Abstract
We investigated ontogenetic and seasonal variations in the diet of the freshwater mullet (Myxus capensis) across a river–estuary interface using dietary tracer (stable isotopes and fatty acids) and stomach content analyses. Two hypotheses were tested: (A) the freshwater mullet diet shifts as individuals grow and migrate from the estuary to the river, and (B) the dominant food resources utilized by freshwater mullet vary through time, mainly as a function of the seasonal changes in the availability of preferred food items in each habitat. Both hypotheses were supported, as our results indicated broad dietary flexibility by M. capensis, with utilized food items ranging from benthic microalgae to insects depending on habitat and seasonal patterns in availability of resources. Given the unexpected importance of invertebrate-derived prey, including some of terrestrial origin (i.e. aerial or semi-aquatic insects), during the freshwater phase of the M. capensis life cycle, we also emphasize a need for a re-assessment of the trophic designation of this species (previously designated as a strict detritivore).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Compared to other aquatic systems, estuaries are often characterized by large proportions of detritivorous relative to herbivorous species (Lin et al., 2007; Franco et al., 2008). The dominance of detritivores in estuaries is due to the large amount of detrital material in these systems, of which the exact nature and origin is usually uncertain (Babler et al., 2010). Determining the nature and origin of detritus consumed by estuarine consumers can assist us in identifying the importance of allochthonous versus autochthonous organic matter in estuarine food webs (Baxter et al., 2005; Babler et al., 2010), a controversial topic in aquatic ecology (Cole et al., 2011; Brett et al., 2012). Specifically, the use of organic carbon of terrestrial origin by aquatic consumers, along with the importance of local primary producers versus imported material to the pool of detritus that is consumed by many species, is an issue that is highly debated (Polis et al., 1997; Carpenter et al., 2005; Brett et al., 2009, 2012; Cole et al., 2011; Jaschinski et al., 2011). Primary producers such as phytoplankton, microphytobenthos, macroalgae and vascular plants (terrestrial or aquatic) all occur in various quantities in estuaries and rivers, but their relative contributions to the detrital pool fuelling local detritivores, including fishes, remain largely unknown (Vaz et al., 1999; Wantzen et al., 2002; Lin et al., 2007; Lopes et al., 2007; Abrantes & Sheaves, 2010).
Among the variety of fish species displaying detritivory in southern African estuaries, the grey mullet (Mugilidae) represent a substantial proportion of the total fish biomass (Harrison & Whitfield, 2006). Mugilids are marine/estuarine-opportunistic species that use estuaries during a major part of their life cycle, especially as nursery areas (Whitfield, 2016). Mullets are often described as ‘mud-eaters’, as their diet is usually dominated by organic matter mixed with sediment (Cardona, 2016). However, associated microphytobenthos, small benthic invertebrates, foraminiferans, filamentous algae, zooplankton and detritus are additional important dietary items of fish within this family (Blaber, 1976, 1977). Mugilidae are characterized by an elevated trophic flexibility, as they are able to switch between deposit- and suspension-feeding according to food availability and they have a complex feeding apparatus that allows them to ingest a variety of resources at the base of the food web (Cardona, 2016). Such dietary flexibility probably contributes to the dominance of this fish family in many estuarine and coastal environments within warm temperate and subtropical regions of the world (Whitfield, 2016).
Estimating the relative importance of different food resources to mugilid diets is complicated by the challenge of obtaining valid quantitative dietary analyses using conventional stomach content techniques for detritivorous species (Cardona, 2016). Most mugilid stomach contents are dominated by a mixture of particulate organic material (POM) and associated sediment (Blaber, 1976, 1977), precluding the application of numerical, biomass or volume-based metrics often applied in stomach content analyses (Hyslop, 1980; Magoro et al., 2015; Cardona, 2016). The nature of the POM or benthic floc is not visually identifiable, and a large proportion is either quickly digested or passed through the alimentary canal without being assimilated (Vaz et al., 1999; Babler et al., 2010; Cardona, 2016). Furthermore, stomach contents provide an ‘instantaneous’ image of a fish diet because only those items consumed immediately prior to capture are recorded (Cortés, 1997). The importance of different food items in the diet of detritivorous mullet therefore needs to be evaluated using a combination of methods that assess the dietary items assimilated along with their origins (e.g. terrestrial versus aquatic, or animal versus plant). Methods like stable isotope and fatty acid analyses are particularly appropriate (Elsdon, 2010; Kelly & Scheibling, 2012; Cardona, 2016), as they allow for a comprehensive picture of resources that are assimilated by a consumer over time (weeks to months depending on species and tissues examined; e.g. Buchleister & Latour, 2010). Additionally, stable isotope and fatty acid analyses permit a multidimensional assessment of dietary variability (Hobson, 1999; Post, 2002; Budge et al., 2006; Elsdon, 2010; Kelly & Scheibling, 2012).
The trophic ecology of mugilids is of major interest to ichthyologists and environmental managers, as these species are often used as biological indicators of the ecological health of aquatic ecosystems, and in particular for tracing pollution levels in estuaries and other coastal waters (Wang et al., 2011). Their migrations between coastal, estuarine and freshwater systems also make some species within this family particularly vulnerable to human-induced habitat alterations such as dams, weirs or other structures that represent obstacles to fish migration routes (Whitfield, 2016). As such, mullet represent a critical component in energy transfer between aquatic ecosystems, and it is essential that we develop an understanding of their trophic dynamics in a variety of coastal systems.
In South Africa, 17 mugilid species have been recorded from coastal, estuarine and freshwater areas (Froese & Pauly, 2016). The grooved mullet (Liza dumerili Steindachner, 1870), southern mullet (Liza richardsonii Smith, 1846), flathead mullet (Mugil cephalus Linnaeus, 1758) and freshwater mullet (Myxus capensis Valenciennes, 1836) are the more abundant taxa and therefore the most studied in the region (Whitfield, 1998). There have been comprehensive studies conducted on the diets of the first three species (Blaber, 1976, 1977; Marais, 1980; Le Loc’h et al., 2015), but little is known about the diet of M. capensis in either estuaries or riverine systems. Based on the limited dietary data available, the freshwater mullet in the estuarine phase of their life cycle are regarded as being dependent upon “diatoms, filamentous green algae and detritus together with some animal prey” (Blaber, 1976, 1977, reviewed in Cardona, 2016). Furthermore, the spatial and temporal variability in M. capensis food preferences, and the origin of its primary food sources, remain largely unknown.
Our objective was to provide quantitative estimations of freshwater mullet dietary composition in the river–estuary interface area of a South African system using a combination of methods. We tested the following hypotheses:
-
(A)
similar to many other migratory species whose ontogenetic movements between freshwater, estuarine and marine environments are known, freshwater mullet change their diet as they grow and migrate from the estuary into the lower riverine area, thereby making use of different food resources depending on habitat; and
-
(B)
because of the dietary flexibility characterizing mugilids, the dominant food resources utilized by freshwater mullet vary among seasons, mainly as a function of the shifting temporal availability of preferred food items in a particular habitat.
Materials and methods
Fish sampling
Myxus capensis specimens were collected seasonally from three sites in the Kowie system (Fig. 1). The sampling sites were located in and just above the ebb and flow region at the head of the Kowie Estuary, as the species congregates in these areas prior to moving upstream into the catchment (Whitfield, 1998). Freshwater Site A was located just above a weir equipped with a fish ladder, while two downstream sites (B and C) were located within 1 km of the upper site and were subject to tidal influence at the head of the estuary (Fig. 1). Other details regarding the study region are documented in Bergamino et al. (2014), Dalu et al. (2014), Bergamino & Richoux (2015), Carassou et al. (2016), Dalu et al. (2016), Moyo (2016) and Moyo et al. (2017).
