenthic invertebrate sampling at three municipal pier sites close together (tens of meters apart) within an urbanized estuarine bay; we refer to this component as the Urban Pier study.
Ferry pier study
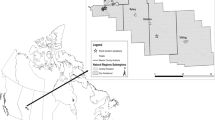
Epibenthic invertebrates were collected from intertidal zones modified by piers associated with Washington State Ferry terminals at Clinton, Bainbridge, and Southworth (Fig. 1). We use the term site to describe the locations where sampling occurred and refer to these sites as Piers A, B, and C, respectively. Piers A and B were constructed of concrete and wood, and Pier C was constructed of wood. The length and width of Piers A, B, and C were 195 × 48 m, 105 × 35 m, and 141 × 16 m, respectively, and they were 4.2, 5.5, and 5.3 m above mean lower low water (MLLW), respectively.
Stratified sampling occurred at each site once per month from March to June at 0-m MLLW. The substrata consisted of sloped sand and gravel beaches with eelgrass Zostera marina occurring at elevations below the sampling strata. The under stratum was defined as the area underneath piers, the near stratum was directly adjacent to piers (0–10 m from the edge), and the away stratum was 100 m away from the piers along the shoreline. Under strata were constantly shaded and disturbed periodically by ferry propeller wash, near strata were shaded depending on the position of the sun and were also disturbed by propeller wash, and away strata were not directly impacted by shading or propeller wash. Fifteen invertebrate samples were taken for each combination of strata, site, and month (n = 540).
Invertebrates were collected by wading from shore using a 7571 l h−1 12-v electric bilge pump, housed at the top of a 14.8-cm-wide PVC sampling cylinder open at the base, sampling an area of 0.018 m2. Inflow ports on the sampling cylinder were covered with 133-µm mesh screen and outflow from the pump was collected in a 106-µm sieve. For each sample, the sampling cylinder was set onto submerged substrate and operated for 20 s. Captured invertebrates were preserved on site with 10% formalin and transported to a laboratory for enumeration and identification. Small crustaceans such as gammarid amphipods and harpacticoid copepods were usually identified to species or genus level, and other groups were usually identified to order level.
Urban pier study
Epibenthic invertebrates were collected from the intertidal zones modified by three wood and concrete municipal piers in the highly urbanized downtown Seattle waterfront along Elliott Bay, Washington (Fig. 1). We refer to these sites as Piers 1, 2, and 3. The length and width of Piers 1, 2, and 3 were approximately 91 × 46, 122 × 38, and 137 × 38 m, respectively, and 4.2 m above MLLW. Pier dimensions are approximate because there is irregularly shaped infrastructure built adjacent to them that shades the water below. Vessels periodically accessed the bayside section of these piers, but were smaller in size than the ferries that used Piers A, B, and C multiple times per day.
Stratified sampling occurred once per month in April and June. Two strata (sun, shade) were sampled. The sun stratum was defined as the area approximately 10 m away from the edge of the pier and was un-shaded throughout the day. The shade stratum was located approximately 10 m under the pier where shade was constantly present. There was no natural shoreline at these sites and samples were taken from the intertidal zone consisting of a concrete vertical seawall. Five samples were taken for each combination of strata, site, and month (n = 30).
Invertebrates were collected from submerged substrate at 0-m MLLW using a manual pump designed for use by snorkelers that operated similarly to the electric pump, except that sampling intensity was standardized by the number of manual pumping cycles per sample (20 repetitions). The opening of this pump was 16 cm in diameter, sampling an area of 0.02 m2, and invertebrates were collected from water collected on a 106-µm mesh screen. Samples were preserved and quantified using the protocol described for the ferry pier study.
