Abstract
The decomposition of leaf litter of terrestrial origin is a fundamental process in aquatic ecosystems in forest contexts. Little is known about what drives leaf litter decomposition in oceanic islands. We examined the relative importance of leaf litter identity (Acacia melanoxylon, Pittosporum undulatum, Morella faya) and environmental conditions on litter decomposition in seven lakes in the oceanic archipelago of Azores for 28 and 56 days. Leaf litter was incubated in coarse and fine mesh bags for the assessment of the relative contribution of macroinvertebrates to leaf litter decomposition. Leaf litter mass loss generally did not differ between mesh sizes, suggesting that in these lakes macroinvertebrates generally have a negligible role on leaf decomposition. Leaf litter decomposition was in the order M. faya < A. melanoxylon < P. undulatum. A negative correlation was found between leaf litter mass loss and lignin concentration. Mass loss of P. undulatum was related to lake elevation and chlorophyll a (taken as surrogates for water temperature and dissolved nutrient availability, respectively), whereas mass loss of M. faya was related to chlorophyll a on day 56. These results suggest that changes in the composition of the leaf litter input and environmental conditions can affect leaf litter decomposition in Azorean lakes, with potential consequences for nutrient cycling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant litter of terrestrial origin has long been recognized as an important source of carbon and energy for aquatic communities in small forest streams, where shading by the riparian vegetation limits instream primary production (Petersen & Cummins, 1974; Wallace et al., 1997). Terrestrial derived plant litter can also be an important resource in the littoral zone of lakes, where the proximity to the riparian vegetation guarantees the input of plant litter to the system (Gasith & Hasler, 1976; France & Peters, 1995). Once in the water, plant litter is mineralized, converted into fine particulate and dissolved organic matter, and incorporated into secondary production, mostly by the activities of microbes and shredder macroinvertebrates (Webster & Benfield, 1986; Gessner et al., 1999). Litter decomposition is thus a fundamental ecosystem process by which dead organic matter is mineralized.
Litter decomposition in aquatic systems is controlled mainly by three factors: intrinsic litter characteristics, environmental conditions, and presence of shredders. Litter with high nutrient concentrations and low concentrations of structural and secondary compounds are generally colonized and decomposed faster than more recalcitrant litter, where biological colonization may be retarded by physical (e.g., high toughness, thick cuticle) and chemical barriers (e.g., essential oils, polyphenols) and microbial activity may be nutrient limited (Ostrofsky, 1997; Canhoto & Graça, 1999; Schindler & Gessner, 2009; Ferreira et al., 2012; Frainer et al., 2015). Environmental conditions can also shape biological colonization and activity on litter and thus control litter decomposition (Webster & Benfield, 1986; Young et al., 2008). Nutrient enrichment is among the most widespread human induced changes affecting freshwater systems and has been widely shown to affect microbial activities and litter decomposition (Woodward et al., 2012; Ferreira et al., 2014b, 2015). Shredders generally accelerate litter decomposition at later stages of leaf decomposition by feeding on microbially conditioned litter (Hieber & Gessner, 2002; Cornut et al., 2010). Thus, the relative importance of litter characteristics, environmental conditions, and presence of shredders on litter decomposition may change over the decomposition process (García-Palacios et al., 2016). To better protect and manage lake ecosystems, it is thus key that we understand the web of interacting forces that supports their functioning.
Lakes are generally under great human pressure, with alteration of riparian forests and eutrophication being among the most pervasive anthropogenic induced changes affecting aquatic communities and processes (Søndergaard & Jeppesen, 2007 and reference therein). These effects may be especially strong in remote islands where human colonization has resulted in dramatic changes in land use (Whittaker & Fernández-Palacios, 2007). In general, native forests were first mainly explored as a source of wood for construction and charcoal production, while at present, they are being replaced by commercial plantations, agriculture and pasture, and invaded by exotic species (Porteiro, 2000). Also, in islands, there is generally a paucity of large shredder macroinvertebrates (Smith et al., 2003; Raposeiro et al., 2012, 2013) as a result from freshwater communities being shaped by biogeographical filters that operate over a range of spatial scales that influence dispersal, colonization, and evolution events (Bilton et al., 2001; Covich 2009).
Here, we investigated the relative importance of macroinvertebrate presence, leaf litter identity and environmental conditions on the decomposition of leaf litter after 28 and 56 days in seven Azorean lakes distributed across a wide gradient of environmental and anthropogenic conditions, ranging from oligotrophic lakes to lakes heavily impacted by human activities. We anticipated (i) low contribution of macroinvertebrates to litter decomposition due to the paucity of shredders (Ferreira et al., 2016), (ii) differences in litter decomposition among species as a result from differences in physical and chemical leaf litter characteristics (Schindler & Gessner, 2009), (iii) differences in litter decomposition among lakes as a result from differences in environmental conditions, especially water temperature and dissolved nutrient concentrations (Ferreira et al., 2015), and (iv) changes in the relative importance of litter identity and environmental conditions on litter decomposition between sampling dates (García-Palacios et al., 2016).
Methods
Study region
The leaf litter decomposition experiment was done in São Miguel island (Azores archipelago, Portugal), an oceanic island located in the middle of the North Atlantic, about 1500 km off Portugal mainland (Fig. 1a). The Azores climate can be considered temperate oceanic, i.e., strongly influenced by topography and the ocean. The climate of the archipelago is characterized by high levels of air humidity, thermal amenity, low rates of insulation, regular and abundant rainfalls, and by strong winds (Climate Atlas, 2011).