Specimens of M. capensis were collected using three monofilament gill nets (10 m × 2 m and stretch mesh sizes of 45, 75 and 100 mm) where depth permitted (i.e. at Site A), and with a cast net (1.6 m diameter and 1 cm bar mesh) in shallower waters (i.e. at sites B and C) (Fig. 1). Each site was visited quarterly between March 2012 and February 2013, for a total of four sampling times: autumn (March/April 2012), winter (August 2012), spring (November 2012) and summer (February 2013). Fish were euthanized using iced water and preserved on ice in a cooler box for transport back to the laboratory at Rhodes University.
Sampling of food sources
Potential food items for M. capensis were collected within the fish sampling area (corresponding to Site B) as part of a larger project investigating energy flow in the Kowie River catchment, so the relevant tracer data were extracted from several studies (Dalu et al., 2014, 2016; Moyo, 2016; Moyo et al., 2017). Food items were selected based on published literature of M. capensis diet (Blaber, 1976, 1977; Cardona, 2016) and from stomach content observations made during this study, following the rationale used in Carassou et al. (2016) for other fish species in the Kowie system. Samples of coarse particulate organic matter (CPOM), benthic microalgae growing on rocks (epilithon) and plants (epiphyton), insects, other aquatic invertebrates, and dominant vascular plants were collected in September (winter) and November (spring) 2012, and February (summer) and May (autumn) 2013. CPOM was used as a surrogate for detrital material, as it was composed mainly of decaying leaf litter (Dalu et al., 2016).
Epiphyton and epilithon were brushed from submerged plants and rocks, respectively (Dalu et al., 2014), and based on their isotopic similarities (Moyo, 2016) and similar visual appearance in fish stomach contents, they were collectively labelled epiphytic microalgae. Larvae of aerial insects, aquatic insects, semi-aquatic (air–water interface) insects (e.g. Hydrometridae) and other freshwater invertebrates were sampled in the river by repetitive kick sampling and sweeping using a South African Standard Scoring (SASS) net (mesh size 80 μm). Dominant emergent and submerged vascular plants such as Juncus effusus [Linnaeus, 1753], Phragmites australis [von Trinius ex. Von Steudel, 1841], Stuckenia pectinata [Börner, 1912] and Schoenoplectus brachyceras [von Hochstetter ex. Richard, 1971] were collected by hand. All samples were placed on ice in plastic containers and transported to the laboratory.
Fish dissection
Fish were measured (standard length (SL) in cm) and their stomachs removed and preserved in 4% formaldehyde for 72 h before being transferred to 70% ethanol. The stomach contents were later placed in a Petri dish and categorized under a dissecting microscope into broad taxonomic groups (usually order), the relative proportions of which were visually estimated (% of total content) according to the area each food type occupied in the dish. For each fish, two samples of dorsal muscle (one sample per tracer method) were extracted and lyophilized (VirTis BenchTop 2K) for ≥30 h at −60°C and stored at −80°C.
Preparation of stable isotope samples
Methods for fish stable isotope analysis (δ13C and δ15N) are detailed in Carassou et al. (2016). Briefly, dried and homogenized fish tissues were weighed to the nearest mg with an analytical microbalance (Mettler Toledo XP205) and packaged into 8 × 5 mm sterile tin capsules. Epiphytic microalgae and detritus (CPOM) were vacuum-filtered onto GF/F glass fibre filters, treated with 1 M hydrochloric acid to remove carbonates, and further processed according to Dalu et al. (2014) and Dalu et al. (2016). Insects and other freshwater invertebrates were identified to the lowest possible taxonomic level (usually family), and all data on the insects were extracted from Moyo (2016). These potential prey items were kept alive for about 6 h to allow for gut clearance before the animals were frozen and lyophilized. For each taxon, one to five individuals were combined to obtain sufficient dry mass for isotopic analysis (i.e. 0.5–1 mg). Additional details on laboratory processing and analysis of insects and invertebrates to produce stable isotope data are available in Moyo (2016).
Analyses of δ13C and δ15N values of fish and food sources were conducted at IsoEnvironmental cc, Grahamstown, using a Europa Scientific 20-20 Isotope Radio Mass Spectrometer linked with an ANCA SL Prep Unit. Ammonium sulphate, casein and beet sugar were used as internal standards, calibrated against the International Atomic Energy references (Vienna Pee Dee Belemnite for carbon, and atmospheric N2 for nitrogen). The analyses were performed with a 0.1‰ precision for both isotopes. Carbon and nitrogen isotopic content of fish and food source samples were based on the standard delta unit notation (δ):
where R = 13C/12C or 15N/14N.
Preparation of fatty acid samples
Lipids were extracted from the food sources and fish, and the fatty acids transformed into fatty acid methyl esters following protocols modified from Folch et al. (1957), Indarti et al. (2005) and Budge et al. (2006). The lipid extraction and fatty acid methyl ester synthesis procedures for fish samples are detailed herein, and the methods for food source preparation and analyses are described in Bergamino et al. (2014), Richoux & Ndhlovu (2014) and Moyo (2016).
Freeze-dried fish samples were homogenized with a mortar and pestle, and dry masses ranging between 14.4 and 87.3 mg (average of 55.3 mg) were placed in 1.5 ml of chloroform with 0.01% butylated hydroxytoluene (used as an antioxidant). To extract lipids, 1 ml of ice-cold methanol was added to each sample before vortexing and sonication for 4 min in an ice-bath. Samples were decanted through a lipid-clean cotton wool plugged pipette to filter out tissue remains and mixed with 1.5 ml of 0.9% potassium chloride, followed by vortexing and centrifugation for 3 min at 3000 rpm, with the aqueous phase then being discarded. Potassium chloride (0.5 ml of 0.9% concentration) and methanol (0.5 ml) were added to the organic phase, which was vortexed and centrifuged for 3 min at 3000 rpm. The aqueous phase was removed a second time, and sodium sulphate added to the samples before filtering through a lipid-clean cotton wool plugged pipette filled with sodium sulphate (the addition of sodium sulphate was to ensure that samples were anhydrous). The remaining organic component was dried under a gentle stream of N2, covered with 0.5 ml of CHCl3 and stored at −20°C.
We used column chromatography to isolate the neutral lipids from the fish samples. Since phospholipids are primarily structural and not readily affected by dietary changes in secondary or higher consumers, we focused only on the neutral lipids that represent stored lipids obtained from the diet (Parrish et al., 2000; Dalsgaard et al., 2003; Budge et al., 2006). Each sample was eluted through activated silica gel with 8 ml of (98:1:0.5) chloroform:methanol:formic acid to obtain a neutral lipid-containing eluant. The solvents were dried under a gentle stream of N2 and topped with 1.5 ml of dry methylene chloride.
Fatty acid methyl esters were synthesized using a standard sulphuric acid method (Budge et al., 2006) and the extracts were stored in hexane and injected (1 μl aliquots) into an Agilent 7890A gas chromatograph equipped with a Zebron-Waxplus 320 column with helium as the carrier gas (see Richoux & Ndhlovu, 2014 for oven protocols). Peaks were integrated with Chemstation chromatography software and identified by an Agilent QQQ mass spectrometer and Masshunter software. Fatty acid composition was expressed qualitatively as individual fatty acid proportions of the total neutral lipids in fish and total lipids in food sources.
Data analysis
Fish size data were plotted (number of fish vs SL) to visualize the size distribution of M. capensis collected over the study period. This distribution plot helped to define three size categories of fish corresponding to successive ontogenetic stages, the ranges of which were estimated based on fish size relative to the lowest estimate of size at first maturity (S m) for the species, i.e. 19 cm SL (Whitfield, 1998). The categories were as follows: small juveniles were smaller than 1/4th S m (J1, ≤5 cm SL), medium-sized juveniles were smaller than 1/2 S m (J2, 5.1–10 cm SL), and adults were larger than S m (Ad, >19 cm SL). Analysis of variance (ANOVA) was applied to test for differences in fish size between seasons (d.f. = 3) and sites (d.f. = 2), and in fish δ13C and δ15N values between seasons (d.f. = 3), sites (d.f. = 2) and size categories (d.f. = 2).