Analysis
We employed a multivariate approach to examine the effects of piers on assemblage composition. Multivariate data describing the epibenthic assemblages were standardized prior to analysis. Species that occurred in less than 5% of samples were excluded; the data were converted to proportions by dividing taxa abundance by total invertebrate abundance, and arcsine square root transformed, a procedure often used for proportion data to spread the higher and lower proportions and compress midlevel proportions (Zar, 2010). Patterns in assemblage composition were visualized by non-metric multidimensional scaling (NMDS). Significant differences in assemblage composition were tested by permutational multivariate analysis of variance (PERMANOVA; Anderson, 2005). The factors considered in these tests were strata, month, and site.
We employed a univariate approach to examine effects of piers on specific invertebrate taxa, overall abundance, and diversity. Univariate data were analyzed by generalized linear models (GLM) following protocol by Zuur et al. (2009). We examined total abundance and taxa richness, which was defined as the total number of monophyletic taxa in a sample. We examined densities of Diptera, Gammaridea, Harpacticoids, and Polychaeta because these taxa are common prey for juvenile salmon in the region (Webb, 1991; Brennan et al., 2004; Toft et al., 2007; Duffy et al., 2010). We also examined the densities of three harpacticoid copepod taxa documented to be primary prey taxa for juvenile pink and chum salmon: Harpacticus uniremis group, Tisbe spp., and Zaus spp. Full models included the terms strata, site, and month. Models were refined via backwards selection using analysis of deviance to compare full models to more parsimonious alternatives and terms were removed if they did not significantly improve model fit. For the ferry piers study, which examined invertebrates at three distances relative to piers, we input GLMs into Tukey’s pairwise comparisons of mean densities and taxa richness among strata. GLMs were fitted with a negative binomial error distribution and log link function. While these variables would typically be treated as random effects, we followed protocol by Bolker et al. (2009) to treat these variables as fixed effects because they were described by fewer than 5 levels per factor. To address heteroscedasticity/type 1 error concern, we looked at plots of the residual vs fitted values for each model and did not observe any obvious trends in variance across the range of fitted values (a poorly fitted model would have shown patterns of greater residual spreads with increasing fitted values). We ordinated observations for all data collected in the urban pier study combined because, unlike the ferry pier study, assemblages were not clustered predominantly by site.
Analyses were performed in R version 3.2.2 (R Core Team, 2015) using the MASS (Venables & Ripley, 2002), Multcomp (Hothorn et al., 2008), and Vegan packages (Oksanen et al., 2012).
Results
Piers had large influences on the composition of the epibenthic invertebrate assemblages. Assemblage composition varied significantly among all strata, including all pairwise comparisons among the three strata in the ferry pier study (all PERMANOVA tests: P < 1 × 10−4, Supplementary Table 1). Ordinations indicated that distinct assemblages occurred under and away from piers (Figs. 2, 3), and that assemblages under piers were proportionally dominated by only a few taxa (NMDS vectors, Supplementary Figs. 1–4, Supplementary Table 2). Among these taxa, the harpacticoid copepods Tisbe spp. were consistently a major component of the assemblages under piers. At urban piers, the harpacticoid family Tegastidae was also a prominent constituent of the under-pier assemblage (Supplementary Fig. 1). In the ferry pier study, invertebrate assemblages under the piers were more similar to those near the piers than those away from the piers. In addition to Tisbe spp., other prominent constituents of the under- and near-pier assemblages at the ferry piers included cyclopoid copepods in the family Cyclopinidae, the harpacticoid family Ameiridae, and polychaete and oligochaete worms (Supplementary Figs. 2–4).
With only two exceptions (polychaetes at ferry piers and Tisbe spp. at urban piers), taxa richness and densities of major invertebrate groupings as well as several selected salmon prey harpacticoid taxa were significantly lower under piers than outside of piers (Fig. 4). This was also true for invertebrates in the stratum near the pier edge at ferry piers. For the large majority of the other individual taxa identified in our study, the trend was toward lower densities under and near piers (Supplementary Table 3).
