Abstract
Fatty acid (FA) analyses of fish tissues offer the potential to gain new knowledge of habitat- or forage-specific energy inputs to fishes in river-floodplain ecosystems, although limited information exists regarding among-habitat differences in FA biomarkers. The goal of this study was to determine if differences in fish FA profiles among main channel and connected and disconnected floodplain lakes exist in large river-floodplain systems. Bluegill Lepomis macrochirus FA profiles were generated to assess differences among two reaches of the Illinois River, USA, and its connected and disconnected floodplain lakes and determine whether FA signatures could be used to reclassify fish to their source habitat. Bluegill FA profiles differed among habitats and river reaches, including differences in levels of individual FAs (e.g., 18:2n−6, an indicator of allochthonous inputs, was higher among main channel fish) and FA groupings (e.g., n−3:n−6 FA ratio, an indicator of aquatic primary productivity, was higher among floodplain lake fish), which enabled >87.5% reclassification accuracy of fish to their source environment. We demonstrated that bluegill FA profiles differed among reaches and laterally among river channel and floodplain habitats, suggesting that FA profiles can be used to infer recent habitat use and habitat-specific foraging of fishes in large river-floodplain ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge of habitats and energy sources used by aquatic organisms is important for management and conservation of species in lentic and lotic environments (Schlosser, 1991; Hamilton et al., 1992; Guegan et al., 1998; Fausch et al., 2002). In large river ecosystems, many fishes rely on the connectivity of the main channel with floodplain lakes for spawning, refuge, and larval nursery habitat (Turner et al., 1994; King et al., 2003; King, 2004; Nunn et al., 2007; Schultz et al., 2007; Zeigler & Whitledge 2010). Fishes may also use these habitats for energy acquisition; however, discerning relative use and importance of habitats within complex river-floodplain lake ecosystems can be difficult due to their energetic complexities (Vannote et al., 1980; Junk et al., 1989; Thorp & DeLong, 1994; Thorp et al., 2006). Fatty acid (FA) analyses of fish tissues may offer the potential to gain new knowledge of habitat- or forage-specific energy inputs to fishes in these river-floodplain lake ecosystems.
Use of FAs as dietary biomarkers has become an increasingly common practice in aquatic food web studies (e.g., Napolitano et al., 1996; Rossi et al., 2006; Perga et al., 2009; Ravet et al., 2010). Aquatic primary producers are often distinguishable from one another based on the levels and ratios of FAs in their tissues; similarly, allochthonous energy sources tend to have different FA levels and ratios than aquatic primary producers (Pohl & Zurheide, 1979; Dalsgaard et al., 2003; Torres-Ruiz et al., 2007; Ravet et al., 2010). Fatty acid biomarkers can be used to identify energy sources of consumers such as fishes because fish lack the ability to transform and synthesize certain FAs, i.e., long-chain poly-unsaturated fatty acids (LC-PUFAs); therefore, LC-PUFAs must be acquired from dietary sources (Sargent et al., 1987; Reuss & Poulsen, 2002; Tocher, 2003; Ravet et al., 2010). Thus, differences in consumption of forage items of fishes can be detected if their forage bases differ in FA composition. However, to detect spatial differences, distinct FA levels and ratios of energy sources must exist among habitats, either as a result of difference in the forage base and/or differences in the composition of the forage among habitats. For example, Czesny et al. (2011) found that fish and invertebrate FA profiles differed spatially among pelagic and benthic zones, which was attributed to FA compositions of pelagic and benthic prey.
Many studies have used FA biomarkers to elucidate food webs in lentic systems (Perga et al., 2009; Ravet et al., 2010; Czesny et al., 2011; Lau et al., 2012), but application of these methods to large river ecosystems is limited. Dayhuff (2004) reported that the FA profiles of white bass Morone chrysops (Rafinesque) differed among navigation pools of the Ohio River, USA. Similarly, Young et al. (2015) found that channel catfish Ictalurus punctatus (Rafinesque) FA profiles differed between channelized and unchannelized reaches of the Kaskaskia River, Illinois, USA; some differences in FA composition of fish from the river and two connected oxbow sloughs were also detected. While these studies indicate that consumer FA profiles can differ longitudinally along rivers, whether fish FA profiles also differ laterally between river channel and floodplain habitats in large rivers that have extensive floodplains has not been assessed. In addition, whether floodplain lake habitats (including those that differ in connectivity to the river channel) may impart distinct FA profiles to fishes based on differences in producer, and fish prey assemblages among floodplain habitats has also not been investigated. Therefore, the goal of this study was determine whether differences in fish FA profiles among main channel and connected and disconnected floodplain lakes occur in large river-floodplain systems. Addressing this objective will provide insight regarding the applicability of FA biomarkers for distinguishing fish with use of river channel and floodplain habitats and habitat-specific nutritional histories of consumers in large river-floodplain ecosystems.