The native Azorean forest (i.e., laurel forest) is mainly composed of evergreen trees and shrubs and is dominated by endemic species such as Laurus azorica ((Seub.) Franco), Ilex perado Aiton subsp. azorica (Loes.), and Morella faya ((Aiton) Wilbur) (Elias & Dias, 2009). After human settlement, beginning in the fifteenth century, other types of vegetation cover have become progressively dominant. In fact, as of today, large areas of native forest have been replaced by commercial conifer plantations (Cryptomeria japonica (L.f. D. Don)) or invaded by the exotic evergreen Pittosporum undulatum (Vent.) and Acacia melanoxylon (R. Brown), especially at 200–800 m above sea level (Lourenço et al., 2011).
Study lakes and macroinvertebrate community
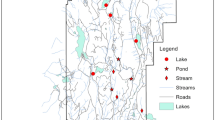
The Azores are particularly rich in lentic habitats, with 88 lakes distributed throughout São Miguel, Terceira, Pico, Flores, and Corvo islands (Porteiro, 2000) (Fig. 1b). For this study, seven lakes were selected in São Miguel island: Rasa, Fogo, Azul, Canário, Verde, Furnas, and São Brás (Fig. 1c). Since 1994, these lakes have been part of a regional biomonitoring program and have different trophic states, ranging from oligotrophic to eutrophic (Gonçalves et al., 2013a; Table 1).
Important changes in catchment land use have occurred in São Miguel island during the last centuries. After human settlement, the main disturbance was deforestation for construction and charcoal production. Later, commercial conifer plantations were explored for the extraction of wood for orange exportation and vegetal material to use in greenhouse beds for pineapple production. The native vegetation in accessible areas at low and middle elevation was also cleared to make way for agriculture and urbanization. Thus, lakes at higher elevation (Rasa, Fogo, and Canário lakes) tend to be surrounded by native forest (e.g., M. faya), or wood production forest (C. japonica) or exotic forest (P. undulatum and A. melanoxylon), while catchments located at lower elevation have increased levels of agriculture, exotic forest (São Brás and Furnas lakes), wood production forest, and urbanization (Verde and Azul lakes). Environmental characterization of the seven lakes is presented in Table 1; water variables (collected in the middle of the lake) for July were compiled from the data collected as part of the European Water Framework Directive—Regional Monitoring Programme (sampled between 2003 and 2012).
The macroinvertebrate community in Azorean lakes is characterized by low taxa richness, which is typical of oceanic islands (Raposeiro et al., 2011, 2012, 2013). Chironomidae, Oligochaeta, Gastropoda, and Odonata are the dominant macroinvertebrate fauna in the study lakes (Gonçalves et al., 2009, 2013a).
Leaf litter
Mature leaves of A. melanoxylon, P. undulatum, and M. faya were collected directly from trees on March 2013 and air dried at room temperature until used. A. melanoxylon and P. undulatum were selected due to their strong presence in the catchment of Azorean lakes, especially at low to mid elevation. In fact, 49% of the forested area is occupied by the P. undulatum, followed by A. melanoxylon (Lourenço et al., 2011). The native M. faya was selected because it is naturally present in the catchment of the studied lakes. Leaves were collected directly from the trees since these are evergreen species and thus waiting for leaves to naturally fall would take too long and could lead to differences in litter characteristics between leaves collected first and last. Nevertheless, using air-dried green leaves is ecologically relevant since the strong winds typical of the region can cause premature litter fall and leaves can then enter the lakes by lateral transport.
Also, these species provide a gradient of toughness, lignin, nitrogen (N), and polyphenols concentrations (Ferreira et al., 2016; Table 2) and thus allow to examine whether these characteristics are important in determining litter decomposition in Azorean lakes. Among the three leaf species, A. melanoxylon was the toughest, had the highest concentration of lignin and N and the lowest concentration of polyphenols. The leaves of P. undulatum had the highest concentration of polyphenols and the lowest concentration of lignin, while the leaves of M. faya were the softest (Ferreira et al., 2016; Table 2).
Leaf litter decomposition
For each species, air-dried leaves were weighed (2.98–3.02 g), sprayed with distilled water to render them soft and less susceptible to break due to handling, and enclosed in experimental bags (10 × 12 cm) of two mesh sizes. The fine mesh (FM; 0.5 mm mesh) excludes most macroinvertebrates and thus litter decomposition is driven by the activities of microbes, whereas the coarse mesh (CM; 5 mm mesh) allows the access of macroinvertebrates to leaf litter and thus litter decomposition is driven by the joint activities of microbes and macroinvertebrates.
For the determination of leaf litter decomposition, six litter bags of each mesh size and leaf species were deployed in each of the seven lakes on 3 June 2013 (252 bags in total), at 1 m depth in the littoral zone where macrophytes were present. After 28 and 56 days, three randomly chosen bags from each leaf species and mesh size were collected from each lake (126 bags per date). In the laboratory, bag contents were gently rinsed with distilled water into a 0.5-mm mesh sieve to retain small leaf fragments. The remaining leaf litter was oven dried (70°C, 48 h) and weighed (0.1 mg precision) for determination of dry mass (DM) remaining. The proportion of DM remaining was given by the ratio between DM remaining and initial DM, and the proportion of DM loss was estimated as one minus the proportion of DM remaining.