Analysis of similarity (ANOSIM) was used to determine any differences in fish stomach content composition according to season, site and size class. All unidentified prey and any minor prey items recorded only once were removed from the stomach content data matrix before analysis. ANOSIM were based on Euclidean distance matrices built upon square-root transformed data to balance the weighting of dominant versus rarer prey in the dataset (Zar, 1999). Analyses of contributions to the dissimilarity (SIMPER) identified the prey items responsible for any observed difference, and the relative proportions of food items in fish stomachs were visualized in box plots.
To make comparisons with the stomach content and fatty acid findings, dietary contributions to the freshwater mullet were estimated based on stable isotope data analysed with Bayesian stable isotope mixing models (Moore & Semmens, 2008; Parnell et al., 2013), specifically using the MixSIAR package in R (Stock & Semmens, 2013). For consistency and simplicity, and to facilitate comparisons among methods, broad food source categories were created to resemble those categories used in stomach content analyses. Food source categories were designed to highlight the ultimate origin of animal prey (Table 1). For example, invertebrates were separated into two groups: one included all animal prey from the aquatic environment (i.e. freshwater invertebrates such as crustaceans or gastropods and strictly aquatic insects) and the other included all animal prey utilizing mainly the terrestrial environment (i.e. aerial flying insects or their larvae and semi-aquatic insects occupying the air–water interface, such as Hydrometridae).
Seven mixing models were run: four ‘seasonal’ models (autumn, winter, spring, summer) in which the fish data were analysed separately for each season, and three ‘life-stage’ models (J1, J2, Ad) in which the fish life stages were analysed separately. Time-averaged food source data were included in all models to overcome species-specific issues related to growth, protein turnover rate, temperature and lags between fish and source sampling times for some seasons (McCutchan et al., 2003; Martínez del Rio et al., 2009; Bergamino et al., 2014). In addition, since all models were run with similar smoothed food source isotope data, comparisons were facilitated between models based on different seasons or fish life stages. Trophic enrichment factors (TEF) were 0.4 ± 0.1 and 2.0 ± 0.3 for carbon and nitrogen, respectively, as recommended by McCutchan et al. (2003) for freshwater aquatic consumers, and after examination of isotopic values from available food source data in the Kowie system (Carassou et al., 2016; Moyo, 2016). These TEFs for consumers fall within the confidence limits summarized by Vanderklift & Ponsard (2003) and Caut et al. (2009).
Proportions of individual fatty acids, saturated fatty acids, monounsaturated fatty acids and polyunsaturated fatty acids in fish and food source samples were summarized. Additionally, several indices were calculated to represent diet-derived fatty acid composition in the fish tissues. The proportion (% total neutral lipids) of essential fatty acids (sum of 20:4ω6, 20:5ω3 and 22:6ω3) was considered an indicator of fish nutritional condition (Dalsgaard et al., 2003; Tocher, 2010). The proportion of bacterial fatty acids (sum of the odd number and branched chains) indicated the consumption of bacterial-derived material (Dalsgaard et al., 2003). The proportion of long-chain (≥20 carbons) saturated fatty acids was associated with the consumption of terrestrial plants (Dalsgaard et al., 2003; Budge et al., 2006). The Σω3/Σω6 ratio was used as a measure of the relative influence of aquatic versus terrestrial material in the fish diet, with higher ratios indicative of higher contributions of terrestrially derived food (Parrish et al., 2000; Brett et al., 2009; Taipale et al., 2013; Antonio & Richoux, 2014). Values of all the fatty acid indices were compared between seasons (d.f. = 3), sites (d.f. = 2) and fish life stages (d.f. = 2) using ANOVA.
Variations in fish total neutral lipid composition between seasons, sites and life stages were statistically tested using ANOSIM based on a Euclidean distance matrix. Two fish samples characterized by abnormally small amounts of neutral lipids were excluded from the matrix due to a problem that arose during laboratory processing. Total fatty acid compositions of the resources and neutral fatty acid compositions of fish were combined into a non-parametric multidimensional scaling analysis (nMDS) to infer trophic relationships within the community. Food source fatty acid data were obtained from concurrent studies at sites and during seasons similar to those of the stable isotope data. The nMDS matrix comprised 35 columns corresponding to the 35 fatty acids identified in fish muscles, and 212 lines including fish observations (N = 33) grouped by season and life-stage and source observations (N = 179) grouped into five categories similar to those used in the stable isotope approach: detritus (n = 4), epiphytic microalgae (n = 41), vascular plants (n = 29), aquatic invertebrates (n = 50) and aerial/interface insects (n = 55).
Results
Fish collection
Seventy-four fish were captured during the study period, including 37, 7, 20 and 10 in autumn, winter, spring and summer, respectively; 29, 26 and 19 at sites A, B and C, respectively; and comprising 26 small juveniles, 27 medium-sized juveniles and 21 adults. Seventy-two fish were dissected for stomach content analysis (7–10 replicates per site and per season), 65 for stable isotope analysis (7–10 replicates per site and per season), and 35 for fatty acid analysis (5 replicates per site and per season). Fish size varied significantly between seasons (F = 144.5, P < 0.0001) and sites (F = 27.2, P < 0.0001). Overall, small (≤5 cm SL) and medium-sized (5.1–10 cm SL) juveniles were more abundant at the downstream sites B and C in autumn and spring, respectively, whereas adult fish (>19 cm SL) were collected mostly from the upstream site (A) during winter and summer (Fig. 2).
Fish size per sampling season (a) and site (b), and overall fish size distribution (c) recorded during the study period. Small letters between brackets under the season and site labels illustrate groups resulting from ANOVA and Student’s post hoc pairwise tests. Labels for the sites and fish life stages are described in the “Materials and methods” section. Upper and lower limits of the boxes (a, b) correspond to the 1st and 3rd quartiles, respectively. The horizontal bars within the boxes indicate the median, the error bars are the 10th and 90th percentiles, and the dots represent values outside this range
Stomach contents
Two of the 72 fish dissected for stomach content analysis had empty stomachs. Particulate organic material (POM) and filamentous algae dominated the stomach contents of fish from all size categories (i.e. they occurred in more than 80% of stomachs for all categories; Table 2). Vascular plant debris (detritus), sand, polychaetes and amphipods were recorded in more than 20% of the adult stomachs, whereas insects, specifically larvae of aquatic breeding flies (chironomids and unidentified flies), occurred in 50% of the small juvenile M. capensis stomachs, and vascular plant debris (detritus) occurred in more than 10% of the medium-sized juvenile stomachs (Table 2).
The ANOSIM performed on the stomach content data detected significant but weak differences in fish diet between sites (R = 0.058, P = 0.026), significant and stronger differences between seasons (R = 0.119, P = 0.027), but no significant difference between fish size classes (P > 0.05). Filamentous algae, POM and Chironomidae always explained more than 15% of the observed significant differences. However, those food items did not display any consistent spatial or temporal patterns, with highly variable proportions at every site and season (Fig. 3). Nevertheless, filamentous algae occurred more frequently in the diet of M. capensis during autumn and winter than spring and summer, while Chironomidae occurred more frequently in the fish from Site B (Fig. 3).
