Abstract
In biparental species, the costs and benefits of parental investment can vary between the sexes and shift over time. However, such sex-specific and temporal changes in territory defense are not well understood. Here, we experimentally investigated parental investment in breeding territory defense in a feral population of the color-polymorphic, biparental cichlid fish, the red devil (Amphilophus labiatus). We presented either gold- or dark-colored conspecific intruder models (i.e., dummy models) to A. labiatus pairs at three key stages during the breeding cycle (i.e., after pair formation, after eggs have been laid, and when fry were free-swimming). We found that males were more aggressive when the pair first formed, whereas females significantly increased their territory defense with time, and were most aggressive when fry were free-swimming. These results show that parental roles in territory defense can markedly shift over key stages of the breeding cycle. Our results demonstrate that parental behaviors may not only vary between the sexes, but can also shift dramatically over the course of the brood cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A key component of parental care in many species is the aggressive defense of a breeding territory and offspring against intruders, such as conspecific and heterospecific competitors and predators (Ridley, 1978; Blumer, 1979; Perrone & Zaret, 1979). Indeed, aggressive defense of a breeding territory can play a key role in parental fitness and offspring success, as shown, for example, in red-backed shrikes (Lanius collurio, Linnaeus, 1758) with more aggressive individuals enjoying higher reproductive success than their less aggressive counterparts (Tryjanowski & Golawski, 2004).
Benefits aside, guarding eggs, offspring or a breeding territory is likely to entail costs for the parents in terms of increased energy expenditure (Haller, 1996), reduced foraging opportunities (Requena et al., 2012), heightened risk of injury and mortality (Marler & Moore, 1988; Lappin & Husak, 2005), as well as lost future mating opportunities (Trivers, 1972; Székely & Cuthill, 2000). For instance, when female crickets (Oecanthus nigricornis, Walker, 1869) are carrying eggs, they are significantly more likely to be taken by predatory wasps (Isodontia mexicana, de Saussure, 1867) (Ercit et al., 2014). Yet, to date, most studies of parental behaviors have tended to focus on offspring provisioning (mostly in birds, see Harrison et al., 2009), with relatively less attention given to the role of territorial defense and offspring guarding in shaping the relative investment of the sexes in offspring care (Clutton-Brock, 1991; Székely & Cuthill, 1999; Harrison et al., 2009; Trnka & Grim, 2012).
When both parents contribute to care, the costs and benefits are often distributed unevenly between the sexes (Houston et al., 2005). Biparental care, therefore, involves elements of not only cooperation but also conflict (Lessells, 1999; Chapman et al., 2003; Harrison et al., 2009). In many species, potential conflicts between the sexes can arise, for example, due to differences between the potential reproductive rates of males and females (Baylis, 1981; Reynolds, 1996), or because males may lack assurance over the paternity of the offspring they are raising (Trivers, 1972; Keenleyside, 1991). As a result, the level of care provided by parents is often not shared equally (Trivers, 1972; Wynne-Edwards, 1995), and may change over time, with a parent sometimes even deserting its partner before offspring become independent (Keenleyside, 1983; McNamara et al., 2002). Despite this, surprisingly few studies have taken an experimental approach to investigate how the sexes might alter their investment in parental behaviors, such as territory defense, over the course of the breeding cycle.