Methods
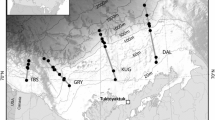
Fish were collected from eight sites along the Illinois River, Illinois, USA, during spring and summer 2009 and 2010. These sampling locations included two reaches of the Illinois River (near Grafton, Illinois, USA; 38°58′21″N, 90°33′01″W, and Havana, Illinois, USA; 40°27′53″N, 89°53′53″W), three connected floodplain lakes near Grafton (Swan Lake, Lower Stump Lake, and Upper Stump Lake), and three disconnected floodplain lakes near Havana (Powerton Lake, South Spring Lake, and Banner Marsh; Fig. 1). The Illinois River at these reaches characterized as a low gradient river (2 cm/km), moderate flow river (~639 m3/s) with a broad floodplain (2.5–5 km wide) that was historically dominated by floodplain forests and backwater areas and floodplain lakes (Starrett, 1971; Koel & Sparks, 2002; US Geological Survey, 2013). These backwater areas and floodplain lakes near Havana have been disconnected via levee system, whereas many of these areas remain near Grafton due to a reduced levee system (Starrett, 1971; Koel & Sparks, 2002). Disconnected floodplain lakes near Havana, IL are primarily shallow (<1.5 m, however, deep areas >3 m exist in all three lakes) and low turbidity (clarity > maximum depth), and aquatic vegetation is present (Stafford et al., 2012). Connected floodplain lakes near Grafton are shallow (mean depth of <1 m), windswept, and highly turbid (clarity < maximum depth), and vegetation is mostly absent (Schultz et al., 2007). These connected floodplain lakes of the Illinois River included representatives with permanent and intermittent (during flooding) connections to the Illinois River and varied in the type of connection to the river (natural channel, ditch or water control structure; Starrett, 1971; Schultz et al., 2007; Zeigler & Whitledge, 2010).
Map showing sites where bluegill were collected for this study. Filled triangles represent disconnected floodplain lake sites, open triangles represent connected floodplain lake sites, and diamonds represent sampling sites on the Illinois River at Havana (filled diamond) and the Illinois River at Grafton (open diamond), respectively
Juvenile bluegill Lepomis macrochirus (Rafinesque) (n = 6–19 per site) ranging from 50–105 mm were collected at each site using three-phase alternating current (AC) electrofishing (250 V, and 7–10 A) at near-shore locations within each site. Fish were euthanized with MS-222, placed on ice for transport to the laboratory, and stored frozen (−80°C) until subsequent analyses. Bluegill were used as the study species because they are readily found throughout the Illinois River-floodplain system (Zeigler & Whitledge, 2010), exhibit small home ranges, and are not particularly mobile (Gunning & Schoop, 1963; Paukert et al., 2004). Thus, bluegill FA profiles are likely representative of the sites in which fish were collected. Additionally, juvenile bluegill were chosen because of dietary consistency and stomach contents data indicated that individuals were consuming similar prey items (zooplankton and aquatic insects) regardless of location (Mittelbach, 1984; Werner & Hall, 1988; Rude, 2012).
Fatty acid profiles were generated from crude lipid samples extracted from individually freeze-dried, pulverized bluegill according to the methods described by Laporte & Trushenski (2011). Briefly, crude lipids were extracted according to the procedures described by Folch et al. (1957) and processed to yield fatty acid methyl esters (FAME) according to the acid-catalyzed transmethylation methods described by Christie (1982). The resultant FAME were separated using a Shimadzu GC-17A gas chromatograph (Shimadzu Scientific Instruments, Kyoto, Japan). Individual FAME were identified by reference to external standards (Supelco 37 Component FAME Mix, PUFA-1, and PUFA-3; Supelco, Bellefonte, PA, USA).
Both univariate and multivariate analyses were used to test the null hypotheses that there were no significant differences in the FA profiles of bluegill from the Illinois River and its connected and disconnected floodplain lakes. One-way analyses of variance (ANOVAs) followed by Tukey’s HSD tests for multiple comparisons were used to assess differences in individual FA abundance in fish among the Illinois River at Grafton and its connected floodplain lakes, along with the ratio of n−3:n−6 FAs. Individual FA levels that differed significantly among site types were used in a multivariate analysis of variance (MANOVA) and a discriminant analysis (CANDISC procedure in SAS®) to characterize the multivariate FA profiles of fish from the different site types. A plot of the first two canonical variates was used to visually depict the differences in FA profiles of fish among site types. Pillai’s trace statistic was used to assess significance of multivariate FA profiles of fish among the sites. Spearman rank correlations were used to assess the relationship between individual FAs and canonical axes 1 and 2 to determine which individual FAs had the greatest influence on the separation found in the discriminant analysis. In addition, linear discriminant function analysis with a leave-one-out jackknife procedure was used to determine the accuracy in which fish could be classified back to the environment in which they were captured based on their FA profiles. Statistical methods described above were repeated using data from the Illinois River at Havana and its disconnected floodplain lakes only as an independent dataset. Additionally, ANOVAs and a MANOVA along with CANDISC procedure in SAS were used to determine differences in FA profiles of fish among each river reach, and also visually depict differences in FA profiles of fish from the Illinois River (both reaches) and connected and disconnected floodplain lakes. All FA data used for statistical analyses were arcsine square root transformed to help meet the assumptions of normality, except for the n−3:n−6 FA ratio. P values were Bonferroni corrected from α = 0.05 by dividing by the number of FAs used in the one-way ANOVAs (26) to account for occurrence of P values < 0.05 due to chance alone. Thus P values ≤ 0.0019 were considered significant for all one-way ANOVAs. Pillai’s Trace statistic was considered significant at α ≤ 0.05. Spearman rank correlations between canonical axes values, and FA levels were also Bonferroni corrected; thus, P values for the canonical axes values and FA level were considered significant at α = 0.0035 for both datasets. A P value of ≤ 0.05 was considered significant for all statistical tests (except where noted), and all statistical analyses were performed using SAS 9.2 (SAS Institute, Inc. Cary, NC).