Taxa richness and densities of main epibenthic invertebrate groups and three harpacticoid taxa important as juvenile salmon prey, compared among locations relative to piers. Different shades of borders around the symbols within a group indicate statistically significant differences among strata for each measure of abundance or taxa richness (e.g., all three strata are significantly different from each other for Gammaridea at ferry piers)
Discussion
In our study, piers within a dense urban landscape of overwater structures and occurring singly in less urbanized settings similarly impacted small invertebrates. Except for polychaetes at ferry piers and the harpacticoids Tisbe species at urban piers, taxa richness and densities of major invertebrate groupings as well as several harpacticoid taxa were significantly lower under both pier types and near the edge of ferry piers. The assemblage structure was also greatly affected by piers, with under-pier assemblages dominated by Tisbe species and a few other taxa and outside-pier assemblages characterized by many taxa. These results are not surprising, because benthic vegetation, including eelgrass, has been shown to be reduced under structures including under the ferry piers that we sampled (Simenstad et al., 1997; Blanton et al., 2001). Many of the harpacticoid copepods characteristic of the area outside the piers (and reduced or eliminated by the piers) are in families and genera associated with seagrass and algal habitats throughout the world (e.g., Hicks, 1980; Arunachalam & Nair, 1988; Ólafsson et al., 2001; Sarmento & Santos, 2012; Mascart et al., 2015). Examples include the genera Harpacticus, Zaus, Diarthrodes, and Diosaccus. Likewise, gammarid amphipods were greatly reduced under piers, and species strongly associated with outside-pier strata were typical of phytal habitats in the region (e.g., Allorchestes angusta, Pontogeneia rostrata, Paracalliopiella pratti—Chapman, 2007). While our results agree with many studies finding negative effects of overwater structures, like some studies (e.g., Blockley, 2007), we found that some taxa were relatively unaffected by or indicative of under-pier habitats. Two harpacticoid taxa—the family Tegastidae and Tisbe species—were associated with under-pier habitats, the former under urban piers and the latter under both urban and ferry piers. While some species of Tegastidae are associated with phytal habitats, others inhabit various other biogenic substrata such as hydroids and corals (Humes, 1981a, b, 1984; Ivanenko et al., 2008), and they may have been associated with sessile invertebrates living under the urban piers. While the genus Tisbe is thought of as being mostly epibenthic, it has less affinity for benthic substrata compared to most harpacticoids, and some species swim actively and enter the water column (Hauspie & Polk, 1973; Marcotte, 1984; Kurdziel & Bell, 1992). Thus, the relatively high numbers of Tisbe under piers may have been transported there by tidal currents that distributed them along the nearshore. Tisbe species may also have been using non-phytal substrata under the piers—they are known to opportunistically occupy a variety of organically enriched environments, even polluted ones (Marcotte & Coull, 1974; Gee et al., 1985; Villano & Warwick, 1995) and can also utilize food sources that do not require light (e.g., dead tunicates—Lopez, 1982).
The elimination of algae and/or seagrasses under our study piers undoubtedly decreased the trophic value of these areas to juvenile salmon. Although un-vegetated habitats can be comparatively productive of fish food organisms (Jenkins et al., 2011), in Puget Sound large reductions in densities of gammarid amphipods, polychaete worms, and harpacticoid copepods under piers resulted in less prey available for juvenile salmon. At the ferry piers, this reduction also occurred at the pier edges, possibly due to the added disturbance of regular ferry propeller wash at these sites in addition to partial shading. Reduction in juvenile salmon prey around urban piers is corroborated by a recent comparison of juvenile chum salmon diets in our urban pier study area to those from more natural man-made beaches, in which salmon from the pier area fed atypically on zooplankton rather than on epibenthic harpacticoid copepods (Munsch et al., 2015). However, this may have also been the result of general lack of prey around urban seawalls compared to the less disturbed beaches—while we did not make statistical comparisons because of differences in methods and years sampled, in our data taxa richness and densities of several taxa were much higher at the single ferry piers compared to the urban piers. The negative impact of piers on fish prey availability is probably not a local phenomenon, because algae and seagrasses in many regions are known to support diverse assemblages of small invertebrates, especially harpacticoid copepods, that dominate the diets of small fish (e.g., Polte & Buschbaum, 2008; Kramer et al., 2012, 2013; Sutherland et al., 2013; Fukuoka & Yamada, 2015).