Among the most species-rich vertebrate family, the cichlid fishes (Cichlidae), there is remarkable interspecific variation in the forms of parental care exhibited (Keenleyside, 1991). For this reason, cichlid fishes are well suited for testing hypotheses on the evolution of parental care (Goodwin et al., 1998). Offspring guarding and territory defense, against both heterospecific and conspecific predators of eggs and fry, are the most common forms of parental care in fishes, including cichlids (Gross & Sargent, 1985). Here, we experimentally investigated parental roles in well-defined phases of the brood cycle in a Neotropical cichlid fish, the red devil (Amphilophus labiatus, Günther, 1864). Amphilophus labiatus is endemic to the two great lakes of Nicaragua, Lake Managua and Lake Nicaragua, and has also been introduced elsewhere as a result of its popularity in the aquarium trade (Meek, 1907; NIWA, 2008). Like other members of the Midas cichlid complex (Barlow, 1976; Rogers, 1988; McKaye & Murry, 2008; Elmer et al., 2009; Lehtonen et al., 2012), A. labiatus displays biparental care and is sexually monomorphic with respect to color. Furthermore, in common with many other members of the species complex, A. labiatus displays a genetically distinct color polymorphism, with both “dark” (i.e., gray through to black) and “gold” (yellow through red) colored individuals. The former, in this regard, is much more abundant in their native habitat, making up approximately 90% of the population (Barlow, 1983; Elmer et al., 2010). When pairs are ready to spawn, they claim a breeding territory, which they actively defend against intruders (McKaye, 1977; Rogers, 1987; Barlow, 2000). Fry of Amphilophus cichlids are highly vulnerable to predators and require the parents’ protection for survival from both heterospecific and conspecific territory intruders (Barlow, 1976; Rogers, 1987; McKaye & Murry, 2008; Lehtonen et al., 2012). During this period, pairs must also subsist mostly upon accumulated fat reserves (Barlow, 2000), which further adds to the costs of parental care. The ability to successfully defend the young is, therefore, critical to reproductive success (McKaye, 1977; Rogers, 1987, 1988; Barlow, 2000). In this regard, a number of studies have addressed sex differences in parental care investment and territory defense in Neotropical cichlids (Itzkowitz 1984; Keenleyside et al., 1990; Budaev et al., 1999; Itzkowitz et al., 2001; Wisenden et al., 2008), including in close relatives of A. labiatus (Holder et al., 1991; Rogers, 1988; McKaye & Murry, 2008). It is surprising, however, that few studies have experimentally controlled either the appearance of territorial intruders or the timing of territorial intrusions throughout a breeding period. In particular, such experimental manipulations are important if we are to gain a more comprehensive understanding of why the sexes might differ in their investment in territory defense, especially as this is a key aspect of parental care in these fish.
Accordingly, in the current study, we investigated how males and females alter their investment in offspring and breeding territory defense at three key stages of the breeding cycle.
Methods
Collection and housing
Amphilophus labiatus were collected using hand-lines in late 2013 from a feral population in Hazelwood Pondage, in South Eastern Australia, where they have been introduced and have established a breeding population over the last 40 years (NIWA, 2008). Fish were transported to Monash University and housed in large stock tanks (4 × 4000 l, 26°C, 12:12 day night cycle, stocking density of one fish per 33 l) furnished with gravel, rocks and PVC pipes for shelter. All fish were fed commercial cichlid pellets (Otohime EP3) daily.
Pair formation
To assess breeding territory defense, we first needed to allow individuals to form breeding pairs. This was done by randomly selecting 6 similarly sized mature fish and putting them together into large experimental tanks of ~1000 l, supplied with terracotta pots as potential spawning sites. A pair bond was deemed to have formed when two individuals were observed interacting around a potential spawning site. At this point, all other fish were removed from the experimental tank. During the course of the study, we were successful in obtaining eleven pairs, eight of which paired assortatively based on color (i.e., four gold–gold pairs and four dark–dark pairs) and three non-assortatively, which gave us a total of 11 gold (5 males and 6 females) and 11 dark individuals (6 males and 5 females). Each pair was used only once. Fish were measured (total length, mm) at the end of the experiment. The average total body length was 182 mm for females (range = 161–214 mm, n = 11) and 198 mm (range = 171–234 mm, n = 11) for males.
Intruder models
We simulated aggressive encounters in a controlled fashion by using models of territory intruders presented to our focal pairs. Each model mimicked either a dark or gold A. labiatus (conspecific) territory intruder. We used models of conspecific territory intruders as models for three reasons. First, A. labiatus is the most abundant species in Hazelwood Pondage (personal observation and unpublished capture data). Second, conspecific cichlids pose a high risk of territory take-overs (McKaye, 1977, 1986; Rogers, 1987, 1988). Third, conspecifics are likely to represent a high predation risk to eggs and especially small juveniles (McKaye, 1977; Rogers, 1987, 1988; personal observations). In this respect, we have found both fish eggs and fish remains in the gut content of A. labiatus individuals from both Hazelwood Pondage and from native Nicaragua populations (unpublished data; also see Colombo et al., 2013). Lastly, previous studies have also used conspecifics as intruders to successfully elicit aggression in the context of breeding territory defense in other closely related cichlid species (Itzkowitch, 1985; Holder et al., 1991; Lehtonen, 2014).