Results
Levels of many individual saturated fatty acids (SFAs) in bluegill tissues were significantly different among the Illinois River and its disconnected floodplain lakes. Levels of even-chain SFAs (14:0, 16:0, and 18:0) were significantly different among sites (ANOVA, F = 60.38, df = 3, 27, P < 0.0001, ANOVA, F = 21.28, df = 3, 27, P < 0.0001, and ANOVA, F = 22.10, df = 3, 27, P < 0.0001, respectively), with SFA levels often lower within fish from the Illinois River compared to its disconnected floodplain lakes (Table 1). Levels of two mono-unsaturated fatty acids (MUFAs; 16:1n−7, and 18:1n−9) in bluegill tissues were significantly different between the Illinois River and its disconnected floodplain lakes (ANOVA, F = 28.97, df = 3, 27, P < 0.0001, and ANOVA, F = 8.24, df = 3, 27, P = 0.0005, respectively), with generally higher concentrations found in the Illinois River (Table 1). Levels of medium chain poly-unsaturated fatty acids (MC-PUFAs; 16:2n−4, 18:2n−6, 18:3n−3, and 18:4n−3) were significantly different between the Illinois River and disconnected floodplain lakes (ANOVA, F = 22.04, df = 3, 27, P < 0.0001, ANOVA, F = 7.47, df = 3, 27, P = 0.0009, ANOVA, F = 10.46, df = 3, 27, P < 0.0001, and ANOVA, F = 37.39, df = 3, 27, P < 0.0001, respectively). Higher levels of 16:2n−4 and 18:2n−6 were found in bluegill from the Illinois River compared to disconnected floodplain lakes, and lower levels of 18:3n−3 and 18:4n−3 were found in the Illinois River compared to disconnected lakes (Table 1). Three long-chain poly-unsaturated fatty acids (LC-PUFAs; 20:4n−6, 20:5n−3, and 22:6n−3) exhibited levels that were significantly different among sites (ANOVA, F = 68.26, df = 3, 27, P < 0.0001, ANOVA, F = 6.30, df = 3, 27, P = 0.0018, and ANOVA, F = 15.29, df = 3, 27, P < 0.0001, respectively). Levels of LC-PUFAs in bluegill were generally lower within fish from the Illinois River compared to its disconnected floodplain lakes (Table 1). The ratio of n−3:n−6 FAs was significantly different among sites (ANOVA, F = 11.87, df = 3, 27, P < 0.0001), with significantly higher values observed in bluegill from disconnected floodplain lakes compared to the Illinois River (Table 1).
Multivariate analysis of bluegill FA profiles indicated that fish from the Illinois River and its disconnected floodplain lakes possessed significantly different FA profiles (MANOVA, Pillai’s trace, F = 12.48, df = 36, 54, P < 0.0001). A plot of the first two canonical variates from the CANDISC procedure in SAS illustrated the distinct FA profiles of bluegill among the Illinois River and disconnected floodplain lakes (Fig. 2a). The first discriminant function (axis 1) from this model accounted for 69.9% of the total dispersion in the dataset. Many of the SFAs were positively correlated with axis 1, whereas many of the MC-PUFAs were both positively (18:3n−3 and 18:4n−3) and negatively (18:2n−6) correlated with axis 1 (Table 2). The second discriminant function (axis 2) from this model accounted for 22.3% of the total dispersion in the dataset. Many of the SFAs and LC-PUFAs were negatively correlated with axis 2, and MUFAs were positively correlated with axis 2 (Table 2). Linear discriminant function analysis with a leave-one-out jackknife procedure indicated that individual bluegill could be classified back to their site type of capture with 87.5% overall accuracy based on their FA profiles (Table 3).
Plot of the first two canonical variates obtained through linear discriminant function analysis including all the FAs that were significantly different among the Illinois River at Havana and its disconnected floodplain lakes (a), the Illinois River at Grafton and its connected floodplain lakes (b), and both reaches of the Illinois River and disconnected and connected floodplain lakes (c)
Bluegill from the Illinois River and three of its connected floodplain lakes had significant differences in levels of four SFAs (14:0, 15:0, 17:0, and 18:0) (ANOVA, F = 16.90, df = 3, 47, P < 0.0001, ANOVA, F = 46.18, df = 3, 47, P < 0.0001, ANOVA, F = 28.08, df = 3, 47, P = 0.0011, and ANOVA, F = 11.67, df = 3, 47, P < 0.0001, respectively), with a general trend of lower levels of both odd- and even-chain SFAs in bluegill from the Illinois River in comparison to its connected floodplain lakes (Table 4). Levels of two MUFAs (16:1n−7 and 18:1n−7) in bluegill tissues were significantly different between fish from the Illinois River and the connected floodplain lakes (ANOVA, F = 14.15, df = 3, 47, P < 0.0001 and ANOVA, F = 38.97, df = 3, 47, P < 0.0001, respectively), with a general trend of lower levels of these MUFAs in bluegill from the Illinois River in comparison to the connected floodplain lakes (Table 4). Levels of three MC-PUFAs (16:2n−4, 18:3n−3 and 18:4n−3) were significantly different between fish from the Illinois River and its connected floodplain lakes (ANOVA, F = 53.23, df = 3, 47, P < 0.0001, ANOVA, F = 52.90, df = 3, 47, P < 0.0001, and ANOVA, F = 78.61, df = 3, 47, P < 0.0001, respectively). Levels of these FAs in tissues of bluegill from the Illinois River and its connected floodplain lakes were highly variable among sites. However, bluegill from the Illinois River generally had lower levels of these FAs than most of fish from the connected lakes (Table 4). Levels of three LC-PUFAs (20:4n−6, 20:5n−3, and 22:6n−3) also differed significantly between fish from the Illinois River and its connected floodplain lakes (ANOVA, F = 15.45, df = 3, 47, P < 0.0001, ANOVA, F = 6.28, df = 3, 47, P = 0.0011, and ANOVA, F = 11.89, df = 3, 47, P < 0.0001, respectively). Levels of these LC-PUFAs in bluegill from the Illinois River sites were highly variable; however, bluegill from one of the connected floodplain lakes (Swan Lake) exhibited significantly lower levels of these FAs than fish from the other sites (Table 4). The ratio of n−3:n−6 FAs was significantly different between bluegill from the Illinois River and its connected floodplain lakes (ANOVA, F = 7.22, df = 3, 47, P = 0.0004), with significantly higher values observed in bluegill from some of the connected floodplain lakes in comparison to the Illinois River (Table 4).