Proportions (%) of filamentous algae (a, d), particulate organic material (b, e) and Chironomidae (c, f) in Myxus capensis stomach contents during each season (a, b, c) and site (d, e, f). Boxes, bars, lines and dots are as in Fig. 2
Stable isotopes
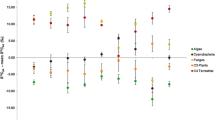
Fish δ13C and δ15N values varied significantly between sites (F = 45.88, P < 0.0001 and F = 6.98, P = 0.0019 for δ13C and δ15N, respectively), seasons (F = 18.63, P < 0.0001 and F = 19.72, P < 0.0001 for δ13C and δ15N, respectively) and fish size categories (F = 85.12, P < 0.0001 and F = 18.25, P < 0.0001 for δ13C and δ15N, respectively). Relatively low δ13C and high δ15N values were observed in fish collected at Site A during winter and summer (Fig. 4a, b), while the smallest juveniles (J1) had much higher δ13C values compared to larger fish (Fig. 4c). The medium-sized juveniles (J2) had the lowest but most variable nitrogen values (Fig. 4c).
Mixing models highlighted clear variations in food resources used by M. capensis during different seasons and life stages (Fig. 5A, B; Supplementary Material 1). Aerial/interface insects were the dominant prey of M. capensis in winter and summer (Fig. 5A; Supplementary Material 1), mainly due to their large contribution to the diets of adult fish (Fig. 5B) that were abundant during those times (Fig. 2a). Aquatic invertebrates were the major food sources of M. capensis in autumn (Fig. 5A), mainly due to their large contribution to the diets of the small juveniles (Fig. 5B) that were abundant during this season (Fig. 2a). Medium-sized juveniles were most abundant in spring (Fig. 2a) and this cohort relied on a more balanced mixture of food resources, dominated by epiphytic microalgae and detritus (Fig. 5A, B). Macrophyte contributions to M. capensis diet never exceeded 20% in any of the seasonal or life-stage models (Fig. 5A, B; Supplementary Material 1).
Contributions (Bayesian posterior density plots for overall population) of aerial/interface insects (‘AI_invert’), aquatic invertebrates (‘AQ_invert’), detritus (=CPOM, ‘detrit’), epiphytic microalgae (=epiphyton + epilithon, “EpPe”) and macrophytes (=vascular plants, “macro”) to the diet of Myxus capensis during different seasons (A) and life stages (B), estimated from stable isotope data analysed using MixSIAR models. Further details on food resource categories are provided in Table 1. Different coloured areas show the distribution of Bayesian estimated proportions for different food resource categories based on multiple runs of the models (after testing, all models set at ‘long runs’, corresponding to a 300,000 chain length of Markov Chain Monte Carlo iterations). Codes for fish life stages are given in the text. Detailed posterior estimates of the models are provided in Supplementary Material 1
Fatty acids
Among the 35 fatty acids identified in M. capensis dorsal muscles, the most abundant were 16:0 (average of 23.4% total neutral lipids) and 16:1ω7 (14.1%), followed by 20:5ω3 (9.7%), 18:1ω9 (8.6%), 18:0 (5.9%), 14:0 (5.3%) and 18:1ω7 (4.5%) (Table 3). All food resources also presented relatively large amounts of 16:0 (always >15% of total fatty acids; Table 3). Detritus and vascular plants had large proportions of 18:3ω3 and 18:2ω6, while 16:1ω7 was typical of epiphytic microalgae, and 20:5ω3 and 18:1ω9 of animal prey (i.e. aquatic invertebrates and aerial/interface insects; Table 3).
Differences in fatty acid indices derived from fish tissues according to season, site and fish life stage were relatively small when significant, as indicated by low F values in corresponding ANOVA (F < 10 in most instances; Table 4). However, the summed essential fatty acids indicative of M. capensis nutritional condition varied significantly between seasons, sites and fish life stages, with the highest values occurring in small juveniles during spring at Site B, and the lowest values in adults during winter at Site A (Table 4). Bacterial fatty acid proportions in M. capensis tissues were always small (<1%), and varied only according to fish life stage, with the highest values occurring in medium juveniles (Table 4). The Σω3/Σω6 ratio indicative of terrestrial contributions to fish diets varied between seasons and fish life stages, with the highest values occurring in adults during summer and the lowest values in small juveniles during autumn (Table 4). Proportions of long-chain saturated fatty acids indicative of vascular plant consumption were small (<1%) and did not vary between seasons, sites or fish life stages (Table 4).
When considering the entire fatty acid compositions in the neutral lipid fraction of fish tissues, some significant differences occurred between seasons (R = 0.277; P = 0.001), sites (R = 0.228; P = 0.001) and fish life stages (R = 0.366; P = 0.001), although small R values derived from the ANOSIM indicated that overlapping occurred. Fish fatty acid compositions were unrelated to those of detritus and vascular plants, but were more closely linked to the compositions of epiphytic microalgae, aquatic invertebrates and aerial/interface insects (Fig. 6).
Non-parametric multidimensional scaling analysis (nMDS) for Myxus capensis (black labels) and its potential food resources (coloured labels) based on fatty acid proportions (% total neutral lipids and total lipids in fish and resources, respectively). The list of fatty acids included in this analysis is provided in Table 3. The MDS plot is shown using two axes, and includes all seasonal data for both fish and food sources. This facilitated a summary of the global relationship between fatty acid profiles of fish and the different food resources
Discussion
The combined use of stomach content, stable isotope ratio and fatty acid analyses allowed us to assess the diet of a poorly studied but abundant mullet species in a South African river–estuary interface region. Overall, M. capensis in the Kowie system had a high frequency of occurrence of POM and filamentous algae in its stomach contents. Assimilated food resources varied seasonally due to fish migration placing M. capensis life stages at different localities along the estuary–river continuum (in support of Hypothesis A: freshwater mullets change their diet as they grow and migrate from the estuary into the lower riverine area, thereby making use of different food resources depending on habitat). Thus, important contributions of detritus and epiphytic microalgae (epiphyton and epilithon) were recorded in the diets of medium-sized juveniles, with dominant contributions of animal-derived food, mainly insects and small aquatic invertebrates, at other life stages.
Variations in diet, together with temporal changes in food resource availability within the study area (Moyo, 2016; discussed below), reinforced the trophic flexibility of this fish family (Cardona, 2016; in support of Hypothesis B: the dominant food resources utilized by freshwater mullet vary between seasons, mainly as a function of the shifting temporal availability of preferred food items in a particular habitat). However, some differences occurred between the datasets produced using different methods, thus reflecting their inherent biases and strengths. Biochemical tracer techniques such as stable isotopes and fatty acids were necessary to estimate the proportions of different foods assimilated by M. capensis, information that is not available from stomach contents. As this study is the first to combine three different methods to address dietary features of any mugilid in the world, we could identify major trophic characteristics that would have remained unresolved using stomach content analysis or stable isotope ratios alone.
The stomach content analyses revealed the ingestion of a variety of food resources by M. capensis, most of which were embedded within a matrix of unidentified organic material of unknown origin. However, many insects and some small aquatic invertebrates were identified, together with filamentous algae and vascular plant detritus. Stomach content analysis therefore provided a useful guideline for the selection of appropriate food resources to be analysed using the biochemical techniques, therefore illustrating the advantage of combining traditional analyses with other methods for fish dietary assessment. As δ15N and δ13C reflect consumer trophic level and origin of food sources (e.g. marine versus freshwater), respectively (Fry & Sherr, 1989; Hobson, 1999; Post, 2002), isotopic ratios measured in the freshwater mullet muscles showed changes in diet and habitat occupancy with fish development. Small juvenile fish were characterized by higher δ15N and δ13C values compared with larger juveniles. Values of δ15N typically increase with trophic level, while δ13C values increase with salinity, i.e. along freshwater-marine gradients (Post, 2002). As with most estuary-associated marine fish species, the early developmental stages of M. capensis utilize zooplankton in the marine environment prior to first recruitment into the estuary (Blaber & Whitfield, 1977). These early juveniles are therefore highly carnivorous, a trophic position highlighted by their high δ15N values, and dependent on marine-derived food sources, as reflected by their high δ13C values (Hobson, 1999; Post, 2002).