“Dummy” models have been successfully used as stimuli to experimentally elicit behaviors in a wide range of fish species (Rowland, 1999), including Amphilophus cichlids – both in the field and in the laboratory (Barlow & Siri, 1994; Lehtonen, 2014; Lehtonen et al., 2015a). We decided to use models instead of live stimulus animals to enable us to control for possible confounding factors that might arise from differences in the behavior of stimulus animals. As with recent studies in other Amphilophus species (Lehtonen, 2014; Lehtonen et al., 2015a), we created realistic-looking models based on photographs of actual fish, rather than the more stylized models that have traditionally been used in the majority of earlier studies (e.g. Barlow & Siri, 1994; Rowland, 1999). We created models of both color morphs (gold and dark) to test whether the level of aggressive territory defense provided by a pair is in anyway influenced by the color of the territory holders and/or conspecific territorial intruders. We did this because biased aggression toward particular individuals, or inherent differences in aggressiveness between phenotypes (e.g., color morphs) could play an important role in the evolution and maintenance of (color) polymorphisms, as observed in Gouldian finches (Erythrura gouldiae, Gould, 1844) (Pryke, 2007) and side-blotched lizards, (Uta stansburiana, Baird & Girard, 1852) (Sinervo et al., 2000).
Specifically, each of the models was made using waterproof, photographic color prints of the lateral side of a live A. labiatus individual. These images (length = 180 mm) were then glued onto both sides of a fish-shaped plastic PVC foam plate (thickness = 6 mm) (see Lehtonen, 2014). Each model was attached to a sinker with a fishing line, which allowed it to float in a natural position approximately 15 cm above the tank bottom (as per Lehtonen, 2014).
Experimental protocol
We used, in total, eight gold and eight dark intruder models to simulate conspecific territory intruders, with each model based on a photograph of a different A. labiatus individual. Individuals whose image was used to create a stimulus model were also excluded from use as potential focal fish (and vice versa). The first model was introduced to the focal pair two hours after the pair first formed and after additional fish had been removed from the tank. The second model was introduced the day after eggs had been laid. The third model was introduced on the day after fry were observed free-swimming. On each occasion, a different stimulus model was used. Thus, each focal pair was exposed to a unique combination of three models (with the color of the model in a given presentation being randomized, resulting in pairs being exposed to a gold intruder, in total, 32 times and a dark intruder 34 times). The three distinct phases for model presentation were chosen to represent key stages in the breeding cycle to allow us to test for differences (if any) in parental investment by males and females over time.
Each replicate was initiated by placing a model approximately 40 cm from the center of the A. labiatus breeding pair’s territory. After an acclimation period of 30 s, we counted the total number of mobile aggressive behaviors (charges and bites) directed by each territory owner (male and female) toward the model over a 2-min observation period, which allowed us to calculate a total ‘aggression rate’ for each pair and each individual (sensu Lehtonen, 2014; Lehtonen et al., 2015b; Oldfield et al., 2015). All trials were filmed with a camcorder positioned on a tripod and watched live, on a closed-circuit system. The trials were run between January and March 2014.
Statistical analysis
To assess the patterns of parental aggressive behavior, we used a generalized mixed model with a negative binomial error distribution using the glmmPQL function of the nlme and MASS packages. In particular, the full model was fitted with reproductive stage (i.e., pair bond, eggs, fry) and sex of the territory owner as explanatory fixed factors, and, with size of the territory owner, color of the dummy model intruder and color (morph) of the territory holder as covariates. To account for the paired design of the experiment (i.e., multiple stimulus presentations to the same individuals) and the potential interdependence between the actions of the paired female and male, both “individual ID” and “pair ID” were added as random effects (as per Pinheiro & Bates, 2000; Lehtonen et al., 2015a).
Given a significant interaction between reproductive stage and sex, we further assessed whether there was an effect of reproductive stage on male or female patterns of parental aggressive behavior by using two separate (one for each sex) generalized mixed models with a negative binomial error distribution, employing the glmmADMB package. Both models were fitted with reproductive stage as a fixed explanatory factor and with size of the territory owner, color of the dummy model intruder and color (morph) of the territory holder as covariates. To account for the design of the experiment in which the same pair was presented with multiple stimuli over time, as well as the potential interdependence between the actions of the paired female and male defending the same territory, “individual ID” was added as a random effect in both models. In both models, we then redefined the reproductive stage reference level, to observe all two-way comparisons between the different stages of the reproductive period. We used R 3.0.0 software (R Development Core Team) for all analyses.
Results
In total, model intruders elicited aggressive responses in 94% of females and 88% of males.
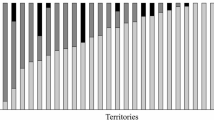
When we applied a generalized mixed model to assess the effects of the reproductive stage and the sex of the focal territory holders on aggression, we found a significant interaction between the stage of the reproductive cycle and the sex of the individual (t 57 = −6.85, P < 0.0001) on the level of aggression. That is, males and females differed in their rates of aggression depending on the stage of the reproductive cycle (Fig. 1). We found no effect of the covariate variables, size of the territory owner (t 57 = 1.52, P = 0.13), color of the dummy model intruder (t 57 = −1.63, P = 0.10, Fig. 2a) or color (morph) of the territory holder (t 57 = −1.02, P = 0.31, Fig. 2b) on patterns of parental aggression.
