Abstract
Fossil shells of the aquatic mollusk Radix are common in the exposed sediments of lake terraces on the Tibetan Plateau. However, the living environment of Radix, and the geochemical characteristics of its shells, is unclear. Here, we report the results of an investigation of the occurrence of modern Radix in lakes of the southeastern and central Tibetan Plateau, as well as measurements of various geochemical characteristics of the shells. The results indicate that the nutritional status of the lake waters is the main limiting factor for the survival of Radix in these lakes. The Sr/Ca ratio of the Radix shells is significantly positively correlated with both the Sr/Ca ratio and the conductivity of the lake water. Initially, Kd Sr decreases rapidly with low values of Sr/Cawater; however, in the case of Sr/Cawater values above 0.0076, Kd Sr exhibits only a small range of variation. The δ 13Cshell values are controlled by the δ 13C of lake water dissolved inorganic carbon (DIC). In addition, the contribution of DIC of organic origin to the Radix shells increases when the lake water is deficient in DIC of inorganic origin. The δ 18O values of the Radix shells provide useful information about the isotopic composition of the ambient waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Tibetan Plateau is one of the major regions of alpine lakes in the world, and although the statistics are incomplete, there are at least 800 lakes with an area >1 km2 (Ma et al., 2011; Fig. 1A). The aquatic gastropod Radix (Montfort, 1810) is common in many of these lakes (von Oheimb et al., 2011); however, its ecological requirements in the lakes of the Tibetan Plateau are not fully understood (Liu, 1963; Zhao et al., 2005; von Oheimb et al., 2011; Taft et al., 2012, 2013). Thus, the limnological characteristics of the sites in which it occurs, as well as the modern Radix biology, warrant further investigation.
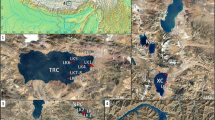
A Location of the sampled lake sites on the Tibetan Plateau. Sites where living specimens of Radix sp. occur at the present day are indicated by triangles, and sites where Radix sp. is absent are indicated by crosses. The Arabic numerals correspond to the Site no. in Table 1; B fossil shells of Radix sp. in exposed lake sediments of terraces of Lake Yamdrok Yumtso, Tibetan Plateau; C shell of Radix sp. from Lake Chen Co; the hole indicates the location of the sample obtained using a dental drill
Radix is one of the few continental gastropod taxa which inhabit extreme boreal and arctic environments (White et al., 2008). Among the aquatic gastropods, besides Radix, only Gyraulus (Pulmonata, Planorbidae) can be found on the Tibetan Plateau (Taft et al., 2012). The pulmonate gastropod Radix prefers calm, shallow water, such as lakes, wetlands, and slow-flowing sections of streams (Økland, 1990). Gaten (1986) measured the shell growth of Radix during all seasons and observed growth rates of 2.41–2.86 mm per month in summer and 0.35 mm per month in winter. Thus, although shell growth during winter is much slower, it does not cease completely. The life span of Radix is approximately one year (Young, 1975; Glöer, 2002). During the winter months, Radix tend to move from shallow areas to deeper water (Gaten, 1986) or remain active under the ice cover (Glöer, 2002). Protozoa, weeds, cyanobacteria, chlorophyta, and diatoms constitute the primary diet of Radix (Knecht & Walter, 1977; Gittenberger et al., 1998). The pH tolerance range of Radix is from 5.8 to 9.9, while values from 7.0 to 9.6 are optimal (Økland, 1990).
Many lake basins on the Tibetan Plateau are characterized by multiple paleo-shorelines and/or lake terraces (e.g., Li, 2000; Zhao et al., 2003; Zheng et al., 2006; Lee et al., 2009; Long et al., 2012; Pan et al., 2012; Stauch et al., 2014), which potentially provide information on the history of past lake level fluctuations. However, there tends to be a lack of suitable archives for studying the paleohydrology and paleoclimatology of these lakes. Our field investigation indicates that fossil shells of Radix are common in the exposed sediments of lake terraces on the Tibetan Plateau (Fig. 1B). The geochemical information recorded in a single Radix shell may reflect annual hydrological and hydrochemical variations which are controlled by distinct climatic parameters. Thus, the study of fossil Radix shells in the lake terraces is potentially useful for reconstructing the paleohydrology and paleoclimatology of these sites.
In the past few decades, an increasing number of studies have focused on the use of δ 13C and δ 18O records from non-marine mollusk shells to infer changes in climatic and environmental conditions (e.g., Linz & Müller, 1981; Abell & Williams, 1989; Zanchetta et al., 1999; Curry & Fallick, 2002; Jones et al., 2002; Baroni et al., 2006; Tütken et al., 2006; Mii et al., 2012). In addition, a large number of studies have been performed on the trace element geochemistry of gastropod shells (e.g., Faure et al., 1967; Eisma et al., 1976; Dodd & Crisp, 1982; Dettman et al., 1999; Anadón et al., 2006; Carroll & Romanek, 2008). However, geochemical studies of the aragonite shells of Radix, including Sr/Ca ratios and the partition coefficient (Kd Sr), as well as their δ 13C and δ 18O isotope values, are comparatively rare.