Medium-sized juveniles occupied the less saline waters of the river–estuary interface of the Kowie system where they consumed a mixture of food items (including both animal- and plant-derived resources), thus contributing to their lower δ15N and δ13C values relative to the smaller fish (i.e. lower δ15N values indicate a lower trophic level, while lower δ13C values reflect food assimilated from less saline environments; Hobson, 1999; Post, 2002). The migration of developing juveniles into the river resulted in further dietary changes, and adults collected from this region displayed a mostly carnivorous diet characterized by high δ15N and low δ13C values, reflecting their consumption of insects and occupation of the freshwater environment, respectively (Hobson, 1999; Post, 2002). Therefore, the stable isotope approach confirmed M. capensis movements across the different habitats in the study system, consistent with the described migratory behaviour of this species in other South African coastal systems (Whitfield, 2016).
Estimations of food resource contributions to the mullet diet based on stable isotope mixing models highlighted the assimilation of animal-derived prey items, with aerial/interface insects (or their larvae) and small aquatic invertebrates (including aquatic insects) making large contributions to the diets of small juveniles and adults during most seasons. Detritus and epiphytic microalgae made important contributions to the diet of M. capensis only in medium-sized juveniles abundant in spring, together with a mixture of other resources. However, microalgae and detritus are traditionally described as the major dietary component of various mugilid species, including M. capensis, in various stomach content studies (Blaber, 1976, 1977; Cardona, 2016). Our MixSIAR approach revealed that in the Kowie system, detritus and epiphytic microalgae contributed to the diet of M. capensis for limited periods and specific life stages, while animal-derived resources dominated the rest of the time.
Mixing models also highlighted relatively small contributions of macrophyte material to the diet of M. capensis, although this food type occurred frequently in the stomach contents (Table 2). Temporal discrepancy between sampling periods for food sources and consumers may have resulted in an over- or under-estimation of the importance of some food sources in the dietary mixing models. However, this explanation is unlikely given the consistently small contributions of macrophytes and corresponding large contributions of animal-derived food to the diet of M. capensis during three out of the four seasons (Fig. 5), a result consistent with our fatty acid approach. Outputs from mixing models can also be affected by the selected trophic fractionation factors (TEFs), an aspect that has been scrutinized in previous studies conducted in the Kowie River system (Bergamino et al., 2014; Bergamino & Richoux, 2015; Carassou et al., 2016). Based on the published sensitivity analyses (Carassou et al., 2016), the TEFs used in our models were appropriately based on the environmental and biological characteristics of our study region.
Contributions by primary producers to the diet of a consumer feeding upon both plant-derived and animal-derived material can be further biased by the premises of stable isotope mixing models themselves, particularly the assumption of consistent carbon and nitrogen contents among food resources (Fry, 2013; Phillips et al., 2014). To deal with this potential bias, some authors recommend the incorporation of concentration-dependent data, although the ecological relevance of this procedure in food web studies remains challenged (e.g. Phillips & Koch, 2002; Koch & Philipps, 2002; Robbins et al., 2002). In the absence of available data on absolute carbon and nitrogen contents of animal prey and other algal or plant-derived sources (including epiphytes), our models were run without inclusion of concentration-dependent data. Furthermore, it can sometimes be difficult to differentiate primary producers from one another based on their stable isotope composition. For example, in a neighbouring South African estuary, stable isotopes could not distinguish vascular plants from epiphytes (Richoux & Froneman, 2007, 2008). However, it is unlikely that any of these issues resulted in under-estimations of macrophyte contributions to the diet of M. capensis in our mixing models, as the fatty acid approach (including both MDS and calculations of fatty acid indices, discussed below) clearly confirmed the lack of a trophic relationship between vascular plants and fish, but a strong reliance of fish on animal-derived food and epiphytic microalgae.
The large contribution of epiphytic microalgae to the diet of M. capensis during spring could be partly the result of aforementioned issues relating to primary producer discrimination. However, this factor seems to have affected only our spring and medium-sized juvenile model, while all other models highlighted a clear dominance of animal-derived prey. The assumption that epiphytic microalgae make substantial contributions to the diet of M. capensis during this specific season and life stage therefore appears reasonable, and probably reflects ontogenetic variations in diet characterizing the species in our study system. Fish collected in spring were indeed mostly medium-sized juveniles, all collected from sites B (n = 13) and C (n = 7), which were the most estuarine-influenced sites in our study area. During the other seasons, between 32% (autumn) and 100% (winter and summer) of fish analysed originated from site A, which is isolated from the estuary by a weir (Fig. 1). Besides chironomid larvae, considerably fewer insects are available in the estuarine area when compared to the riverine portion of the Kowie system (Moyo, 2016), and this may explain why M. capensis from the lower sites of our sampling gradient fed mostly upon microalgae during spring.
According to Blaber (1976, 1977), POM dominating M. capensis stomach contents in estuaries generally contains sand granules of 0.2–0.3 mm mean diameter, thus providing evidence that M. capensis juveniles in estuaries forage primarily on the bottom, i.e. not at the air/water interface. This behaviour could also explain the small contributions of insects and the large contributions of benthic microalgae in the diet of juvenile M. capensis that dominated our spring collection. Consumption of aerial/surface insects in riverine areas during the other seasons and life stages can therefore be viewed as a secondary opportunistic foraging behaviour that is energetically beneficial to M. capensis because of the higher energy, fat and protein contents of insect food.
Besides considerations relating to the spatial origin of analysed specimens, the large contribution of epiphytic microalgae to the diet of M. capensis during spring is consistent with the temporal variations in food resource availability in the study system (in support of our hypothesis B, as already discussed above). In spring, microalgae in most aquatic ecosystems begin to multiply due to improved growing conditions, especially increased light penetration within the water column. Therefore, it is not surprising that M. capensis utilized benthic primary producers to a significant extent during this season. Furthermore, insect numbers begin building up during spring but only reach maximum abundance (both larval and adult forms) in summer; hence, M. capensis utilized greater proportions of insects during summer than spring. The latter explanation was further confirmed by the fatty acid nutritional condition index results, as discussed below.
The fatty acid composition of freshwater mullet tissues was consistent with that typically recorded in muscles of bony fish from a variety of aquatic systems (Osman et al., 2001; Robin et al., 2003; Turner & Rooker, 2005, 2006; Skadullah & Tsuchiya, 2009; van der Bank et al., 2011; Iitembu & Richoux, 2016). Proportions of essential fatty acids measured in M. capensis, which were used as indicators of fish nutritional condition (Dalsgaard et al., 2003; Tocher, 2010), were largely above the minimum requirements reported through feeding experiments on various reared fish species (Takeuchi, 1997; Izquierdo, 2005; Tocher, 2010), although values from the latter studies are usually expressed as a percentage of total unsaturated fatty acids or total lipids, and are therefore difficult to compare with our estimates from wild fish based on the percentage of total neutral lipids. However, essential fatty acids, together with other fatty acid indices used in this study, highlighted the variability in mullet diet and nutritional condition with season and fish life stage. More specifically, the largest amounts of essential fatty acids occurred in small juvenile fish collected during spring, while the smallest amounts occurred in adults collected during winter (Table 4). Besides ontogenetic variations associated with fish nutritional metabolism (Tocher, 2010), this trend may reflect a decreased nutritional condition in M. capensis during winter, consistent with decreased availability of nutrient-rich food resources such as larval insects. For instance, biomass estimates of emergent larval insects and infalling terrestrial insects into the Kowie system varied from 3–28 mg m−2 day−1 and 11–220 mg m−2 day−1 in winter, to between 169–1402 mg m−2 day−1 and 413–679 mg m−2 day−1 during summer (Moyo et al., 2017), resulting in decreased availability of nutrient-rich resources and therefore decreased fish nutritional condition during winter. This explanation is further supported by the larger amounts of essential fatty acids (and therefore better nutritional condition) recorded in adult fish during summer compared to winter (Table 4).