In addition to decreasing prey, other effects of piers on juvenile fish may be cumulative. For example, effects of dense urban piers on juvenile salmon also include interrupting natural along-shore movements and drastically lowering feeding activity due to shading (Munsch et al., 2014). Duffy-Anderson & Able (2001) also found that feeding by young-of-the-year winter flounder caged under piers was significantly depressed even though sufficient prey was available. It would be informative to examine pier impacts on juveniles of other commercially and recreationally important fishes in shallow waters that visually feed on phytal invertebrates and occur where large overwater structures cast large shade footprints (e.g., the King George whiting Sillaginodes punctatus in Australia—Jenkins et al., 2011 and tusk fishes Choerodon schoenleinii and C. anchorago in Japan—Fukuoka & Yamada, 2015). Given the few studies quantifying pier effects on small fish, studies of these and other species will be important in informing decisions made by fishery managers regarding harvesting, permitting of shoreline development, and habitat restoration.
Habitat restoration can be challenging along urban shorelines, although there has been some success in increasing the diversity of epibenthic invertebrates by enhancing intertidal areas along seawalls (Toft et al., 2013). Another promising enhancement method is adding light to the shaded environment. For juvenile salmon, reducing shade under piers may improve habitat access in areas such as Elliott Bay where piers decrease feeding opportunity. For example, in an experiment using fiber optic and halogen lighting systems under a ferry dock, salmon swam closer to the dock, but also appeared to avoid areas lit by both full sun and artificial light (Ono and Simenstad, 2014). However, using and maintaining artificial lights are problematic in marine environments, and may require light systems having a natural light spectrum and encompassing the entire shade footprint (Ono et al., 2010). Another more practical solution is using passive light penetrating surfaces (LPS) in overwater structures (Cordell et al., 2017; Munsch et al., 2017). For example, a preliminary study in Elliott Bay suggested that LPS (glass panels, metal grating, skylight) in a large urban pier allowed juvenile salmon to increase the use of under-pier areas (Cordell et al., 2017). In Seattle, Washington, glass block LPS were included in sidewalks along the waterfront to reduce shading effects associated with a new seawall (Cordell et al., 2017). The ability of LPS to mitigate shading is largely unknown, but given that many shallow water fish visually feed and orient themselves, providing light under piers may benefit migrating species such as juvenile salmon as well as non-migratory species (Munsch et al., 2017). Another important question is whether adding light can provide food web support for fish. As many studies have demonstrated, small fish often feed on invertebrates associated with phytal habitats, and artificial light or LPS may not support the primary production that such invertebrates require. For example, the LPS along Seattle’s seawall provide only a few percent of ambient photosynthetically active radiation (Cordell et al., 2017, J. Cordell, unpublished data). However, it is notable that some invertebrate prey taxa appear to be relatively unaffected by pier shading (this study, Duffy-Anderson & Able, 2001), and these taxa may be available to small fish after shaded areas are lit.
In conclusion, our study found that piers negatively impacted the abundance and diversity of small invertebrates, including prey species for fish. While densities of invertebrates in shaded areas were often much lower, a few taxa appeared to be relatively abundant in shade, suggesting that adaptation to shade varies among invertebrate taxa. Given the foundational role of sunlight in determining abiotic (e.g., temperature) and biotic (e.g., primary productivity) functions of shallow waters and the lack of natural analogs to large shaded areas, effects of piers on small epibenthic invertebrates and the fish that feed on them are likely widespread and negative. Piers are often aggregated in urban areas that are already of conservation concern (e.g., shorelines that are polluted or structurally transformed) where they also affect larger scale factors such as habitat connectivity and fish migrations (Munsch et al., 2014, 2017). This presents an opportunity to incorporate pier effects into existing management frameworks. An example of this occurs in our study region, where the Washington Department of Fish and Wildlife’s Hydraulic Code recognizes that larger overwater structures have more impacts than smaller residential docks, which is considered during regulatory review for new projects and redevelopments. Studies like ours provide further impetus to develop, test, and incorporate methods to mitigate pier effects on small invertebrates and fish.