Fatty acid profiles of bluegill from the Illinois River and its connected floodplain lakes were significantly different (MANOVA, Pillai’s Trace Statistic: F = 16.33, df = 36, 114, P < 0.0001). A plot of the first two canonical variates from the CANDISC procedure in SAS illustrated the distinct FA profiles of bluegill between sites (Fig. 2b). The first discriminant function (axis 1) from this model accounted for 64.19% of the total dispersion in the dataset. All SFAs were negatively correlated with axis 1 except for 14:0. Many of the 16 and 18 carbon MUFAs and PUFAs were positively correlated with axis 1, whereas 20:4n−6 and 22:6n−3 were negatively correlated with axis 1 (Table 5). The second discriminant function (axis 2) from this model accounted for 18.37% of the total dispersion in the dataset. Only 17:0 was positively correlated with axis 2 and 16:1n−7 was negatively correlated with axis 2 (Table 5). Linear discriminant function analysis with a leave-one-out jackknife procedure indicated that individual fish could be classified back to their environment of capture with 88% accuracy based on their FA profiles (Table 6).
Bluegill from both reaches of the Illinois River had significant differences in levels of four SFAs (15:0, 16:0, 17:0, and 18:0) (ANOVA, F = 5.87, df = 1, 16, P = 0.0277, ANOVA, F = 83.40, df = 1, 16, P < 0.0001, ANOVA, F = 19.14, df = 1, 16, P = 0.0005 and ANOVA, F = 7.37, df = 1, 16, P = 0.0153, respectively), with a higher levels of SFAs in bluegill from the Illinois River at Grafton (Table 7). Levels of three MC-PUFAs (18:2n−6, 18:3n−3 and 18:4n−3) were significantly different between reaches of the Illinois River (ANOVA, F =8.57, df = 1, 16, P = 0.0099, ANOVA, F = 15.32, df = 1, 16, P = 0.0012, and ANOVA, F = 18.75, df = 1, 16, P = 0.0005, respectively). Bluegill from the Illinois River at Grafton had higher levels on n−3 MC-PUFAs and lower levels of n−6 MC-PUFAS in comparison to the Illinois River at Havana (Table 7). The ratio of n−3:n−6 FAs in bluegill tissues was significantly different between reaches of the Illinois River (ANOVA, F = 10.66, df = 1, 16, P = 0.0048), with significantly higher values observed in bluegill from the Illinois River at Grafton (Table 7).
Fatty acid profiles of bluegill from both reaches of the Illinois River and connected and disconnected floodplain lakes were significantly different (MANOVA, Pillai’s Trace Statistic: F = 8.08, df = 36, 207, P < 0.0001). A plot of the first two canonical variates from the CANDISC procedure in SAS illustrated the distinct FA profiles of bluegill among the sites (Fig. 2c). The first discriminant function (axis 1) from this model accounted for 73.1% of the total dispersion in the dataset, and the second discriminant function (axis 2) from this model accounted for 26.3% of the total dispersion in the dataset. Linear discriminant function analysis with a leave-one-out jackknife procedure indicated that individual fish could be classified back to their environment of capture with 91.4% accuracy based on their FA profiles and 88.8% accuracy with fish from only the Illinois River reaches.
Discussion
Results indicated that bluegill from the Illinois River and its connected and disconnected floodplain lakes could be distinguished with a high degree of accuracy based on their FA profiles. Spatial differences in FA profiles of bluegill among environments can be attributed to differences in FA availability within these environments, which is likely due to differences in basal energy sources among environments (Vannote et al., 1980; Twombly & Lewis, 1987; Junk et al., 1989; Thorp & DeLong, 1994; Garcia de Emiliani, 1997; Thomaz et al., 2007; Lehman et al., 2008), as FA compositions of both basal energy sources and invertebrate prey can influence the FA profiles within fish tissues (Sargent et al., 1987; Reuss & Poulsen 2002; Tocher 2003; Ravet et al., 2010; Burns et al., 2011). These differences in FA profiles are not likely due to major diet differences, as bluegill stomach contents were similar across sites and were comprised primarily of cladocerans, aquatic insects (chironomidae, corixidae, ephemeroptera, odonota; see, Rude, 2012), and bluegill tend to feed similarly in different habitats (Mittelbach, 1984; Werner & Hall, 1988). Our results are similar to other studies investigating spatial differences in FA profiles of fish in both lentic (Czesny et al., 2011; Lau et al., 2012) and longitudinal differences in lotic systems (Dayhuff, 2004; Young et al., 2015). Although studies of spatial differences in FA profiles of organisms in lotic environments are limited, our classification success rates for individual fish to environment of capture in this study were greater than one published study using FA profiles of channel catfish to distinguish location of capture in the Kaskaskia River and its connected oxbow lakes (Young et al., 2015).