Initial DM was estimated by multiplying the initial air-dried mass by a conversion factor derived from extra sets of six mesh bags for each mesh size and leaf species. These extra bags were taken to the field on day 0, immersed in water for approximately 10 min, and taken back to the laboratory for determination of DM, as described above. The initial air-dried mass to initial DM conversion factor, given by the ratio between initial DM and initial air-dried mass, were 0.92 for P. undulatum (both mesh sizes), 0.96 for A. melanoxylon (both mesh sizes), and 0.94 for M. faya (both mesh sizes).
Leaf litter macroinvertebrates
In addition to the above, another set of six litter bags of coarse mesh per leaf species was concomitantly deployed in each lake (126 bags in total) to determine the macroinvertebrate assemblage associated with the decomposing litter. As above, three randomly chosen bags from each leaf species were collected from each lake after 28 and 56 days (63 bags per date). In the laboratory, bag contents were gently rinsed with distilled water into a 0.5-mm mesh sieve to retain macroinvertebrates. Macroinvertebrates associated with decomposing litter were saved and preserved in 70% ethanol until identified and counted under a stereo microscope (Zeiss Stemi, Göttingen, Deutschland). Identification was made to the lowest possible taxonomic level (Tachet et al., 2006; Oscoz et al., 2011). Abundance was expressed as number of individuals per bag (no. ind bag−1) and taxa richness was expressed as number of taxa per bag (no. taxa bag−1).
Data analyses
Data analyses were done separately for days 28 and 56 as we anticipated that the responses of litter mass loss and macroinvertebrate colonization to leaf species and lakes would differ between litter decomposition stages. To test the hypothesis that leaf litter mass loss differs among mesh sizes, lakes, and leaf species, we used a three-way ANOVA with mesh size, lake, and leaf species as factors (all fixed and orthogonal), followed by Tukey’s honest significant difference (HSD) test when significant effects were detected in the ANOVAs. Total macroinvertebrate abundance and taxa richness associated with leaf litter were compared among treatments by two-way ANOVA (lake and leaf species as categorical variables), followed by Tukey’s HSD test when significant effects were detected in the ANOVAs. Homogeneity of variances (checked with Cochran’s test) and normality (checked with Shapiro–Wilk’s test) assumptions were met, and no data transformation was required prior to analysis. Data analyses were performed on STATISTICA 11 software (StatSoft Inc., Tulsa, OK, USA).
Macroinvertebrate communities were compared among litter species and lakes by permutational multivariate analysis of variance (PERMANOVA) based on Bray–Curtis similarity matrix (Anderson, 2001; McArdle & Anderson, 2001). As PERMANOVA is sensitive to differences in dispersion, PERMDISP was performed to test for homogeneity of multivariate dispersion across groups (Anderson et al., 2008).
Distance-based analyses (DistLM) were first performed estimating fine and coarse bags separately (data not show). However, since the main results were similar and leaf litter mass loss generally (39 out of 42 cases) did not differ between mesh sizes, distance-based analyses (DistLM) were estimated by pooling CM and FM bags together, to investigate (i) the influence of spatial variation in environmental variables among lakes on leaf mass loss and macroinvertebrate assemblage structure and (ii) the influence of the physical and chemical characteristics of leaf species on leaf mass loss. To avoid multicollinearity, a non-redundant subset of variables from an initial list of ten possible environmental predictor variables (Table 1) and six physical and chemical leaf predictor variables (Table 2) were selected using Pearson correlation coefficients. If two or more variables were correlated (threshold value of r > |0.4| and P < 0.001), they were excluded from analyses (Tables S1 and S2). Selected environmental predictor variables were elevation and chlorophyll a (chl a) concentration, while the selected physical and chemical leaf predictor variables were lignin, carbon (C), and phosphorous (P) concentration, and penetrance. Prior to analyses, predictor variables were normalized according to Clarke & Warwick (2001). DistLM routine allows predictor variables to be fitted individually or together in specified sets (Legendre & Anderson, 1999; McArdle & Anderson, 2001). The routine was based on R 2 model selection criteria using “all specified” procedure and was performed using PRIMER 6 v6.1.11 & PERMANOVA + v1.0.1 (Primer-E Ltd, Plymouth, UK). For visual interpretation of the resulting model in multi-dimensional space, we used distance-based redundancy analysis (dbRDA) to investigate the relationship between physical and chemical variables (Anderson et al., 2008). Each vector begins at the center of the circle (the origin) and ends at the coordinates (x and y). The length and direction of each vector indicates the strength and sign of the relationship between the given variable and the dbRDA axes.
Results
Leaf litter decomposition
After 28 days of deployment, leaf litter mass loss varied between 25 and 45% for P. undulatum, 13 and 21% for M. faya, and 13 and 26% for A. melanoxylon (across lakes and mesh sizes; Fig. 2). After 56 days, leaf litter mass loss varied between 40 and 74% for P. undulatum, 20 and 30% for M. faya, and 20 and 38% for A. melanoxylon (across lakes and mesh sizes; Fig. 2).