References
Able, K. W. & J. T. Duffy-Anderson, 2005. A synthesis of impacts of piers on juvenile fishes and selected invertebrates in the lower Hudson River. Rutgers University, Institute of Marine and Coastal Sciences Technical Report #2005-13, New Brunswick, NJ.
Able, K. W., J. P. Manderson & A. L. Studholme, 1998. The distribution of shallow water juvenile fishes in an urban estuary: the effects of man-made structures in the lower Hudson River. Estuaries 21: 731–744.
Anderson, M. J., 2005. PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. Department of Statistics, University of Auckland, New Zealand.
Arunachalam, M. & N. B. Nair, 1988. Harpacticoid copepods associated with the seagrass Halophila ovalis in the Ashtamudi Estuary, south-west coast of India. Hydrobiologia 167(168): 151–522.
Blanton, S. L., R. M. Thom & J. A. Southard, 2001. Documentation of ferry terminal shading, substrate composition, and algal and eelgrass coverage. Battelle, Pacific Northwest Division of Battelle Memorial Institute.
Blockley, D. J., 2007. Effect of wharves on intertidal assemblages on seawalls in Sydney Harbour, Australia. Marine Environmental Research 63: 409–427.
Bolker B. M., M. E. Brooks, C. J. Clark, S. W. Geange, J. R Poulsen, M. H. H. Stevens & J. S. S. White, 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution 24: 127–135.
Brennan, J. S., K. F. Higgins, J. R. Cordell & V. A. Stamatiou, 2004. Juvenile salmon composition, timing distribution, and diet in marine nearshore waters of central Puget Sound in 2001-2002. King County Department of Natural Resources and Parks, Seattle.
Burdick, D. M. & F. T. Short, 1999. The effects of boat docks on eelgrass beds in coastal waters of Massachusetts. Environmental Management 23: 231–240.
Chapman, J. W., 2007. Gammaridea. In Carlton, J. T. (ed), The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon, 4th ed. University of California Press, Berkeley: 545–618.
Chebanova, V. V., S. E. Frenkel & G. S. Zelenikhina, 2015. Feeding and food relationships of juvenile chum salmon (Oncorhynchus keta) and pink salmon (O. gorbuscha) in coastal waters of Prostor Bay (Iturup Island). Journal of Ichthyology 55: 671–678.
Cordell, J. R., J. D. Toft, S. H. Munsch & M. Goff, 2017. Benches, beaches, and bumps, how habitat monitoring and experimental science can inform urban seawall design. In Bilkovic, D. M., M. M. Mitchell, M. K. LaPeyre & J. D. Toft (eds), Living Shorelines: The Science and Management of Nature-Based Coastal Protection. CRC Press, Routledge: 421–438.
D’Amours, D., 1987. Trophic phasing of juvenile chum salmon (Oncorhynchus keta Walbaum) and harpacticoid copepods in the Fraser River Estuary. PhD Thesis, University of British Columbia, Vancouver, BC
Duffy, E. J., D. A. Beauchamp, R. M. Sweeting, R. J. Beamish & J. S. Brennan, 2010. Ontogenetic diet shifts of juvenile Chinook salmon in nearshore and offshore habitats of Puget Sound. Transactions of the American Fisheries Society 139: 803–823.
Duffy-Anderson, J. T. & K. W. Able, 1999. Effects of municipal piers on the growth of juvenile fish in the Hudson River estuary: a study across a pier edge. Marine Biology 133: 409–418.
Duffy-Anderson, J. T. & K. W. Able, 2001. An assessment of the feeding success of young-of-the-year winter flounder (Pseudopleuronectes americanus) near a municipal pier in the Hudson River estuary, USA. Estuaries 24: 430–440.