The rate of aggression a directed toward either dark (n = 8) or gold (n = 8) intruder models averaged across the three key stages of the reproductive cycle, b displayed by individual dark (n = 11) and gold (n = 11) territory holders toward intruder models averaged across the three key stages of the reproductive cycle. Whiskers indicate standard error
Next, when we applied a generalized mixed model to assess the effect of reproductive stage on patterns of male parental aggressive behavior, we found a significant effect of reproductive stage on male aggressive behavior (z = 48.45, P < 0.0001, Fig. 1). Furthermore, there was a significant difference in the level of male aggression (P < 0.001) in each two-way comparison between the stages of the reproductive cycle (Fig. 1). The covariate variables, size of the territory owner (z = 0.59, P = 0.43), color of the dummy model intruder (z = 0.04, P = 0.83) and color (morph) of the territory holder (z = −1.92, P = 0.16) had no significant effect on patterns of male parental aggression.
Similarly, when we applied a generalized mixed model to assess the effect of reproductive stage on patterns of female parental aggressive behavior, we found a significant effect of reproductive stage on female aggressive behavior (z = 53.07, P < 0.0001, Fig. 1). Similar to males, female aggression significantly differed (P < 0.001) in each two-way comparison between the stages of the reproductive cycle (Fig. 1). The effect of the covariate variables, size of the territory owner (z = 2.63, P = 0.10), color of the dummy model intruder (z = 1.50, P = 0.21) or color (morph) of the territory holder (z = 0.03, P = 0.85) was not significant on patterns of female parental aggression.
Discussion
We observed a significant shift in parental investment in territory defense over the course of the breeding cycle. In other words, investment in territory defense at key stages was not divided equally between the sexes. In particular, females were most aggressive toward territorial intruders later in the breeding cycle, when fry were free-swimming. In contrast, the level of territorial defense displayed by males was highest when the pair had first formed and subsequently tapered with time. We also found that the size of a territory owner did not have a significant effect on the level of territory defense. Moreover, in the current study, we found no evidence that territorial aggression was significantly affected by the color of the intruder or parent.
A temporal increase in parental investment by females is concordant with studies in other taxa, such as birds, in which most of the research has so far been carried out (Schipper, 1973; Newton, 1979; Carere & Alleva, 1998; Watts, 2014). For example, female common swifts (Apus apus, Linnaeus, 1758) attend to nests and offspring at a higher rate than males from the nestling stage onwards (Carere & Alleva, 1998). The results of our current experimental study are also in accordance with observational findings in some other cichlid species (McKaye, 1977; Rogers, 1988; McKaye & Murry, 2008). In particular, these previous studies suggest that females of biparental cichlids focus less on territory defense and more on other parental activities in the early stages of the breeding cycle, while males specialize in territorial defense right from the onset of the brood cycle (Rogers, 1988; Murry et al., 2001; Itzkowitz et al., 2005; McKaye & Murry, 2008).
We provide three explanations to account for the subsequent increase in territorial defense intensity by females (sensu Redondo & Carranza, 1989). First, females may be compensating for temporal changes in susceptibility of offspring to predation. Indeed, Amphilophus cichlid fry become more vulnerable to predators when they start to swim actively (the free-swimming phase) and consequently require continual protection for survival (McKaye, 1977; McKaye & Murry, 2008). Second, the reproductive value of offspring increases as the breeding cycle progresses. Specifically, older offspring are more valuable to parents due to their increased probability of reaching maturity and the parental investment that would be required to replace them (Salfert & Moodie, 1985; Rytkönen et al., 1995; Jaroensutasinee & Jaroensutasinee, 2003). Females may, therefore, be adjusting their level of territory defense as a direct response to these specific selection pressures, particularly as males are simultaneously lowering their level of territorial defense at this stage (sensu Hammerstein & Parker, 1987). Finally, if no change is required in the overall level of territory defense, as the relatively constant sum of female and male territorial aggression seems to suggest, females may have been compensating for the males’ reduced effort (see below) in the later stages of the brood cycle.