Differences in bluegill FA profiles from the Illinois River and its disconnected floodplain lakes were detected, which are resultant from differential energy sources and FA availability. Many FAs contributed to differences among these environments; however, certain FA and ratios were key contributors to these differences. The n−3:n−6 ratio was significantly lower in fish from the river compared to disconnected lake fish. This ratio is built on the fact that aquatic primary producers synthesize large amounts of n−3 FAs (e.g., 18:3n−3, 20:5n−3, and 22:6n−3; Ahlgren et al., 2009), and terrestrial primary producers contain elevated levels of n−6 FAs (e.g., 18:2n−6 and 20:4n−6; Napolitano, 1999; Ahlgren et al., 2009). Thus, a low n−3:n−6 ratio is a putative marker of a diet more based on terrestrial inputs (Torres-Ruiz et al., 2007; Ahlgren et al., 2009), suggesting that fish from the river received an increased degree of allochthonous production in comparison to its disconnected floodplain lakes. Further supporting increased allochthonous energy sources to fish in the river was significantly higher 18:2n−6 levels in river fish, as this FA is associated with terrestrially derived diet (Maazouzi et al., 2007; Koussoroplis et al., 2008; Brett et al., 2009; Perga et al., 2009; Young et al., 2015). Similarly, Young et al. (2015) observed elevated levels of 18:2n−6 in channel catfish from the Kaskaskia River main channel compared to fish from its oxbow lakes. We also detected increased levels of MUFAs 16:1n−7 and 18:1n−9 in fish from the river, which have been shown to be related to increased microbial and detritus energetic contributions (Gonzalez-Baro & Pollero, 1988; Wakeham & Canuel, 1990; Scholz & Boon, 1993; Boon et al., 1996; Torres-Ruiz et al., 2007), which are major constituents of allochthonous energy pathways (see, Cummins, 1974; Vannote et al., 1980; Roach, 2013). Similarly, the riverine ecosystem synthesis suggests a link between microbial and fish production (Thorp et al., 2006). In contrast to fish from the river, bluegill from disconnected lakes exhibited increased n−3:n−6 ratio, suggestive of more aquatic-origin energy sources, but also the LC-PUFA 22:6n−3 was significantly higher in fish from disconnected lakes compared to the river. High levels of 22:6n−3 are often associated with autochthonous energy pathways via aquatic primary production (Perga et al., 2009; Ravet et al., 2010), and is an important component for overall fish health, reproduction, growth, and many physiological processes (Brett & Muller-Navarra, 1997; Ahlgren et al., 2009). Young et al. (2015) also observed elevated 22:6n−3 levels in channel catfish from oxbow lakes compared to main channel fish. This study and Young et al. (2015) suggest these floodplain lake environments may be a potential source of important FAs (e.g., 22:6n−3) for fish in large river-floodplain systems; however, the lack of connectivity between the Illinois River at Havana and its floodplain lakes limits potential transfer of energy and potentially important FAs.
Increased river-floodplain connectivity allows organisms to actively (or passively) move and feed among habitats, and allow exchange of energy sources, which may create a more homogeneous system in terms of energetic contributions to fish and other organisms (Junk et al., 1989; Tockner et al., 2000; Amoros & Bornette, 2002). Despite the potential for increased homogeneity in energy sources, bluegill FA levels and ratios differed among connected environments of the Illinois River and floodplain lake system near Grafton. Similar to the river and its disconnected floodplain lakes, bluegill from the river had lower n−3:n−6 ratios compared to fish from connected floodplain lakes, indicating an increased influence of terrestrial dietary inputs (Torres-Ruiz et al., 2007; Ahlgren et al., 2009). However, other FAs indicative of a more terrestrial-origin diet (e.g., 18:2n−6, 18:1n−9, and 16:1n−7; Gonzalez-Baro & Pollero, 1988; Wakeham & Canuel, 1990; Scholz & Boon, 1993; Boon et al., 1996; Maazouzi et al., 2007; Torres-Ruiz et al., 2007; Koussoroplis et al., 2008; Brett et al., 2009; Perga et al., 2009) were not substantially different between the river and its connected floodplain lakes, and FAs indicative of increased autochthonous energy sources (e.g., 22:6n−3; Perga et al., 2009; Ravet et al., 2010) were not substantially higher in fish from floodplain lakes compared to fish from the main channel. Much of the multivariate differences in bluegill FA levels and ratios between the main channel and its connected floodplain lakes occurred within floodplain lake habitats. The FA biomarkers commonly associated with primary producers (n−3 FAs such as 18:3n−3, 20:5n−3, and 22:6n−3; Perga et al., 2009; Ravet et al., 2010) differed between floodplain lakes and can be attributed to site-specific differences in primary producer assemblages. Lateral habitats of large river-floodplain lake systems differ in depth, fluvial geomorphology, and connectivity resulting in different energy production dynamics among sites (Thorp et al., 2006). These physical differences among habitats result in site-specific succession of primary producer communities (Garcia de Emiliani, 1993; Huszar & Reynolds, 1997; Miranda, 2005), leading to different FA availability in each lake, resulting in different FA levels and ratios in fish tissues among habitats (Zenebe et al., 1998; Dayhuff, 2004; Czesny et al., 2011; Young et al., 2015).