Fukuoka, K. & H. Yamada, 2015. Food habits of juvenile tuskfishes (Choerodon schoenleinii and C. anchorago) in relation to food availability in the shallow waters of Ishigaki Island. Southwestern Japan. Fisheries Science 81: 331–344.
Gee, J. M., R. M. Warwick, M. Schaanning, J. A. Berge & W. G. Ambrose, 1985. Effects of organic enrichment on meiofaunal abundance and assemblage structure in sublittoral soft sediments. Journal of Experimental Marine Biology and Ecology 91: 247–262.
Gladstone, W. & G. Courtenay, 2014. Impacts of docks on seagrass and effects of management practices to ameliorate these impacts. Estuarine, Coastal and Shelf Science 136: 53–60.
Glasby, T. M., 1999. Effects of shading on subtidal epibiotic assemblages. Journal of Experimental Marine Biology and Ecology 234: 275–290.
Hauspie, R. & P. H. Polk, 1973. Swimming behaviour patterns in certain benthic harpacticoids (Copepoda). Crustaceana 25: 95–103.
Healey, M. C., 1979. Detritus and juvenile salmon production in the Nanaimo Estuary: I. Production and feeding rates of juvenile chum salmon (Oncorhynchus keta). Journal of the Fisheries Board of Canada 36: 488–496.
Hicks, G. R. F., 1980. Structure of phytal harpacticoid copepod assemblages and the influence of habitat complexity and turbidity. Journal of Experimental Marine Biology and Ecology 44:157–192.
Hothorn, T., F. Bretz, P. Westfall & R. M. Heiberger, 2008. Multcomp: simultaneous inference in general parametric models. R package version 1.0-0. R. Foundation for Statistical Computing. Vienna, Austria.
Humes, A. G., 1981a. A new species of Tegastes (Copepoda: Harpacticoida) associated with a scleractinian coral at Eniwetok atoll. Proceedings of the Biological Society of Washington 94: 254–263.
Humes, A. G., 1981b. Harpacticoid copepods associated with Cnidaria in the Indo West Pacific. Journal of Crustacean Biology 94: 227–240.
Humes, A. G., 1984. Harpacticoid copepods associated with cnidarians in the tropical Pacific Ocean. Zoologica Scripta 13: 209–221.
Ivanenko, V. N., F. D. Ferrari & H.-U. Dahms, 2008. Copepodid development of Tegastes falcatus (Copepoda, Harpacticoida, Tegastidae) with a discussion of the male genital somite. Proceedings of the Biological Society of Washington 121: 191–225.
Ivankov, V. N. & V. V. Andreyeva, 1996. Strategy for culture, breeding and numerous dynamics of Sakhalin salmon populations. In Report of the PICES Workshop on the Okhotsk Sea and Adjacent Areas, 332–336.
Jenkins, G. P., A. Syme & P. I. Macreadie, 2011. Feeding ecology of King George whiting Sillaginodes punctatus (Perciformes) recruits in seagrass and unvegetated habitats. Does diet reflect habitat utilization? Journal of Fish Biology 78: 1561–1573.
Kramer, M. J., D. R. Bellwood & O. Bellwood, 2012. Cryptofauna of the epilithic algal matrix on an inshore coral reef, Great Barrier Reef. Coral Reefs 31: 1007–1015.
Kramer, M. J., O. Bellwood & D. R. Bellwood, 2013. The trophic importance of algal turfs for coral reef fishes: the crustacean link. Coral Reefs 32: 575–583.
Kurdziel, J. P. & S. S. Bell, 1992. Emergence and dispersal of phytal-dwelling meiobenthic copepods. Journal of Experimental Marine Biology and Ecology 163: 43–64.
Landingham, J. H., 1982. Feeding ecology of pink and chum salmon fry in the nearshore habitat of Auke Bay. University of Alaska, Alaska.