Proportional dry mass loss of the three leaf species incubated in coarse and fine mesh bags in seven Azorean lakes for 28 and 56 days. Values are mean ± SE. Proportional dry mass loss of the three leaf species were compared among treatments by three-way ANOVA; treatments with the same letter do not significantly differ (Turkey’s HSD test, P > 0.050). Lakes are ranked by trophic state, from oligotrophic to eutrophic (Gonçalves et al., 2013a)
Analysis of the leaf litter mass loss showed a significant interaction between mesh size, leaf species, and lake (Table 3). Inspection of post hoc tests (Tukey’s test; Fig. 2) showed that (i) there were significant differences (3 out of 42 cases) in mass loss between mesh sizes only on day 56 and only for A. melanoxylon in São Brás and Fogo lakes and for P. undulatum in Verde lake, with mass loss being faster in coarse than in fine mesh bags (Fig. 2), (ii) mass loss was generally significantly greater in P. undulatum compared to the other two species, among which mass loss was generally similar, and this was consistent among lakes and mesh sizes on both days 28 and 56 (Fig. 2), and (iii) there was significant spatial (among lakes) variation in mass loss in coarse mesh bags for P. undulatum and A. melanoxylon but not for M. faya, while in fine mesh bags there was significant spatial variation in mass loss only for P. undulatum, and this was consistent on days 28 and 56 (Fig. 2).
Analyses of the spatial variation on leaf litter mass loss in relation to lake’s environmental conditions showed a significant and negative correlation between P. undulatum leaf litter mass loss and elevation and a positive correlation between mass loss and chl a on both days 28 and 56 (Table 4). In contrast, no significant correlation was found between the tested environmental variables and A. melanoxylon leaf litter mass loss on either day 28 or 56 (Table 4). For M. faya, there was no significant correlation between leaf litter mass loss and the tested variables after 28 days, while a significant and positive correlation between leaf litter mass loss and chl a was found after 56 days (Table 4).
Analysis of the influence of initial leaf lignin, C and P concentration and penetrance on leaf litter mass loss showed a significant and negative correlation between mass loss and initial lignin and C concentration and a positive correlation between mass loss and P concentration on days 28 and 56 (Table 4). No significant correlation was found between mass loss and penetrance (Table 4).
Leaf litter macroinvertebrates
Macroinvertebrate abundance increased with time (182 ind bag−1 on day 28 and 263 ind bag−1 on day 56, mean across leaf species and lakes; Fig. 3). Values for Verde and São Brás lakes were particularly high with 604–773, 641–727 and 136–839 ind bag−1 for A. melanoxylon, M. faya, and P. undulatum, respectively, after 56 days (Fig. 3). Macroinvertebrate taxa richness varied between 3 and 14 for A. melanoxylon and M. faya and 4–13 for P. undulatum over the incubation period, with a total of 30 taxa being recorded in the entire experiment (Table S5). Taxa richness tended to decrease from day 28 (7.8 bag−1, mean across leaf species and lakes) to day 56 (6.0 bag−1) (Fig. 3).
Total macroinvertebrate abundance and taxa richness associated with three leaf litter species incubated in coarse mesh bags in seven Azorean lakes for 28 and 56 days. Values are means ± 1SE. Total macroinvertebrate abundance and taxa richness were compared among treatments by two-way ANOVA; treatments with the same letter do not significantly differ (Turkey’s HSD test, P > 0.050). Lakes are ranked by trophic state, from oligotrophic to eutrophic (Gonçalves et al., 2013a)
Macroinvertebrate abundance and taxa richness did not significantly differ among leaf species on days 28 and 56, while it significantly differed among lakes (Table 3). There was a significant interaction between leaf species and lake for macroinvertebrate abundance on day 56 indicating that differences among lakes may depend on litter species (Table 3).
Macroinvertebrate assemblages associated with leaf litter were numerically dominated by midges (average relative abundance: 39% Chironomini, 19% Orthocladiinae, 8% Tanytarsini), followed by Naididae (19%) (Table S3). No shredders were found associated with leaf litter during the study period (Table S3). Macroinvertebrate assemblage structure varied significantly among lakes but not among leaf species after 28 days, while a significant interaction between lake and species was found after 56 days (Fig. 4; Tables 5, S4). The two dbRDA axes explained 44.5 and 62.2% of the relationship between the macroinvertebrate assemblages and the environmental variables, and 58.4 and 70.3% of the total variability in the community data, for days 28 and 56, respectively (Fig. 4). Analyses of the spatial variation in the structure of macroinvertebrate assemblages in relation to lake’s environmental conditions showed a significant correlation with elevation and chl a, which was consistent on both days 28 and 56 (Table 4; Fig. 4).
Discussion
The role of macroinvertebrates on leaf litter decomposition on insular lentic ecosystems
Our results showed that leaf litter decomposition was generally similar (39 out of 42 cases) between fine mesh and coarse mesh bags, suggesting that macroinvertebrates have little influence on leaf litter decomposition in Azorean lakes. Shredders generally colonize decomposing litter when it is already fully conditioned by microbes (Hieber & Gessner, 2002; Cornut et al., 2010), and since litter mass loss did not reach 50% for M. faya (≤30%) and A. melanoxylon (≤38%), this could justify the lack of shredders. However, P. undulatum mass loss attained >50% in several lakes, but shredders were also not recorded. This lack of shredders associated with decomposing litter together with previous reports of the paucity of shredders in Azorean lakes (Gonçalves et al., 2009, 2013a) contribute to sustain our suggestion that macroinvertebrates have little influence on litter decomposition in these systems. Other studies addressing litter decomposition in lakes have found similar decomposition rates between litter exposed to and protected from macroinvertebrates (Ágoston-Szabó & Dinka, 2008; Bassett et al., 2010). Such similar decomposition rates found regardless of the presence or absence of macroinvertebrates might result from compensatory mechanisms driving leaf decomposition in the absence of macroinvertebrates. For instance, Sabetta et al. (2000) found similar litter decomposition rates between fine and coarse mesh bags in a volcanic lake in central Italy, but there were significant positive correlations between decomposition rates in coarse mesh bags and detritivore biomass and between decomposition rates in fine mesh bags and fungi species richness, suggesting that while detritivores may promote litter decomposition, fungi can compensate for their absence. We believe that in our case the lack of differences in decomposition rates between the two mesh sizes are most likely due to the overall paucity of large detritivores, i.e., shedders, in Azorean aquatic systems. In line with this argument, van Dokkum et al. (2000) found faster litter decomposition rates in coarse mesh than in fine mesh bags in a Dutch lake where gammarid shredders were abundant, and Tuchman (1993) found faster decomposition rates for litter exposed than protected from invertebrates in alkaline lakes where shredders comprised 65% of total invertebrates but not in acidic lakes where shredders were rare. Our findings are also in line with those reported by Raposeiro et al. (2014) and Ferreira et al. (2016) for Azorean lotic systems, reinforcing the idea that in oceanic islands, such as the Azores, macroinvertebrates generally have little influence on the decomposition of leaf litter in freshwater ecosystems.