What about males? Although the above-mentioned selection pressures should equally affect males, we nevertheless found an opposite pattern. In other words, we found that males were most aggressive toward model intruders when the pair had first formed and, then, reduced their level of investment in aggressive defense as the breeding cycle progressed. A commonly observed behavior in many socially monogamous species is mate guarding (Komdeur et al., 1999; Saino et al., 1999; Chuang-Dobbs et al., 2001), where males actively guard females from sexual rivals. We consider the possibility that male A. labiatus may be mate-guarding females from conspecific competitors early in the breeding cycle. However, in Amphilophus cichlids, competition for breeding territories can be intense (both in the wild and introduced ranges), and success in aggressive territory defense against conspecifics and heterospecifics is critical for brood survival (McKaye & Barlow, 1976; McKaye, 1977; Rogers, 1987, 1988). Hence, male aggression toward territorial intruders, independent of his motivations, should contribute toward territory defense and, hence, survival of the offspring. Another commonly observed behavior in monogamous taxa is the desertion of mates and offspring by males (Keenleyside, 1983; Keenleyside, 1991; Amat et al., 2000). In many Amphilophus and related cichlid species, single females are frequently found occupying territories in the wild (Lehtonen et al., 2011), and, by the time the young become independent, they are commonly guarded by only one parent (presumably the female) (Barlow, 1976), suggesting that mate desertion is widespread in this group. Field studies conducted on other Neotropical cichlids have shown that if males abandon their brood prematurely, they usually do it only after offspring have become free-swimming (Wisenden, 1994; Jennions & Polakow, 2001; Vélez et al., 2002). Desertion may also be more common in areas of high brood success and low predation levels (Townshend & Wootton, 1985), which may allow young to survive with only one parent (Wisenden, 1994). In our study, we observed that the territorial defense of males was at its lowest during this free-swimming stage, suggesting that some males may be shifting their behavior toward self-maintenance and possibly preparing for additional reproductive opportunities (Jennions & Telford, 2002).
To conclude, the results of our experimental stimulus manipulations showed a significant change in parental investment in aggressive defense during the progression of the breeding cycle in a feral population of the red devil cichlid. In particular, by controlling the appearance of intruders and timing of their presentation, we found that, at key stages, territorial defense was not shared equally between the sexes. In this regard, it is possible that the sexes may be investing more in territorial defense when it is most profitable for them to do so. Together, our results show that parental behavior may not only vary between the sexes, but can also shift dramatically over the course of the breeding period.
References
Amat, J. A., G. H. Visser, A. Pérez-Hurtado & G. M. Arroyo, 2000. Brood desertion by female shorebirds: a test of the differential parental capacity hypothesis on Kentish plovers. Proceedings of the Royal Society London B 267: 2171–2176.
Barlow, G. W., 1976. The Midas Cichlid In Nicaragua. In Thorson, T. B. (ed.), Investigation of the Ichthyofauna of Nicaraguan Lakes. University of Nebraska-Lincoln, Lincoln.
Barlow, G. W., 1983. Do gold midas cichlid fish win fights because of their colour, or because they lack normal coloration? A logistic solution. Behavioral Ecology and Sociobiology 13: 197–204.
Barlow, G. W., 2000. The cichlid fishes: nature’s grand experiment in evolution. Perse us Publishing, Cambridge.
Barlow, G. W. & P. Siri, 1994. Polychromatic midas cichlids respond to dummy opponents: color, contrast and context. Behaviour 130: 77–112.
Baylis, J. R., 1981. The evolution of parental care in fishes, with reference to Darwin’s rule of male sexual selection. Environmental Biology of Fishes 6: 223–251.
Blumer, L. S., 1979. Male parental care in the bony fishes. The Quarterly Review of Biology 54: 149–161.
Budaev, S. V., D. D. Zworykin & A. D. Mochek, 1999. Individual differences in parental care and behaviour profile in the convict cichlid: a correlation study. Animal Behaviour 58: 195–202.
Carere, C. & E. Alleva, 1998. Sex differences in parental care in the common swift (Apus apus): effect of brood size and nestling age. Canadian Journal of Zoology 76: 1382–1387.
Chapman, T., G. Arnqvist, J. Bangham & L. Rowe, 2003. Sexual conflict. Trends in Ecology and Evolution 18: 41–47.
Chuang-Dobbs, H. C., M. S. Webster & R. T. Holmes, 2001. The effectiveness of mate guarding by male black-throated blue warblers. Behavioral Ecology 12: 541–546.
Clutton-Brock, T. H., 1991. The Evolution of Parental Care. Princeton University Press, Princeton.