We observed longitudinal differences in FA profiles of bluegill across both Illinois River reaches. This finding is consistent with the results of Young et al. (2015) and Dayhuff (2004) as they documented distinct FA profiles of fish within channelized and unchannelized reaches of the Kaskaskia River, and among pools of the Ohio River, respectively. These differences can be attributed to changes in taxonomic composition of primary producers and prey items among different river reaches (Dayhuff, 2004; Young et al., 2015). More specifically in this study, bluegill from the Illinois River at Havana had a lower n−3:n−6 FA ratio in comparison to the Illinois River at Grafton, which is indicative of increased allochthonous energy production available to bluegill at this site. These observed differences in bluegill FAs can be attributed to differences in river-floodplain connectivity at each reach, as productivity in lateral habitats influence productivity in main channel habitats, resulting in longitudinal differences in food web structure among reaches (Thorp et al., 2006). Our results suggest that floodplain energy inputs are important for bluegill in river reaches with extensive connectivity to floodplain lakes, further highlighting the potential importance of maintaining river-floodplain connectivity to support production of riverine consumers such as bluegill.
This study demonstrates that the FA profiles of fish differed longitudinally among reaches, and laterally among floodplain habitats in a large river-floodplain system. Consistent with the riverine ecosystem synthesis view of rivers as a set of linked hydrogeomorphic patches that can result in both longitudinal and lateral differences in food web structure and function (Thorp et al., 2006). Our data suggest FA profiles of fish tissues can potentially be used to identify recent habitat use of fishes in large river-floodplain systems, similar to use of FA profiles to distinguish energy sources of fish in lentic systems (Czesny et al., 2011). Furthermore, these methods may potentially be used to assess spatially explicit energy sources of fish (or other organisms) in large river-floodplain systems. However, further research is needed to assess inter-annual variability in habitat or river reach FA profiles of fishes, along with differences in primary producer and consumer taxa within these distinct habitats in river-floodplain systems to determine whether these differences persist among river reaches (Dayhuff, 2004; Young et al., 2015), and river-floodplain lakes. Furthermore, research is needed to facilitate efforts to quantify energy subsidies and lipid allocation to fish in large river-floodplain systems, particularly in areas with high connectivity among habitats, and of fishes that utilize both main channel and floodplain lake environments (Polis et al., 1997).
References
Ahlgren, G., T. Vrede & W. Goedkoop, 2009. Fatty acid ratios in freshwater fish, zooplankton and zoobenthos – are there specific optima? In Arts, M. T., M. T. Brett & M. J. Kainz (eds), Lipids in aquatic ecosystems. Springer, New York, NY: 147–178.
Amoros, C. & G. Bornette, 2002. Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshwater Biology 47: 761–776.
Boon, P. I., P. Virtue & P. D. Nichols, 1996. Microbial consortia in wetland sediments: a biomarker analysis of the effects of hydrological regime, vegetation and season on benthic microbes. Marine and Freshwater Research 47: 27–41.
Brett, M. T. & D. C. Muller-Navarra, 1997. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshwater Biology 38: 483–499.
Brett, M. T., D. C. Muller-Navarra & J. Persson, 2009. Crustacean zooplankton fatty acid composition. In Arts, M. T., M. T. Brett & M. J. Kainz (eds), Lipids in aquatic ecosystems. Springer, New York, NY: 115–146.
Burns, C. W., M. T. Brett & M. Schallenberg, 2011. A comparison of the trophic transfer of fatty acids in freshwater plankton by cladocerans and calanoid copepods. Freshwater Biology 56: 889–903.
Christie, W. W., 1982. Lipid Analysis, 2nd ed. Pergamon, Oxford.
Cummins, K. W., 1974. Structure and function of stream ecosystems. BioScience 24: 631–641.
Czesny, S., J. Rinchard, S. D. Hansen, J. M. Dettmers & K. Dabrowski, 2011. Fatty acid signatures of Lake Michigan prey fish and invertebrates: among species differences and spatiotemporal variability. Canadian Journal of Fisheries and Aquatic Sciences 68: 1211–1230.
Dalsgaard, J., M. St, G. John, D. C. Kattner, D. C. Muller-Navarra & W. Hagen, 2003. Fatty acid trophic markers in the pelagic marine food environment. Advanced Marine Biology 46: 226–340.
Dayhuff, L., 2004. Chemometric analyses of fatty acids in sauger, white bass, and paddlefish from the Ohio River as indicators of species, season, and subpopulations. Dissertation, Tennessee Technological University, Cookeville, Tennessee, USA.
Fausch, K. D., C. E. Torgersen, C. V. Baxter & H. W. Li, 2002. Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes. BioScience 52: 483–498.
Folch, J., M. Lees & G. H. Sloane-Stanley, 1957. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 276: 497–507.
Garcia de Emiliani, M. O., 1993. Seasonal succession of phytoplankton in a lake of the Paraná river floodplain, Argentina. Hydrobiologia 264: 101–114.