This lack of influence of the macroinvertebrate assemblage on leaf litter decomposition likely results from the overall depauperate diversity of macroinvertebrate communities found in oceanic islands (Larned et al., 2003; Benstead et al., 2009), which, in turn, likely results from macroecological filters such as the long distance to a source of colonization (i.e., mainland) and the relative young age and small area of the islands (Covich, 2009). Shredders, which are known to play an important role in leaf litter decomposition (Hieber & Gessner, 2002; Cornut et al., 2010), are generally absent from lakes in the Azorean islands (Gonçalves et al., 2013a) and were also not found during this study. The few cases where differences in leaf litter decomposition were found between mesh sizes are likely due to greater physical abrasion in coarse mesh bags, especially in Verde and São Brás lakes where the water level dropped between 0.5 and 0.8 m during the course of the experiment (personal observation).
Our results also showed no particular association between macroinvertebrate assemblages and any of the three leaf litter species, suggesting that macroinvertebrates were not attracted to any particular leaf species, even though the three leaf species differed in physical and chemical characteristics. The macroinvertebrate assemblages colonizing litter bags comprised mostly collectors/gatherers (Chironomidae and Oligochaeta). Collectors/gatherers may use the leaves as an indirect food resource, provider of fine particulate organic matter as a result from the microbial activity, or use it as a substrate and/or refugee from larger predators (e.g., fishes) (Pope et al., 1999) but appear to have little direct influence on leaf decomposition despite their high abundance, especially in Verde and São Brás lakes. Microbes are key players in leaf litter decomposition (Hieber & Gessner, 2002; Taylor & Chauvet, 2014) and may be of particular importance in aquatic systems that lack shredders, such as those found in the Azores (Ferreira et al., 2016). A better knowledge of the role and structure of microbial assemblages in Azorean lakes is thus essential for understanding the functioning of insular lentic ecosystems.
Effects of leaf species on leaf litter decomposition on insular lentic ecosystems
Litter mass loss was generally higher for P. undulatum than for A. melanoxylon and M. faya, among which there were generally no significant differences, for both sampling dates suggesting that the same litter characteristics likely determined litter mass loss on days 28 and 56. In fact, litter mass loss was strongly and negatively correlated with the initial litter lignin concentration on both days 28 and 56. Initial lignin concentration was positively correlated with initial N concentration, but since litter decomposition rates varied in the opposite direction of N concentration, differences in structural compounds concentration among leaf species were likely more important than differences in N concentration in controlling litter decomposition (Gessner & Chauvet, 1994; Schindler & Gessner, 2009; Frainer et al., 2015; Ferreira et al., 2016). A strong positive correlation was also found between litter mass loss and initial litter P concentration, which is often a limiting nutrient for biotic activity (Ferreira et al., 2012). However, P concentration in lake water was likely enough to sustain microbial activity (Rosemond et al., 2002; Gulis et al., 2006).
The stronger role of structural compounds relative to nutrient concentration in determining litter decomposition may have been a consequence of the already moderate to high nutrient availability in lake water. Microbes colonizing submerged litter are able to retrieve nutrients from both the organic substrates and the water (Suberkropp, 1995), but inorganic nutrients in the water are preferred as they are ready to use while the extraction of nutrients from the leaf matrix requires higher energy investment as extracellular enzymes need to be produced (Gulis & Suberkropp, 2003; Ferreira et al., 2006; Fernandes et al., 2014). Thus, microbes and consequently litter decomposition may be less sensitive to nutrient concentration in the organic substrate when dissolved nutrient availability is high (Gulis & Suberkropp, 2003; Gulis et al., 2006; but see Lima-Fernandes et al., 2014). In this case, the quality of the carbon may be more important than litter nutrient concentration, with high-quality carbon substrates being decomposed faster than substrates richer in structural compounds.
Effects of environmental conditions on leaf litter decomposition on insular lentic ecosystems
Differences in litter decomposition among lakes suggest that environmental conditions were also important in controlling leaf litter decomposition, especially for P. undulatum. In our study, P. undulatum litter decomposition was faster at lower than at higher elevation, likely a result of the stimulation of microbial activities at warmer temperature. Laboratory experiments have shown a stimulation of overall microbial metabolism (with C mineralization), fungal reproductive activity (with the release of reproductive propagules), and fungal biomass build-up on decomposing litter with increases in temperature, which lead to litter mass loss (Ferreira & Chauvet, 2011; Fernandes et al., 2014). However, temperature effects on microbial activities and litter decomposition may depend on litter identity (Gonçalves et al., 2013b; Ferreira et al., 2014a), which may help explaining the relative greater variation in leaf litter decomposition among lakes found for P. undulatum that was likely better conditioned than the other two species.