Colombo, M., E. T. Diepeveen, M. Muschick, M. E. Santos, A. Indermaur, N. Boileau, M. Barluenga & W. Salzburger, 2013. The ecological and genetic basis of convergent thick-lipped phenotypes in cichlid fishes. Molecular Ecology 22: 670–684.
Elmer, K. R., T. K. Lehtonen & A. Meyer, 2009. Color assortative mating contributes to sympatric divergence of neotropical cichlid fish. Evolution 63: 2750–2757.
Elmer, K. R., H. Kusche, T. K. Lehtonen & A. Meyer, 2010. Local variation and parallel evolution: morphological and genetic diversity across a species complex of neotropical crater lake cichlid fishes. Proceedings of the Royal Society London B 365: 1763–1782.
Ercit, K., A. Martinez-Novoa & D. T. Gwynne, 2014. Egg load decreases mobility and increases predation risk in female black-horned tree crickets (Oecanthus nigricornis). PLoS One 9: e110298.
Goodwin, N. B., S. Balshine-Earn & J. D. Reynolds, 1998. Evolutionary transitions in parental care in cichlid fish. Proceedings of the Royal Society London B 265: 2265–2272.
Gross, M. R. & R. C. Sargent, 1985. The evolution of male and female parental care in fishes. American Zoologist 25: 807–822.
Haller, J., 1996. Biochemical background for an analysis of cost-benefit interrelations in aggression. Neuroscience & Biobehavioral Reviews 19: 599–604.
Hammerstein, P. & G. A. Parker, 1987. Sexual selection: games between the sexes. In Bradbury, J. W. & M. B. Andersson (eds), Sexual selection: testing the alternatives. Wiley, Chichester: 119–142.
Harrison, F., Z. Barta, I. Cuthill & T. Székely, 2009. How is sexual conflict over parental care resolved? A meta-analysis. Journal of Evolutionary Biology 22: 1800–1812.
Holder, J. L., G. W. Barlow & R. C. Francis, 1991. Differences in aggressiveness in the Midas cichlid (Cichlasoma citrinellum) in relation to sex, reproductive state and the individual. Ethology 88: 297–306.
Houston, A. I., T. Székely & J. M. McNamara, 2005. Conflict between parents over care. Trends in Ecology and Evolution 20: 33–38.
Itzkowitz, M., 1984. Parental division of labor in a monogamous fish. Behaviour 89: 251–260.
Itzkowitz, M., 1985. Sexual differences in offspring defense in a monogamous cichlid fish. Zeitschrift für Tierpsychologie 70: 247–255.
Itzkowitz, M., N. Santangelo & M. Richter, 2001. Parental division of labour and the shift from minimal to maximal role specializations: an examination using a biparental fish. Animal Behaviour 61: 1237–1245.
Itzkowitz, M., N. Santangelo, A. Cleveland, A. Bockelman & M. Richter, 2005. Is the selection of sex-typical parental roles based on an assessment process? A test in the monogamous convict cichlid fish. Animal Behaviour 69: 95–105.
Jaroensutasinee, M. & K. Jaroensutasinee, 2003. Type of intruder and reproductive phase influence male territorial defence in wild-caught Siamese fighting fish. Behavioural Processes 64: 23–29.
Jennions, M. D. & D. A. Polakow, 2001. The effect of partial brood loss on male desertion in a cichlid fish: an experimental test. Behavioral Ecology 12: 84–92.
Jennions, M. D. & S. Telford, 2002. Life-history phenotypes in populations of Brachyrhaphis episcopi (Poeciliidae) with different predator communities. Oecologia 132: 44–50.
Keenleyside, M. H., 1983. Mate desertion in relation to adult sex ratio in the biparental cichlid fish (Herotilapia multispinosa). Animal Behaviour 31: 683–688.
Keenleyside, M. H. (ed.), 1991. Cichlid Fishes: Behaviour, Ecology and Evolution, 2nd ed. Springer, Netherlands.
Keenleyside, M. H., R. C. Bailey & V. H. Young, 1990. Variations in the mating system and associated parental behaviour of captive and free-living Cichlasoma nigrofasciatum (Pisces, Cichlidae). Behaviour 112: 202–221.
Komdeur, J., F. Kraaijeveld-Smit, K. Kraaijeveld & P. Edelaar, 1999. Explicit experimental evidence for the role of mate guarding in minimizing loss of paternity in the Seychelles warbler. Proceedings of the Royal Society London B 266: 2075.
Lappin, A. K. & J. F. Husak, 2005. Weapon performance, not size, determines mating success and potential reproductive output in the collared lizard (Crotaphytus collaris). The American Naturalist 166: 426–436.