Garcia de Emiliani, M. O., 1997. Effects of water level fluctuations on phytoplankton in a river–floodplain lake system (Parana River, Argentina). Hydrobiologia 357: 1–15.
Gonzalez-Baro, M. & R. J. Pollero, 1988. Lipid characterization and distribution among tissues of freshwater crustacean Macrobrachium borellii during an annual cycle. Comparative Biochemical Physiology 91B: 711–715.
Guegan, J. F., S. Lek & T. Oberdorff, 1998. Energy availability and habitat heterogeneity predict global riverine fish diversity. Nature 391: 382–384.
Gunning, G. E. & C. R. Schoop, 1963. Occupancy of home range by longear sunfish, Lepomis m. megalotis (Rafinesque), and bluegill. Lepomis m. macrochirus Rafinesque. Animal Behaviour 11: 325–330.
Hamilton, S. K., W. M. Lewis Jr. & S. J. Sippel, 1992. Energy sources for aquatic animals in the Orinoco River floodplain: evidence from stable isotopes. Oecologia 89: 324–330.
Huszar, V. L. M. & C. S. Reynolds, 1997. Phytoplankton periodicity and sequences of dominance in an Amazonian flood–plain lake (Lago Batata, Pará, Brasil): responses to gradual environmental change. Hydrobiologia 346: 169–181.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river–floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106: 110–127.
King, A. J., 2004. Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river. Journal of Fish Biology 65: 1582–1603.
King, A. J., P. Humphries & P. S. Lake, 2003. Fish recruitment on floodplains: the roles of patterns of flooding and life history characteristics. Canadian Journal of Fisheries and Aquatic Sciences 60: 773–786.
Koel, T. M. & R. E. Sparks, 2002. Historical patterns of river stage and fish communities as criteria for operations of dams on the Illinois River. River Research and Applications 18: 3–19.
Koussoroplis, A. M., C. Lemarchand, A. Bec, C. Desvilettes, C. Amblard, C. Fournier, P. Berny & G. Bourdier, 2008. From aquatic to terrestrial food webs: decrease of the docosahexaenoic acid/linoleic acid ratio. Lipids 43: 461–466.
Laporte, J. & J. T. Trushenski, 2011. Growth performance and tissue fatty acid composition of largemouth bass fed diets containing fish oil or blends of fish oil and soy–derived lipids. North American Journal of Aquaculture 73: 435–444.
Lau, D. C. P., T. Vrede, J. Pickova & W. Goedkoop, 2012. Fatty acid composition of consumers in boreal lakes: variation across species, space and time. Freshwater Biology 57: 24–38.
Lehman, P. W., T. Sommer & L. Rivard, 2008. The influence of floodplain habitat on the quantity and quality of riverine phytoplankton carbon produced during the flood season in San Francisco Estuary. Aquatic Ecology 42: 363–378.
Maazouzi, C., G. Masson, M. S. Izquierdo & J. C. Pihan, 2007. Fatty acid composition of the amphipod Dikerogammarus villosus: feeding strategies and trophic links. Comparative Biochemistry and Physiology 147: 868–875.
Miranda, L. E., 2005. Fish assemblages in oxbow lakes relative to connectivity with the Mississippi River. Transactions of the American Fisheries Society 134: 1480–1489.
Mittelbach, G. G., 1984. Predation and resource partitioning in two sunfishes (Centrarchidae). Ecology 65: 449–513.
Napolitano, G. E., 1999. Fatty acids as trophic and chemical markers in freshwater ecosystems. In Arts, M. T. & B. C. Wainman (eds), Lipids in freshwater ecosystems. Springer-Verlag, New York, NY: 21–44.
Napolitano, G. E., N. C. Shantha, W. R. Hill & A. E. Luttrell, 1996. Lipid and fatty acid composition of stream periphyton and stoneroller minnows (Campostoma anomalum): trophic and environmental applications. Archiv fuer Hydrobiologie 137: 211–225.
Nunn, A. D., J. P. Harvey & I. G. Cowx, 2007. Benefits to 0+ fishes of connecting man–made waterbodies to the lower River Trent, England. River Research and Applications 23: 361–376.
Paukert, C. P., D. W. Willis & M. A. Bouchard, 2004. Movement, home range, and site fidelity of bluegills in a Great Plains Lake. North American Journal of Fisheries Management 24: 154–161.
Perga, M. E., A. Bec & O. Anneville, 2009. Origins of carbon sustaining the growth of whitefish Coregonus lavaretus early larval states in Lake Annecy: insights from fatty–acid biomarkers. Journal of Fish Biology 74: 2–17.
Pohl, P. & F. Zurheide, 1979. Fatty acids and lipids of marine algae and the control of their biosynthesis by environmental factors. In Hoppe, H. A. & T. Levring (eds.), Marine algae in pharmaceutical science. Walter de Gruyter, Berlin: 65–80.
Polis, G. A., W. B. Anderson & R. D. Holt, 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28: 289–316.
Ravet, J. L., M. T. Brett & G. B. Arhonditsis, 2010. The effects of seston lipids on zooplankton fatty acid composition in Lake Washington, Washington, USA. Ecology 91: 180–190.
Reuss, N. & L. Poulsen, 2002. Evaluation of fatty acids and biomarkers for natural plankton community. A field study of a spring bloom and post bloom period of west Greenland. Marine Biology 141: 423–434.
Roach, K. A., 2013. Environmental factors affecting incorporation of terrestrial material into large river food webs. Freshwater Science 32: 283–298.