Litter decomposition of P. undulatum and M. faya was positively correlated with chl a concentration. Chlorophyll a concentration, which is commonly used to infer lake trophic state (Carlson, 1977) and nutrient enrichment (OCDE, 1982), was significantly and positively correlated with total P and total N concentrations. Dissolved inorganic nutrients, in particular, have been widely shown to stimulate microbial activities (Gulis & Suberkropp, 2003; Gulis et al., 2006; Ferreira & Chauvet, 2011) and consequently litter decomposition (Ferreira et al., 2015). Nutrient stimulation of litter decomposition, however, depends on litter identity with stronger effects for litter with lower nutrient concentration and where microbial activity is likely nutrient limited compared with litter with high nutrient concentration (Gulis & Suberkropp, 2003; Ferreira et al., 2006; Gulis et al., 2006). This also partially explains the relatively stronger effect of spatial variability on litter decomposition found for P. undulatum and M. faya (with the lowest N concentration) compared to A. melanoxylon (with the highest N concentration), especially for day 56, when microbial colonization is likely higher compared with day 28.
The likely reduced microbial colonization of A. melanoxylon leaf litter, as a result of its higher initial lignin concentration (see above), may have made it less sensitive to differences in dissolved nutrient concentrations and masked the effects of spatial variation in environmental variables on litter decomposition. Thus, the possibility that the effects of differences in dissolved nutrient availability would be also noted in A. melanoxylon litter decomposition at later stages of the decomposition process, when microbial biomass increases, cannot be ruled out.
In summary, leaf litter decomposition in Azorean lakes appears to be mainly driven by microbes and depends on leaf identity and environmental conditions (temperature and nutrient availability in particular). This suggests that changes in the composition of the riparian vegetation may affect nutrient cycling within aquatic food webs. This is a matter of concern in Azorean islands where large areas of the native laurel forest have been replaced by conifer plantations or invaded by exotic species. Changes in environmental conditions driven by human activities, as the increase in nutrient concentrations as a result from agriculture and pasture, may also affect nutrient cycling in Azorean lakes. Thus, mitigation measures are needed, which could include the maintenance of native riparian vegetation that would contribute with native litter to the littoral zone of lakes and act as a buffer limiting the effects of land use changes on lake communities and processes.
References
Ágoston-Szabó, E. & M. Dinka, 2008. Decomposition of Typha angustifolia and Phragmites australis in the littoral zone of a shallow lake. Biologia 63(6): 1104–1110.
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 35: 32–46.
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth.
APHA, 1995. Standard Methods for the Examination of Water and Wastewater, 19th ed. American Public Health Association, Washington DC.
Bassett, I. E., J. R. Beggs & Q. Paynter, 2010. Decomposition dynamics of invasive alligator weed compared with native sedges in a Northland lake. New Zealand Journal of Ecology 34(3): 324–331.
Benstead, J. P., J. G. March, C. M. Pringle, K. C. Ewel & J. W. Short, 2009. Biodiversity and ecosystem function in species-poor communities: community structure and leaf litter breakdown in a Pacific island stream. Journal of the North American Benthological Society 28(2): 454–465.
Bilton, D. T., J. R. Freeland & B. Okamura, 2001. Dispersal in freshwater invertebrates. Annual Review of Ecology and Systematics 32(1): 159–181.
Canhoto, C. & M. A. S. Graça, 1999. Leaf barriers to fungal colonization and shredders (Tipula lateralis) consumption of decomposing Eucalyptus globulus. Microbial Ecology 37: 163–172.
Carlson, R. E., 1977. A trophic stste index for lakes’. Limnology and Oceanography 22: 361–369.
Clarke, K. R. & R. N. Gorley, 2001. PRIMER v5: User Manual/Tutorial. Plymouth, UKPRIMER-E: 91.
Climate-Atlas, 2011. Climate atlas of the archipelagos of Canary islands, Madeira and the Azores-Air temperature and precipitation. Agência Estatal de Meterologia e Agência Estatal de Meterologia, Lisboa.
Cornut, J., A. Elger, D. Lambrigot, P. Marmonier & E. Chauvet, 2010. Early stages of leaf decomposition are mediated by aquatic fungi in the hyporheic zone of woodland streams. Freshwater Biology 55: 2541–2556.
Covich, A. P., 2009. Freshwater ecology. In Gillespie, R. G. & D. A. Clague (eds), Encyclopedia of Islands. University of California Press, Berkeley: 343–347.
Elias, R. & E. Dias, 2009. Gap dynamics and regeneration strategies in Juniperus-Laurus forests of the Azores Islands. Plant Ecology 200: 179–189.
Fernandes, I., S. Seena, C. Pascoal & F. Cássio, 2014. Elevated temperature may intensify the positive effects of nutrients on microbial decomposition in streams. Freshwater Biology 59: 2390–2399.
Ferreira, V. & E. Chauvet, 2011. Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Global Change Biology 17: 551–564.
Ferreira, V., V. Gulis & M. S. Graça, 2006. Whole-stream nitrate addition affects litter decomposition and associated fungi but not invertebrates. Oecologia 149: 718–729.
Ferreira, V., A. C. Encalada & M. A. S. Graça, 2012. Effects of litter diversity on decomposition and biological colonization of submerged litter in temperate and tropical streams. Freshwater Science 31: 945–962.