Lehtonen, T. K., 2014. Colour biases in territorial aggression in a Neotropical cichlid fish. Oecologia 175: 85–93.
Lehtonen, T. K., B. B. M. Wong, K. Lindström & A. Meyer, 2011. Species divergence and seasonal succession in rates of mate desertion in closely related Neotropical cichlid fishes. Behavioral Ecology and Sociobiology 65: 607–612.
Lehtonen, T. K., J. K. McCrary & A. Meyer, 2012. Introduced predator elicits deficient brood defence behaviour in a crater lake fish. PLoS One 7: e30064.
Lehtonen, T. K., W. Sowersby, K. Gagnon & B. B. M. Wong, 2015a. Cichlid fish use color as a cue to assess the threat status of heterospecific intruders. The American Naturalist 186: 547–552.
Lehtonen, T. K., W. Sowersby & B. B. M. Wong, 2015b. Heterospecific aggression bias towards a rarer colour morph. Proceedings of the Royal Society London B 282: 20151551.
Lessells, K. 1999. Sexual conflict. Wiley Online Library, eLS.
Marler, C. & M. Moore, 1988. Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behavioral Ecology and Sociobiology 23: 21–26.
Meek, S. E. 1907. Synopsis of the fishes of the great lakes of Nicaragua. Field Columbian Museum, 121, Zoological Series 7: 97–132
McKaye, K. R., 1977. Competition for breeding sites between the cichlid fishes of Lake Jiloa, Nicaragua. Ecology 58: 291–302.
McKaye, K. R., 1986. Mate choice and size assortative pairing by the cichlid fishes of Lake Jiloá, Nicaragua. Journal of Fish Biology 29: s135–s150.
McKaye, K. R. & G. W. Barlow, 1976. Competition between color morphs of the Midas cichlid, Cichlasoma citrinellum, in Lake Jiloa, Nicaragua. In Thorson, T. B. (ed.), Investigation of the Ichthyofauna of Nicaraguan Lakes. University of Nebraska-Lincoln, Lincoln.
McKaye, K. R. & B. A. Murry, 2008. Sex role differentiation in brood defense by a Nicaraguan cichlid fish, Amphilophus xiloanensis. Caribbean Journal of Science 44: 13–20.
McNamara, J. M., A. I. Houston, T. Székely & J. N. Webb, 2002. Do parents make independent decisions about desertion? Animal Behaviour 64: 147–149.
Murry, B. A., E. P. Van Den Berghe & K. R. McKaye, 2001. Brood defense behavior of three sibling species in the Amphilophus citrinellus species complex in Lake Xiloá, Nicaragua. Journal of Aquariculture and Aquatic Science 9: 134–149.
Newton, I., 1979. Population Ecology of Raptors. T and AD Poyser, Berkhamsted.
NIWA, 2008. Review of the impacts of introduced ornamental fish species that have established wild populations in Australia. Department of the Environment, Water, Heritage and the Arts, Canberra
Oldfield, R., K. Mandrekar, M. X. Nieves, D. A. Hendrickson, P. Chakrabarty, B. O. Swanson & H. A. Hofmann, 2015. Parental care in the Cuatro Ciénegas cichlid, Herichthys minckleyi (Teleostei: Cichlidae). Hydrobiologia 48: 233–257.
Perrone Jr., M. & T. M. Zaret, 1979. Parental care patterns of fishes. The American Naturalist 113: 351–361.
Pinheiro, J. C. & D. M. Bates, 2000. Mixed-Effects Models in S and S-PLUS, Statistics and Computing Series. Springer, New York.
Pryke, S. R., 2007. Fiery red heads: female dominance among head color morphs in the Gouldian finch. Behavioral Ecology 18: 621–627.
Redondo, T. & J. Carranza, 1989. Offspring reproductive value and nest defense in the magpie (Pica pica). Behavioral Ecology and Sociobiology 25: 369–378.
Requena, G. S., B. A. Buzatto, E. G. Martins & G. Machado, 2012. Paternal care decreases foraging activity and body condition, but does not impose survival costs to caring males in a Neotropical arachnid. PLoS One 7: e46701.
Reynolds, J. D., 1996. Animal breeding systems. Trends in Ecology and Evolution 11: 68–72.
Ridley, M., 1978. Paternal care. Animal Behaviour 26: 904–932.
Rogers, W., 1987. Sex ratio, monogamy and breeding success in the Midas cichlid (Cichlasoma citrinellum). Behavioral Ecology Sociobiology 21: 47–51.
Rogers, W., 1988. Parental investment and division of labor in the Midas cichlid (Cichlasoma citrinellum). Ethology 79: 126–142.