Lopez, G. W., 1982. Short-term population dynamics of Tisbe cucumariae (Copepoda: Harpacticoida). Marine Biology 168(3): 33–41.
Marcotte, B. M., 1984. Behaviourally defined ecological resources and speciation in Tisbe (Copepoda: Harpacticoida). Journal of Crustacean Biology 4: 404–416.
Marcotte B.M., & B. C. Coull, 1974. Pollution, diversity and meiobenthic assemblages in the north Adriatic (Bay of Piran, Yugoslavia). Vie Milieu(B) 24: 281–300.
Mascart, T., G. Lepoint, S. Deschoemaeker, M. Binard, F. Remy & M. M. De Troch, 2015. Seasonal variability of meiofauna, especially harpacticoid copepods, in Posidonia oceanica macrophytodetritus accumulations. Journal of Sea Research 95: 149–160.
Mayama, H. & Y. Ishida, 2003. Japanese studies on the early ocean life of juvenile salmon. North Pacific Anadromous Fish Commission Bulletin 3: 41–67.
Munsch, S. H., J. R. Cordell, J. D. Toft & E. E. Morgan, 2014. Effects of seawalls and piers on fish assemblages and juvenile salmon feeding behavior. North American Journal of Fisheries Management 34: 814–827.
Munsch, S. H., J. R. Cordell & J. D. Toft, 2015. Effects of seawall armoring on juvenile Pacific salmon diets in an urban estuarine embayment. Marine Ecology Progress Series 535: 213–229.
Munsch, S. H., J. R. Cordell & J. D. Toft, 2017. Effects of shoreline armouring and overwater structures on coastal and estuarine fish: opportunities for habitat improvement. Journal of Applied Ecology. doi:10.1111/1365-2664.12906.
Oksanen, J. F., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’Hara, R. B., Simpson, G. L., Solymos, P., Stevens, M. H. M., & H. Wagner, 2012 Vegan: Assemblage Ecology Package.
Ólafsson, E., A. Ingólfsson & M. B. Steinarsdóttir, 2001. Harpacticoid copepod assemblages of floating seaweed: controlling factors and implications for dispersal. Hydrobiologia 453: 189–200.
Ono, K. & C. A. Simenstad, 2014. Reducing the effect of overwater structures on migrating juvenile salmon: an experiment with light. Ecological Engineering 71: 180–189.
Ono, K., C. Simenstad, J. Toft, S. Southard, K. Sobocinski & A. Borde, 2010. Assessing and mitigating dock shading impacts on the behavior of juvenile Pacific salmon (Oncorhynchus spp.): can artificial light mitigate the effects? Washington State Department of Transportation Research Report. WA-RD, 755.
Polte, P. & C. Buschbaum, 2008. Native pipefish Entelurus aequoreus are promoted by the introduced seaweed Sargassum muticum in the northern Wadden Sea, North Sea. Aquatic Biology 3: 11–18.
R Core Team, 2015. R: A Language and Environment for Statistical Computing
Sarmento, V. C. & P. J. Santos, 2012. Species of Harpacticoida (Crustacea, Copepoda) from the phytal of Porto de Galinhas coral reefs, northeastern Brazil. Check List 8: 936–939.
Sibert, J. R., 1979. Detritus and juvenile salmon production in the Nanaimo estuary: II. Meiofauna available as food to juvenile chum salmon (Oncorhynchus keta). Journal of the Fisheries Board of Canada 36: 497–503.
Simenstad C. A., Thom R. M., & A. M. Olson (eds), 1997. Mitigation between regional transportation needs and preservation of eelgrass beds. Washington State Department of Transportation.
Simenstad C. A., Ramirez M., Burke J., Logsdon M., Shipman H., Tanner C., Toft J., Craig B., Davis C., Fung J., Bloch P., Fresh K., Myers D., Iverson E., Bailey A., Schlenger P., Kiblinger C., Myre P., Gerstel W., & A. MacLennan. 2011. Historical change of puget sound shorelines: puget sound nearshore ecosystem project change analysis. Puget Sound Nearshore Report No. 2011-01. Washington Department of Fish and Wildlife, Olympia, Washington, and U.S. Army Corps of Engineers, Seattle, Washington.