Ferreira, V., E. Chauvet & C. Canhoto, 2014a. Effects of experimental warming, litter species, and presence of macroinvertebrates on litter decomposition and associated decomposers in a temperate mountain stream. Canadian Journal of Fisheries and Aquatic Sciences 72: 206–216.
Ferreira, V., V. Gulis, C. Pascoal & M. A. S. Graça, 2014b. Chapter 18: Stream pollution and fungi. In Jones, G., K. Hyde & K.-L. Pang (eds), Freshwater Fungi and Fungus-like Organisms. De Gruyter Series: Marine and Freshwater Botany. De Gruyter, Berlin: 389–412.
Ferreira, V., B. Castagneyrol, J. Koricheva, V. Gulis, E. Chauvet & M. A. S. Graça, 2015. A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biological Reviews 90: 669–688.
Ferreira, V., P. M. Raposeiro, A. Pereira, A. Cruz, A. C. Costa, M. A. S. Graça & V. Gonçalves, 2016. Leaf litter decomposition in remote oceanic islands streams is driven by microbes and depends on litter quality and environmental conditions. Freshwater Biology 61(5): 783–799.
Frainer, A., J. Jabiol, M. O. Gessner, A. Bruder, E. Chauvet & B. G. McKie, 2015. Stoichiometric imbalances between detritus and detritivores are related to shifts in ecosystem functioning. Oikos. doi:10.1111/oik.02687.
France, R. L. & R. H. Peters, 1995. Predictive model of the effects on lke metabolism of decreased airborne litterfall through riparian deforestation. Conservation Biology 9: 1578–1586.
García-Palacios, P., E. A. Shaw, D. H. Wall & S. Hättenschwiler, 2016. Temporal dynamics of biotic and abiotic drivers of litter decomposition. Ecology Letters. doi:10.1111/ele.12590.
Gasith, A. & A. D. Hosier, 1976. Airborne litterfall as a source of organic matter in lakes1. Limnology and Oceanography 21: 253–258.
Gessner, M. O. & E. Chauvet, 1994. Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75: 1807–1817.
Gessner, M. O., E. Chauvet & M. Dobson, 1999. A perspective on leaf litter breakdown in streams. Oikos 85: 377–384.
Goering, H. K. & P. J. Van Soest, 1970. Forage fiber analysis (apparatus, reagents, procedures and some applications). Agricultural Handbook No. 379. ARS-USDA, Washington DC.
Gonçalves, V., A. Costa, P. Raposeiro, H. Marques, A. Cunha, J. Ramos, A. Cruz & C. Pereira, 2009. Caracterização biológica das massas de água interiores das Ilhas de São Miguel e Santa Maria. CCPA/Departamento de Biologia, Universidade dos Açores, Ponta Delgada.
Gonçalves, V., A. Costa, P. Raposeiro, H. Marques, A. Cunha, J. Ramos, A. Cruz, C. Pereira & J. Vilaverde, 2013a. Monitorização das massas de água interiores da Região Hidrográfica Açores. Relatório Anual de 2012 (R5/2012). CIBIO Açores, Departamento de Biologia, Universidade dos Açores, Ponta Delgada.
Gonçalves, A. L., M. A. S. Graça & C. Canhoto, 2013b. The effect of temperature on leaf decomposition and diversity of associated aquatic hyphomycetes depends on the substrate. Fungal Ecology 6: 546–553.
Graça, M. A. S., F. Bärlocher & M. O. Gessner, 2005. Methods to study litter decomposition. A practical guide. Springer, Dordrecht.
Gulis, V. & K. Suberkropp, 2003. Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshwater Biology 48: 123–134.
Gulis, V., V. Ferreira & M. A. S. Graça, 2006. Stimulation of leaf litter decomposition and associated fungi and invertebrates by moderate eutrophication: implications for stream assessment. Freshwater Biology 51: 1655–1669.
Hieber, M. & M. O. Gessner, 2002. Contribution of stream detrivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83: 1026–1038.
Larned, S. T., R. A. Kinzie, A. P. Covich & C. T. Chong, 2003. Detritus processing by endemic and non-native Hawaiian stream invertebrates: a microcosm study of species-specific effects. Archiv für Hydrobiologie 156: 241–254.
Legendre, P. & M. J. Anderson, 1999. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69: 1–24.
Lima-Fernandes, E., I. Fernandes, A. Pereira, P. Geraldes, F. Cássio & C. Pascoal, 2014. Eutrophication modulates plant -litter diversity effects on litter decomposition in streams. Freshwater Science 3: 31–41.
Lourenço, P., V. Medeiros, A. Gil & L. Silva, 2011. Distribution, habitat and biomass of Pittosporum undulatum, the most important woody plant invader in the Azores Archipelago. Forest Ecology and Management 262: 178–187.
McArdle, B. H. & M. J. Anderson, 2001. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82: 290–297.
Oscoz, J., D. Galicia & R. Miranda, 2011. Identification Guides of Freshwater Macroinvertebrates of Spain. Springer, Dordrecht.
Ostrofsky, M. L., 1997. Relationship between chemical characteristics of autumn-shed leaves and aquatic processing Rrtes. Journal of the North American Benthological Society 16: 750–759.
Petersen, R. C. & K. W. Cummins, 1974. Leaf processing in a woodland stream. Freshwater Biology 4: 343–368.
Pope, R. J., A. M. Gordon & N. K. Kaushik, 1999. Leaf litter colonization by invertebrates in the littoral zone of a small oligotrophic lake. Hydrobiologia 392: 99–112.