Values for other fatty acid indices derived from M. capensis were low, typical of many high-order consumers in aquatic environments (Budge et al., 2001; Dalsgaard et al., 2003), and they varied relatively weakly through space and time. For instance, sums of bacterial (odd-numbered and branched chains) and vascular plant-associated (long saturated chains) fatty acids in M. capensis never exceeded 1% of the total neutral lipids. Both indices did not vary or varied weakly between seasons and fish life stages in the Kowie system, reflecting little consumption of bacterial-derived and vascular plant material (Parrish et al., 2000; Budge et al., 2006). This finding was consistent with outputs from our mixing models that showed small contributions of vascular plants to the nutrition of M. capensis. The Σω3/Σω6 ratio of the mullet (putative marker for consumption of autochthonous versus allochthonous resources) also ranged within typically low values for aquatic consumers (Budge et al., 2001; Dalsgaard et al., 2003), including marine fishes (Osman et al., 2001). However, the ratio highlighted a larger contribution of terrestrial-derived food to the adult mullet diet during spring and summer compared to autumn and winter, a finding consistent with the greater availability of nutrient-rich insects in the study system during spring and summer (Moyo et al., 2017). The large contribution of insects to the diet of adult mullet highlighted by the above method is supported by the M. capensis stomach content and stable isotope datasets.
The most striking outcome regarding the diet of freshwater mullet arose from our multivariate analysis of fatty acid compositions from both the fish neutral lipids and the food source total lipids, which illustrated clear similarities between the fish and three major food resources available in the Kowie system, i.e. aquatic invertebrates (including aquatic insects), aerial/interface insects and epiphytic microalgae, whereas detritus and vascular plants had divergent compositions. This result affirmed our interpretations from the stomach content analysis and mixing model outputs, specifically in terms of the dominance of animal-derived and epiphytic algal material in the diet of M. capensis. The importance of those dominant resources to the fish varied temporally in accordance with seasonal changes in fish size, larval insect emergence and terrestrial insect infall (Moyo et al., 2017), as well as with epiphytic algae growth in the study system.
Another important outcome of this study was evidence of a strong reliance by M. capensis on flying (aerial) or interface (semi-aquatic) insects, which are terrestrially derived food sources. This finding implies that a strong trophic connection exists between the aquatic environment and adjacent riparian zones that may allow for the elevated feeding success of freshwater mullet, an important consumer in the Kowie River (Bok, 1979). Similar examples of strong connectivity between water and land occur in other riverine systems (e.g. Nakano et al., 1999; Nakano & Murakami, 2001; Pusey & Arthington, 2003; Baxter et al., 2005; Marcarelli et al., 2011), leading to increased interest in the importance of connectivity for aquatic ecosystem functioning (Richardson & Sato, 2015). The abundance of insects in the Kowie catchment (Moyo, 2016) may therefore partly explain why M. capensis favours the freshwater reaches of this system as a nursery area, i.e. the fish can benefit from insects falling into the river from the riparian area or occupying the water–air interface (Baxter et al., 2005).
Stream environments characterized by relatively undegraded riparian vegetation favour terrestrial subsidization of food resources into the water body, thus benefitting the recipient fish communities (Pusey & Arthington, 2003; Wang et al., 2003; Richardson et al., 2010). A similar situation may pertain to the Kowie River, which has large numbers of trees in the riparian zone and many deep pools that are suitable for occupation by fish species such as M. capensis (Bok, 1979). Furthermore, the Kowie system has a permanently open estuary mouth, a relatively large catchment (769 km2), and a perennial river flow. These characteristics permit free access by recruiting juvenile M. capensis from the sea into the river at any time of the year and the return migration of sexually mature fish to the marine environment where spawning occurs. In the riverine reaches of the Kowie system, M. capensis also benefits from lower competition for food resources from other mullet species that are confined to the estuary, and from the absence of estuary-associated marine piscivorous fish. Unfortunately, the introduction of largemouth bass (Micropterus salmoides), an important consumer of migrating juvenile freshwater mullet (Magoro et al., 2015) in the lower Kowie River system, reduces this predator-free advantage of river occupation by M. capensis.
To conclude, our results clearly highlight the need for a re-assessment of the freshwater mullet trophic guild designation as a strictly detritivorous species, as our data indicate that M. capensis in the Kowie River assimilated mostly microphytobenthos and invertebrates, with a heavy reliance on animal-derived food. This result is consistent with a study of other mugilid species in Aiguillon Bay on the French Atlantic coast, where a higher than expected reliance by Liza ramada and L. aurata on invertebrates such as benthic meiofauna and small macrofauna was documented (Lebreton et al., 2011). Furthermore, although mullets are often associated with the detritivore trophic guild (Cardona, 2016; Crosetti & Blaber, 2016), it seems that different species within the family display strong trophic niche segregation, with each species relying on different carbon sources within the microphytobenthos or organic detrital matter pools (Le Loc’h et al., 2015). Based on these different lines of evidence, we recommend that additional tracer-based studies be conducted on detritivorous fishes such as Mugilidae to clarify their relative dietary dependence on detritus/POM, plants and animals.
References
Abrantes, K. G. & M. Sheaves, 2010. Importance of freshwater flow in terrestrial-aquatic energetic connectivity in intermittently connected estuaries of tropical Australia. Marine Biology 157: 2071–2086.
Antonio, E. S. & N. B. Richoux, 2014. Trophodynamics of three decapod crustaceans in a temperate estuary using stable isotope and fatty acid analyses. Marine Ecology Progress Series 504: 193–205.
Babler, A. L., A. Pilati & M. J. Vanni, 2010. Terrestrial support of detritivorous fish populations decreases with watershed size. Ecosphere 2: 1–23.
Baxter, C. V., K. D. Fausch & W. C. Saunders, 2005. Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshwater Biology 50: 201–220.
Bergamino, L. & N. B. Richoux, 2015. Spatial and temporal changes in estuarine food web structure: differential contributions of marsh grass detritus. Estuaries and Coasts 38: 367–382.
Bergamino, L., T. Dalu & N. B. Richoux, 2014. Spatial and temporal patterns in sediment organic matter composition within an estuarine environment: stable isotope and fatty acid signatures. Hydrobiologia 732: 133–145.
Blaber, S. J. M., 1976. The food and feeding ecology of Mugilidae in the St Lucia lake system. Biological Journal of the Linnean Society 8: 267–277.
Blaber, S. J. M., 1977. The feeding ecology and relative abundance of mullet (Mugilidae) in Natal and Pondoland estuaries. Biological Journal of the Linnean Society 9: 259–275.
Blaber, S. J. M. & A. K. Whitfield, 1977. The feeding ecology of juvenile mullet (Mugilidae) in south-east African estuaries. Biological Journal of the Linnean Society 9: 277–284.
Bok, A. H., 1979. The distribution and ecology of two mullet species in some freshwater rivers in the eastern Cape, South Africa. Journal of the Limnology Society of South Africa 5: 97–102.
Brett, M. T., M. J. Kainz, S. J. Taipale & H. Seshan, 2009. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Science 106: 21197–21201.
Brett, M. T., G. B. Arhonditsis, S. Chandra & M. Z. Kainz, 2012. Mass flux calculations show strong allochthonous support of freshwater zooplankton in unlikely. PLoS ONE 7: e39508.
Buchleister, A. & R. J. Latour, 2010. Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Canadian Journal of Fisheries and Aquatic Sciences 67: 445–461.
Budge, S. M., C. C. Parrish & C. H. Mckenzie, 2001. Fatty acid composition of phytoplankton, settling particulate matter and sediments at a sheltered bivalve aquaculture site. Marine Chemistry 2001: 285–303.