Rowland W. J., 1999. Studying visual cues in fish behavior: A review of ethological techniques. Environmental Biology of Fishes 56: 285–305.
Rytkönen, S., M. Orell, K. Koivula & M. Soppela, 1995. Correlation between two components of parental investment: nest defence intensity and nestling provisioning effort of willow tits. Oecologia 104: 386–393.
Saino, N., C. R. Primmer, H. Ellegren & A. P. Møller, 1999. Breeding synchrony and paternity in the barn swallow (Hirundo rustica). Behavioral Ecology and Sociobiology 45: 211–218.
Salfert, I. G. & G. E. E. Moodie, 1985. Filial egg-cannibalism in the brook stickleback, Culaea inconstans (Kirtland). Behaviour 93: 82–100.
Sinervo, B., D. B. Miles, W. A. Frankino, M. Klukowski & D. F. DeNardo, 2000. Testosterone, endurance, and Darwinian fitness: natural and sexual selection on the physiological bases of alternative male behaviors in side-blotched lizards. Hormones and Behavior 38: 222–233.
Schipper, W. J. A., 1973. A Comparison of Prey Selection in Sympatric Harriers, Circus, in Western Europe. Institut Royal des Sciences naturelles de Belgique, Brussels.
Székely, T. & I. C. Cuthill, 1999. Brood desertion in Kentish plover: the value of parental care. Behavioral Ecology 10: 191–197.
Székely, T. & I. C. Cuthill, 2000. Trade-off between mating opportunities and parental care: brood desertion by female Kentish plovers. Proceedings of the Royal Society London B 267: 2087–2092.
Townshend, T. J. & R. J. Wootton, 1985. Variation in the mating system of a biparental cichlid fish, Cichlasoma panamense. Behaviour 95: 181–197.
Trivers, R., 1972. Parental investment and sexual selection. In Campbell, B. (ed.), Sexual Selection and the Descent of Man 1871–1971. Aldine Publication, Chicago.
Trnka, A. & T. Grim, 2012. To compensate or not to compensate: testing the negotiation model in the context of nest defense. Behavioral Ecology 24: 223–228.
Tryjanowski, P. & A. Goławski, 2004. Sex differences in nest defence by the red-backed shrike Lanius collurio: effects of offspring age, brood size, and stage of breeding season. Journal of Ethology 22: 13–16.
Vélez, M. J., M. D. Jennions & S. R. Telford, 2002. The effect of an experimental brood reduction on male desertion in the Panamanian blue acara cichlid Aequidens coeruleopunctatus. Ethology 108: 331–340.
Watts, S. H., 2014. A study of nesting sparrowhawks Accipiter nisus using video analysis. Bird Study 61: 428–437.
Wisenden, B. D., 1994. Factors affecting mate desertion by males in free-ranging convict cichlids (Cichlasoma nigrofasciatum). Behavioral Ecology 5: 439–447.
Wisenden, B. D., J. L. Snekser, A. D. Stumbo & J. M. Leese, 2008. Parental defence of an empty nest after catastrophic brood loss. Animal Behaviour 76: 2059–2067.
Wynne-Edwards, K. E., 1995. Biparental care in Djungarian but not Siberian dwarf hamsters (Phodopus). Animal Behaviour 50: 1571–1585.
Acknowledgments
We thank Fiona Kang, Rowan Jacques-Hamilton, Nicholas Deal, Rachel Fetherston, Eeling Ng, Ruby Albury, Andrej Hohmann and Matthew Simpson for logistic support and animal collection. We also thank members of the Wong and Chapple Labs at Monash University, who assisted with animal husbandry. Lastly, we thank Professor Sefc and two anonymous reviewers for their helpful suggestions to improve the manuscript. Funding was provided by the Holsworth Wildlife Endowment Fund, and the Linnean Society of New South Wales.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving in Human and animal rights
All applicable national and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Animal Ethics Committee of Monash University, Australia, BSCI/2012/23).
Additional information
Guest editors: S. Koblmüller, R. C. Albertson, M. J. Genner, K. M. Sefc & T. Takahashi / Advances in Cichlid Research II: Behavior, Ecology and Evolutionary Biology
Rights and permissions
About this article
Cite this article
Sowersby, W., Lehtonen, T.K. & Wong, B.B.M. Temporal and sex-specific patterns of breeding territory defense in a color-polymorphic cichlid fish. Hydrobiologia 791, 237–245 (2017). https://doi.org/10.1007/s10750-016-2889-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2889-1