Porteiro, J., 2000. Lagoas dos Açores: elementos de suporte ao planeamento integrado. Dissertação para a obtenção do grau de Doutor em Geografia. Departamento de Biologia, Universidade dos Açores, Ponta Delgada, 344 pp.
Raposeiro, P. M., A. C. Costa & S. H. Hughes, 2011. Environmental factors-spatial and temporal variation of chironomid communities in oceanic island streams (Azores archipelago). Annales de Limnologie-International Journal of Limnology 47: 325–338.
Raposeiro, P. M., A. M. Cruz, S. J. Hughes & A. C. Costa, 2012. Azorean freshwater invertebrates: status, threats and biogeographic notes. Limnetica 31: 13–22.
Raposeiro, P. M., S. J. Hughes & A. C. Costa, 2013. Environmental drivers–spatial and temporal variation of macroinvertebrate communities in island streams: the case of the Azores Archipelago. Fundamental and Applied Limnology/Archiv fur Hydrobiologie 182: 337–350.
Raposeiro, P. M., G. M. Martins, I. Moniz, A. Cunha, A. C. Costa & V. Gonçalves, 2014. Leaf litter decomposition in remote oceanic islands: the role of macroinvertebrates versus microbial decomposition of native versus exotic plant species. Limnologica-Ecology and Management of Inland Waters 45: 80–87.
Rosemond, A. D., C. M. Pringle, A. Ramírez, M. J. Paul & J. L. Meyer, 2002. Landscape variation in phosphorus concentration and effects on detritus-based tropical streams. Limnology and Oceanography 47: 278–289.
Sabetta, L., M. L. Costantini, O. Maggi, A. M. Persiani & L. Rossi, 2000. Interactions between detritivores and microfungi during the leaf detritus decomposition in a volcanic lake (Lake Vico–central Italy). Hydrobiologia 439: 49–60.
Schindler, M. H. & M. O. Gessner, 2009. Functional leaf traits and biodiversity effects on litter decomposition in a stream. Ecology 90: 1641–1649.
Smith, G. C., A. P. Covich & A. M. D. Brasher, 2003. An ecological perspective on the biodiversity of tropical island streams. BioScience 53: 1048–1051.
Sondergaard, M. & E. Jeppesen, 2007. Anthropogenic impacts on lake and stream ecosystems, and approaches to restoration. Journal of Applied Ecology 44: 1089–1094.
Suberkropp, K., 1995. The influence of nutrients on fungal growth, productivity, and sporulation during leaf breakdown in streams. Canadian Journal of Botany 73: 1361–1369.
Tachet, H., M. Bournand & P. Richoux, 2006. Introduction à l´étude des macroinvertebrés des eaux douces. Université Claude Bernard, Lyon: 155 pp.
Taylor, B. R. & E. E. Chauvet, 2014. Relative influence of shredders and fungi on leaf litter decomposition along a river altitudinal gradient. Hydrobiologia 721: 239–250.
Tuchman, N. C., 1993. Relative importance of microbes versus macroinvertebrate shredders in the process of leaf decay in lakes of differing pH. Canadian Journal of Fisheries and Aquatic Sciences 50: 2707–2712.
van Dokkum, H. P., D. M. E. Slijkerman, L. Rossi & M. L. Costantini, 2002. Variation in the decomposition of Phragmites australis litter in a monomictic lake: the role of gammarids. Hydrobiologia 482: 69–77..
Wallace, J. B., S. L. Eggert, J. L. Meyer & J. R. Webster, 1997. Multiple trophic levels of a forest stream linked to terrestrial litter onputs. Science 277: 102–104.
Webster, J. R. & E. F. Benfield, 1986. Vascular plant breakdown in freshwater ecosystems. Annual Review of Ecology and Systematics 17: 567–594.
Whittaker, R. J. & J. M. Fernández-Palacios, 2007. Island Biogeography. Oxford University Press, Oxford.
Woodward, G., M. O. Gessner, P. S. Giller, V. Gulis, S. Hladyz, A. Lecerf, B. Malmqvist, B. G. McKie, S. D. Tiegs, H. Cariss, M. Dobson, A. Elosegi, V. Ferreira, M. A. S. Graça, T. Fleituch, J. O. Lacoursière, M. Nistorescu, J. Pozo, G. Risnoveanu, M. Schindler, A. Vadineanu, L. B. M. Vought & E. Chauvet, 2012. Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336: 1438–1440.
Young, R. G., C. D. Matthaei & C. R. Townsend, 2008. Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health. Journal of the North American Benthological Society 27: 605–625.
Acknowledgments
Part of this study was financed by the Fundo Regional da Ciência e Tecnologia (M3.1.7/F/009/2011). PMR, GMM, and VF were supported by Fundação para a Ciência e Tecnologia (SFRH/BPD/99461/2014, SFRH/BPD/108114/2015 and IF/00129/2014, respectively). We thank the Freshwater Ecology Research Group of the University of the Azores for the support provided during the field and laboratory work and CIGPT for helping in making the maps. This work was also funded by FEDER funds through the Operational Programme for Competitiveness Factors - COMPETE and by National Funds through FCT - Foundation for Science and Technology under the UID/BIA/50027/2013 and POCI-01-0145-FEDER-006821.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luz Boyero
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raposeiro, P.M., Ferreira, V., Guri, R. et al. Leaf litter decomposition on insular lentic systems: effects of macroinvertebrate presence, leaf species, and environmental conditions. Hydrobiologia 784, 65–79 (2017). https://doi.org/10.1007/s10750-016-2852-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2852-1