Budge, S. M., S. A. Iverson & H. N. Koopman, 2006. Studying trophic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Marine Mammal Science 22: 759–801.
Carassou, L., A. K. Whitfield, L. Bergamino, S. Moyo & N. B. Richoux, 2016. Trophic dynamics of the Cape stumpnose (Rhabdosargus holubi, Sparidae) across three adjacent aquatic habitats. Estuaries and Coasts 39: 1221–1233.
Cardona, L., 2016. Food and feeding of Mugilidae. Chapter 9 pp. In Crosetti, D. & S. J. M. Blaber (eds), Biology, Ecology and Culture of Grey Mullets (Mugilidae). CRC Press, Boca Raton: 165–195.
Carpenter, S. R., J. J. Cole, M. L. Pace, M. Van de Bogert, D. L. Bade, D. Bastviken, C. M. Gille, J. R. Hodgson, J. F. Kitchell & E. Kritzberg, 2005. Ecosystem subsidies: terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology 86: 2737–2750.
Caut, S., E. Angelo & F. Courchamp, 2009. Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic value and applications for diet reconstruction. Journal of Applied Ecology 46: 443–453.
Cole, J. J., S. R. Carpenter, J. Kitchell, M. L. Pace, C. T. Solomon & B. Weidel, 2011. Strong evidence for terrestrial support of zooplankton in small lakes based on stable isotopes of carbon, nitrogen, and hydrogen. Proceedings of the National Academy of Sciences 108: 1975–1980.
Cortés, E., 1997. A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Sciences 54: 726–738.
Crosetti, D. & S. J. M. Blaber (eds), 2016. Biology, Ecology and Culture of Grey Mullets (Mugilidae). CRC Press, Boca Raton.
Dalsgaard, J., M. St John, G. Kattner, D. Müller-Navarra & W. Hagen, 2003. Fatty acid trophic markers in the pelagic marine environment. Advances in Marine Biology 46: 225–340.
Dalu, T., P. W. Froneman & N. B. Richoux, 2014. Using multivariate analysis and stable isotopes to assess the effects of substrate type on phytobenthos communities. Inland Waters 4: 397–412.
Dalu, T., N. B. Richoux & P. W. Froneman, 2016. Nature and source of suspended particulate organic matter and detritus along an austral temperate river-estuary continuum assessed using stable isotope analysis. Hydrobiologia 767: 95–110.
Elsdon, T. S., 2010. Unraveling diet and feeding histories of fish using fatty acids as natural tracers. Journal of Experimental Marine Biology and Ecology 386: 61–68.
Folch, J., M. Lees & G. H. Sloane Stanley, 1957. A simple method for the isolation and purification of total lipids from animal tissues. The Journal of Biological Chemistry 226: 497–509.
Franco, A., M. Elliott, P. Franzoi & P. Torricelli, 2008. Life strategies of fishes in European estuaries: the functional guild approach. Marine Ecology Progress Series 354: 219–228.
Froese, R. & D. Pauly, 2016. Fishbase. World Wide Web electronic publication [available on internet at www.fishbase.org]. Accessed July 2016
Fry, B., 2013. Alternative approaches for solving underdetermined isotope mixing problems. Marine Ecology Progress Series 472: 1–13.
Fry, B. & E. B. Sherr, 1989. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. In Rundel, P. W., J. R. Ehleringer & K. A. Nagy (eds), Stable Isotopes in Ecological Research. Springer, New York: 196–229.
Harrison, T. D. & A. K. Whitfield, 2006. Estuarine typology and the structuring of fish communities in South Africa. Environmental Biology of Fishes 75: 269–293.
Hobson, K. A., 1999. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120: 314–326.
Hyslop, E. J., 1980. Stomach contents analysis – a review of methods and their application. Journal of Fish Biology 17: 411–429.
Iitembu, J. A. & N. B. Richoux, 2016. Characterisation of the dietary relationships of two sympatric hake species, Merluccius capensis and M. paradoxus, in the northern Benguela region using fatty acid profiles. African Journal of Marine Science 38: 39–48.
Indarti, E., M. I. A. Majid, R. Hashim & A. Chong, 2005. Direct FAME synthesis for rapid total lipid analysis from fish oil and cod liver oil. Journal of Food Composition and Analysis 18: 161–170.
Izquierdo, M., 2005. Essential fatty acid requirements in Mediterranean fish species. Cahiers Options Méditerranéennes 63: 91–102.
Jaschinski, S., D. C. Brepoll & U. Sommer, 2011. The trophic importance of epiphytic algae in a freshwater macrophyte system (Potamogeton perfoliatus L.): stable isotope and fatty acid analyses. Aquatic Sciences 73: 91–101.
Kelly, J. R. & R. E. Scheibling, 2012. Fatty acids as dietary tracers in benthic food webs. Marine Ecology Progress Series 446: 1–22.
Koch, P. L. & D. L. Phillips, 2002. Incorporating concentration dependence in stable isotope mixing models: a reply to Robbins, Hilderbrand and Farley (2002). Oecologia 133: 14–18.
Lebreton, B. P., P. Richard, E. P. Parlier, G. Guillou & G. F. Blanchard, 2011. Trophic ecology of mullets during their spring migration in a European saltmarsh: a stable isotope study. Estuarine Coastal and Shelf Science 91: 502–510.
Le Loc’h, F., J.-D. Durand, K. Diop & J. Panfili, 2015. Spatio-temporal isotopic signatures (δ13C and δ15N) reveal that two sympatric West African mullet species do not feed on the same basal production sources. Journal of fish Biology 86: 1444–1453.
Lin, H.-J., W.-Y. Kao & Y.-T. Wang, 2007. Analyses of stomach contents and stable isotopes reveal food sources of estuarine detritivorous fish in tropical/subtropical Taiwan. Estuarine Coastal and Shelf Science 73: 527–537.
Lopes, C. A., E. Benedito-Cecilio & L. A. Martinelli, 2007. Variability in the carbon isotope signature of Prochilodus lineatus (Prochilodontidae, Characiformes) a bottom-feeding fish of the Neotropical region. Journal of Fish Biology 70: 1649–1659.
Magoro, M. L., A. K. Whitfield & L. Carassou, 2015. Predation by introduced largemouth bass, Micropterus salmoides on indigenous marine fish in the lower Kowie River, South Africa. African Journal of Aquatic Science 40: 81–88.
Marais, J. F. K., 1980. Aspects of food intake, food selection, and alimentary canal morphology of Mugil cephalus (Linnaeus, 1958), Liza tricuspidens (Smith, 1935), L. richardsoni (Smith, 1846), and L. dumerili (Steindachner, 1869). Journal of Experimental Marine Biology and Ecology 44: 193–209.
Marcarelli, A. M., C. V. Baxter, M. M. Mineau & R. O. Hall, 2011. Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92: 1215–1225.
Martínez del Rio, C., N. Wolf, S. A. Carleton & L. Z. Gannes, 2009. Isotopic ecology ten years after a call for more laboratory experiments. Biological Reviews 84: 91–111.
McCutchan, J. H. J., W. M. J. Lewis, C. Kendall & C. C. McGrath, 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen and sulfur. Oikos 102: 378–390.
Moore, J. W. & B. X. Semmens, 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters 11: 470–480.
Moyo, S., 2016. Aquatic-terrestrial linkages via riverine invertebrates in a South African catchment. Ph.D. Dissertation, Department of Zoology and Entomology, Rhodes University, Grahamstown. Available at Rhodes University eRepository [ available on internet at http://contentpro.seals.ac.za/iii/cpro/]. Accessed on March 18th 2017.
Moyo, S., L. D. Chari, M. H. Villet & N. B. Richoux, 2017. Decoupled reciprocal subsidies of biomass and fatty acids in fluxes of invertebrates between a temperate river and the adjacent riparian land. Aquatic Sciences: in press. doi:10.1007/s00027-017-0529-0.