Struck, S. D., C. B. Craft, S. W. Broome, M. D. Sanclements & J. M. Sacco, 2004. Effects of bridge shading on estuarine marsh benthic invertebrate assemblage structure and function. Environmental Management 34: 99–111.
Sutherland, T. F., R. W. Elner & J. D. O’Neill, 2013. Roberts Bank: ecological crucible of the Fraser River estuary. Progress in Oceanography 31: 171–180.
Thom, R. M., S. L. Southard, A. B. Borde & P. Stoltz, 2008. Light requirements for growth and survival of eelgrass (Zostera marina L.) in Pacific Northwest (USA) estuaries. Estuaries and Coasts 31: 969–980.
Toft, J. D., J. R. Cordell, C. A. Simenstad & L. A. Stamatiou, 2007. Fish distribution, abundance, and behavior along city shoreline types in Puget Sound. North American Journal of Fisheries Management 27: 465–480.
Toft, J. D., A. S. Ogston, S. M. Heerhartz, J. R. Cordell, & E. E. Flemer, 2013. Ecological response and physical stability of habitat enhancements along an urban armored shoreline. Ecological Engineering 57: 97–108.
Vasilas, B., J. Bowman, A. Rogerson, A. Chirnside & W. Ritter, 2011. Environmental impact of long piers on tidal marshes in Maryland-vegetation, soil, and marsh surface effects. Wetlands 31: 423–431.
Venables, W. N. & B. D. Ripley, 2002. Modern Applied Statistics with S, 4th ed. Springer, New York.
Villano, N. & R. M. Warwick, 1995. Meiobenthic assemblages associated with the seasonal cycle of growth and decay of Ulva rigida Agardh in the Palude Della Rosa, Lagoon of Venice. Estuarine, Coastal and Shelf Science 41: 181–194.
Webb, D. G., 1991. Effect of predation by juvenile Pacific salmon on marine harpacticoid copepods. I. Comparisons of patterns of copepod mortality with patterns of salmon consumption. Marine Ecology Progress Series 72: 25–36.
Zar, J. H., 2010. Biostatistical Analysis, 5th ed. Pearson, New York.
Zuur, A., E. N. Ieno, N. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed effects models and extensions in ecology with R. Springer, New York.
Acknowledgements
This research was funded by the Washington State Department of Transportation and Seattle Department of Transportation. S.H.M. was additionally supported by a National Science Foundation Graduate Research Fellowship (Grant DGE 1256082).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Jonne Kotta
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2017_3262_MOESM1_ESM.eps
Supplementary Fig. 1 NMDS of invertebrate taxa at Pier A with vectors indicating taxa with statistically significant gradients in ordination space (EPS 346 kb)
10750_2017_3262_MOESM2_ESM.eps
Supplementary Fig. 2 NMDS of invertebrate taxa at Pier B with vectors indicating taxa with statistically significant gradients in ordination space (EPS 312 kb)
10750_2017_3262_MOESM3_ESM.eps
Supplementary Fig. 3 NMDS of invertebrate taxa at Pier C with vectors indicating taxa with statistically significant gradients in ordination space (EPS 378 kb)
10750_2017_3262_MOESM4_ESM.eps
Supplementary Fig. 4 NMDS of invertebrate taxa at three urban piers with vectors indicating taxa with statistically significant gradients in ordination space (EPS 291 kb)
Rights and permissions
About this article
Cite this article
Cordell, J.R., Munsch, S.H., Shelton, M.E. et al. Effects of piers on assemblage composition, abundance, and taxa richness of small epibenthic invertebrates. Hydrobiologia 802, 211–220 (2017). https://doi.org/10.1007/s10750-017-3262-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3262-8