Rossi, S., A. Sabates, M. Latasa & E. Reyes, 2006. Lipid biomarkers and trophic linkages between phytoplankton, zooplankton and anchovy (Engraulis encrasicolus) larvae in NW Mediterranean. Journal of Plankton Research 28: 551–562.
Rude, N. P, 2012. Tracing energy flow pathways to fish using fatty acids and stable isotopes of hydrogen and oxygen. M.S. Thesis, Department of Zoology, Southern Illinois University, Carbondale, IL.
Sargent, J. R., R. J. Parkes, I. Mueller-Harvey & R. J. Henderson, 1987. Lipid biomarkers in marine ecology. In Sleigh, M. A. (ed.), Microbes in the sea. Ellis Horwood, Chichester: 119–138.
Schlosser, I. J., 1991. Stream fish ecology: a landscape perspective. BioScience 41: 704–712.
Scholz, O. & P. I. Boon, 1993. Biofilms on submerged River Red gum (Eucalyptus camaldulensis Dehnh, Myrtaceae) wood in billabongs: an analysis of bacterial assemblages using phospholipid profiles. Hydrobiologia 259: 169–178.
Schultz, D. W., J. E. Garvey & R. C. Brooks, 2007. Backwater immigration by fishes through a water control structure: implications for connectivity and restoration. North American Journal of Fisheries Management 27: 172–180.
Stafford, J. D., M. W. Eichholz & A. C. Phillips, 2012. Impacts of mute swans (Cygnus olor) on submerged aquatic vegetation in Illinois River valley backwaters. Wetlands 32: 851–857.
Starrett, W. C., 1971. Man and the Illinois River. In Ogelsby, R. T., C. A. Carlson & J. A. McCann (eds), River Ecology and the Impact of Man. Academic Press, New York: 131–169.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river–floodplain systems. Hydrobiologia 579: 1–13.
Thorp, J. H. & M. D. DeLong, 1994. The riverine productivity model: an heuristic view of carbon source and organic processesing in large river ecosystems. Oikos 70: 305–308.
Thorp, J. H., M. C. Thoms & M. D. Delong, 2006. The river ecosystem synthesis: biocomplexity in river networks across space and time. River Research and Application 22: 123–147.
Tocher, D. R., 2003. Metabolism and functions of lipids and fatty acids in teleost fish. Reviews in Fisheries Science 11: 107–184.
Tockner, K., F. Malard & J. V. Ward, 2000. An extension of the flood pulse concept. Hydrological Processes 14: 2861–2883.
Torres-Ruiz, M., J. D. Wehr & A. A. Perrone, 2007. Trophic relations in a stream food web: importance of fatty acids for macroinvertebrate consumers. Journal of the North American Benthological Society 26: 509–522.
Turner, T. F., J. C. Trexler, G. L. Miller & K. E. Toyer, 1994. Temporal and spatial dynamics of larval and juvenile fish abundance in temperate floodplain river. Copeia 1994: 174–183.
Twombly, S. & W. M. Lewis Jr., 1987. Zooplankton abundance and species composition in Laguna La Orsinera, a Venezuelan flood–plain lake. Arch Hydrobiol/Suppl 79: 87–107.
U.S. Geological Survey, 2013. Water-resources data for the United States, water year 2012: U.S. Geological Survey water-data report WDR-US-2012, site 05586100. Available at: http://wdr.water.usgs.gov/wy2012/pdfs/05586100.2012.pdf. Accessed 2015.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Wakeham, S. G. & E. A. Canuel, 1990. Fatty acids and sterols of particulate matter in a brackish and seasonally anoxic coastal salt pond. Organic Geochemistry 16: 703–713.
Werner, E. E. & D. J. Hall, 1988. Ontogenetic habitat shifts in bluegill: the foraging rate–predation risk trade–off. Ecology 69: 1352–1366.
Young, M. P., G. W. Whitledge & J. T. Trushenski, 2015. Fatty acid profiles distinguish channel catfish from three reaches of the Lower Kaskaskia River and its floodplain lakes. River Research and Applications. Advance online publication. doi: 10.1002/rra.2856
Zeigler, J. M. & G. W. Whitledge, 2010. Assessment of otolith chemistry for identifying source environment of fishes in the lower Illinois River, Illinois. Hydrobiologia 638: 109–119.
Zenebe, T., G. Ahlgren, I. B. Gustafsson & M. Boberg, 1998. Fatty acid and lipid content of Oreochromis niloticus L. in Ethiopian lakes – dietary effects of phytoplankton. Ecology of Freshwater Fish 7: 146–158.
Acknowledgments
We would like to thank Kurt Smith and Paul Hitchens of the Southern Illinois University Center for Fisheries, Aquaculture, and Aquatic Sciences, and Wayne Herndon and Rob Hilsabeck of the Illinois Department of Natural Resources for field assistance and collection of fish. We would also like to thank Heidi Hill and Brian Gause of the Southern Illinois University Center for Fisheries, Aquaculture, and Aquatic Sciences for lab assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: David J. Hoeinghaus
Rights and permissions
About this article
Cite this article
Rude, N.P., Trushenski, J.T. & Whitledge, G.W. Fatty acid profiles are biomarkers of fish habitat use in a river-floodplain ecosystem. Hydrobiologia 773, 63–75 (2016). https://doi.org/10.1007/s10750-016-2679-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2679-9