Nakano, S. & M. Murakami, 2001. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Science 98: 166–170.
Nakano, S., H. Miyasaka & N. Kuhara, 1999. Terrestrial-aquatic linkages: riparian arthropod inputs alter trophic cascades in a stream food web. Ecology 80: 2435–2441.
Osman, H., A. R. Suriah & E. C. Law, 2001. Fatty acid composition and cholesterol content of selected marine fish in Malaysian waters. Food Chemistry 73: 55–60.
Parnell, A., D. L. Phillips, S. Bearshop, B. X. Semmens, E. J. Ward, J. W. Moore, A. L. Jackson, J. Grey, D. J. Kelly & R. Inger, 2013. Bayesian stable isotope mixing models. Environmetrics 24: 387–399.
Parrish, C. C., T. A. Abrajano, S. M. Budge, R. J. Helleur, E. D. Hudson, K. Pulchan & C. Ramos, 2000. Lipid and phenolic biomarkers in marine ecosystems: analysis and interpretation. In Wangersky, P. (ed.), The Handbook of Environmental Chemistry Part D Marine Chemistry, Chapter 8, Vol. 5. Springer-Verlag, Berlin: 193–223.
Phillips, D. L. & P. L. Koch, 2002. Incorporating concentration dependence in stable isotope mixing models. Oecologia 130: 114–125.
Philipps, D. L., R. Inger, S. Bearshop, A. L. Jackson, J. W. Moore, A. Parnell, B. X. Semmens & E. J. Ward, 2014. Best practices for use of stable isotope mixing models in food-web studies. Canadian Journal of Zoology 92: 823–835.
Polis, G. A., W. B. Anderson & R. D. Holt, 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annual Review of Ecology, Evolution and Systematics 28: 289–316.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods and assumptions. Ecology 83: 703–718.
Pusey, B. J. & A. H. Arthington, 2003. Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research 54: 1–16.
Richardson, J. S. & T. Sato, 2015. Resource subsidy flows across freshwater-terrestrial boundaries and influence on processes linking adjacent ecosystems. Ecohydrology 8: 406–415.
Richardson, J. S., Y. Zhang & L. B. Marczak, 2010. Resource subsidies across the land-freshwater interface and responses in recipient communities. River Research and Applications 26: 55–66.
Richoux, N. B. & P. W. Froneman, 2007. Assessment of spatial variation in carbon utilization by benthic and pelagic invertebrates in a temperate South African estuary using stable isotope signatures. Estuarine Coastal and Shelf Science 71: 545–558.
Richoux, N. B. & P. W. Froneman, 2008. Trophic ecology of dominant zooplankton and macrofauna in a temperate and oligotrophic South African estuary: a fatty acid approach. Marine Ecology Progress Series 357: 121–137.
Richoux, N. B. & R. T. Ndhlovu, 2014. Temporal shifts in the fatty acid profiles of rocky intertidal invertebrates. Marine Biology 161: 2199–2211.
Robbins, C. T., G. V. Hilderbrand & S. D. Farley, 2002. Incorporating concentration dependence in stable isotope mixing models: a response to Phillips and Koch (2002). Oecologia 133: 10–13.
Robin, J. H., C. Regost, J. Arzel & S. J. Kaushik, 2003. Fatty acid profile of fish following a change in dietary fatty acids source: model of fatty acid composition with a dilution hypothesis. Aquaculture 225: 283–293.
Skadullah, A. & M. Tsuchiya, 2009. The origin of particulate organic matter and the diet of tilapia from an estuarine ecosystem subjected to domestic wastewater discharge: fatty acid analysis approach. Aquatic Ecology 43: 577–589.
Stock, B.C. & B.X. Semmens, 2013. MixSIAR GUI User Manual. Version 3.1.
Taipale, S., U. Strandberg, E. Peltomaa, A. W. E. Galloway, A. Ojala & M. T. Brett, 2013. Fatty acid composition as biomarkers of freshwater microalgae: analysis of 37 strains of microalgae in 22 genera and in seven classes. Aquatic Microbial Ecology 71: 165–178.
Takeuchi, T., 1997. Essential fatty acid requirements of aquatic animals with emphasis on fish larvae and fingerlings. Reviews in Fisheries Science 5: 1–25.
Tocher, D. R., 2010. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquaculture Research 41: 717–732.
Turner, J. P. & J. R. Rooker, 2005. Effects of dietary fatty acids on the body tissues of larval and juvenile cobia and their prey. Journal of Experimental Marine Biology and Ecology 322: 13–27.
Turner, J. P. & J. R. Rooker, 2006. Effect of diet on fatty acid compositions in Sciaenops ocellatus. Journal of Fish Biology 67: 1119–1138.
Van der Bank, M. G., A. C. Utne-Palme, K. Pittman, A. K. Sweetman, N. B. Richoux, V. Brüchert & M. J. Gibbons, 2011. Dietary success of a ‘new’ key fish in an overfished ecosystem: evidence from fatty acid and stable isotope signatures. Marine Ecology Progress Series 428: 219–233.
Vanderklift, M. A. & S. Ponsard, 2003. Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136: 169–182.
Vaz, M. M., M. Jr, L. A. Partinelli Petrere & A. A. Moreto, 1999. The dietary regime of detritivorous fish from the River Jacaré, Brazil. Fisheries Management and Ecology 6: 121–132.
Wang, L., J. Lyons, P. Rasmussen, P. Seelbach, T. Simon, M. Wiley, P. Kanehl, E. Baker, S. Niemela & P. W. Stewart, 2003. Watershed, reach, and riparian influences on stream fish assemblages in the Northern Lakes and Forest Ecoregion, USA. Canadian Journal of Fisheries and Aquatic Sciences 60: 491–505.
Wang, C.-H., C.-C. Hsu, W.-N. Tzeng, C.-F. You & C.-W. Chang, 2011. Origin of the mass mortality of the flathead grey mullet (Mugil cephalus) in the Tanshui River, northern Taiwan, as indicated by otolith elemental signatures. Marine Pollution Bulletin 62: 1809–1813.
Wantzen, K. M., F. de Arruda Machado, M. Voss, H. Boriss & W. J. Junk, 2002. Seasonal isotopic shifts in fish of the Pantanal wetland, Brazil. Aquatic Sciences 64: 239–251.
Whitfield, A. K., 1998. Biology and Ecology of Fishes in southern African estuaries. JLB Institute of Ichthyology, South Africa.
Whitfield, A. K., 2016. Ecological role of Mugilidae in the coastal zone. In Crosetti, D. & S. J. M. Blaber (eds), Biology, Ecology and Culture of Grey Mullets (Mugilidae), Chapter 14. CRC Press, Boca Raton: 324–348.
Zar, J. H., 1999. Biostatistical Analysis, 4th ed. Prentice Hall, Upper Saddle River, NJ.
Acknowledgements
This study was funded by the Water Research Commission (WRC) of South Africa, the National Research Foundation (NRF) of South Africa, Rhodes University’s Sandisa Imbewu Initiative, and the South African Institute for Aquatic Biodiversity (SAIAB). This project received ethics clearance from Rhodes University (RU Ethics Clearance ZOOL-02-2012) and the South African Institute for Aquatic Biodiversity (SAIAB Ethics Clearance 2012/04). We thank Paul Cowley, Mandla Magoro, Tatenda Dalu, Leandro Bergamino and Bernadette Hubbart for their assistance with field and/or laboratory work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Michael Power
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carassou, L., Whitfield, A.K., Moyo, S. et al. Dietary tracers and stomach contents reveal pronounced alimentary flexibility in the freshwater mullet (Myxus capensis, Mugilidae) concomitant with ontogenetic shifts in habitat use and seasonal food availability. Hydrobiologia 799, 327–348 (2017). https://doi.org/10.1007/s10750-017-3230-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3230-3