Taft et al. (2012, 2013) were the first to demonstrate that Radix shells were a useful archive for reconstructing hydrological and climatic conditions on the Tibetan Plateau. They investigated the sclerochronological δ 13C and δ 18O patterns of the Radix shells on a sub-seasonal scale in several modern lakes across the Tibetan Plateau and discussed the possible mechanisms affecting carbon and oxygen stable isotopes in the shells. Nevertheless, the geochemical characteristics of Radix shells, and their relationship with the hydrochemistry of lakes on the Tibetan Plateau, are largely unknown. In addition, the average elevation of the Tibetan Plateau is above 4 km, and it covers a region of almost 3 million km2 (Royden et al., 2008; Yao et al., 2013); thus, the lake environments on the Tibetan Plateau are diverse and complex. Therefore, further studies are needed to document and improve our understanding of the environmental implications of the Sr/Ca, δ 13C, and δ 18O characteristics of Radix shells in order to provide a sound basis for their use for environmental reconstruction.
The present study is an investigation of modern Radix and its ambient aquatic environment in lakes on the Tibetan Plateau. The objectives were (1) to characterize the modern environments of Radix; (2) to measure the Sr/Ca, Kd Sr, δ 13C, and δ 18O values of the Radix shells and their habitat water; and thereby (3) to assess the potential of geochemical analyses of fossil Radix shells as paleo-hydrochemical and paleo-environmental proxies on the Tibetan Plateau.
Materials and methods
Field sampling
Twenty-two lakes on the southeastern and central Tibetan Plateau were investigated during two field trips from May to October 2013 (Fig. 1A). We collected water samples from all of the lakes and obtained specimens of living Radix (Fig. 1C) from 10 lakes. The specimens were collected from shallow water (<1.5 m) close to the shoreline. In addition, we collected 5 lake water samples for δ 13C measurements of dissolved inorganic carbon (DIC) in the Yamdrok Yumtso lake basin, during July 2015. All water samples were filtered through a 0.45-μm membrane in situ. Samples for δ 13C analysis of DIC were treated with trace amounts of HgCl2 in situ to minimize the influence of microorganisms on the δ 13C values (Wachniew & Różański, 1997). The locations of the lakes are shown in Fig. 1A, and more detailed information about the sampling sites and the measured geochemical parameters are listed in Table 1.
Analytical methods
Hydrochemical analysis
In situ electrical conductivity (EC), pH, and water temperature (T) were measured using a YSI ProPlus Multi-probe Water Quality Sonde made by YSI., USA. The accuracies of the EC, pH, and T measurements are ±0.001, ±0.10, and ±0.2, respectively. The concentrations of dissolved cations (K+, Na+, Mg2+ and Ca2+) were measured by Inductively Coupled Plasma Optical Emission Spectrometry (Prodigy, Teledyne Leeman Labs), with analytical relative standard deviation (RSD) <0.5%. The concentrations of dissolved anions (F−, Cl−, \( {\text{NO}}_{3}^{ - } \) and \( {\text{SO}}_{4}^{2 - } \)) were determined by ion chromatography (Metrohm Compact IC 761), with RSD <1%. The concentrations of \( {\text{HCO}}_{3}^{ - } \) and \( {\text{CO}}_{3}^{2 - } \) were measured by automatic titration (Metrohm), with relative error <1.8% and RSD <1.2%. The concentrations of non-purgeable organic carbon (NPOC), total nitrogen (TN), and DIC in the lake water were measured using a Shimadzu SSM 5000A/TOC-V CPH meter, with RSD <3%. The concentration of Sr was measured by Inductively Coupled Plasma Optical Mass Spectrometry (X-7 series; Thermo Elemental); the detection limit was 0.004 ppb for Sr, and RSD <1%. δ 18O and δD were analyzed using a Wave Scan-Cavity Ring Down Spectrometer (L1102i), and the results are reported using the δ‰ notation relative to V-SMOW; RSD for δ 18O and δD are <0.1‰ and <0.6‰, respectively. The δ 13CDIC was determined using the MAT253 + GasBench at the State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering, Hohai University. The results are reported using the δ‰ notation relative to V-PDB, with RSD <0.2‰.
Analysis of shell geochemistry
Because Radix has not so far been identified to species level on the Tibetan Plateau (Taft et al., 2012), we use Radix sp. to represent all of the species belonging to the genus. The following procedure was used to prepare the shells for geochemical analysis. Firstly, all obvious inorganic and organic material was removed from the shells using a fine brush; secondly, the shells were treated in H2O2 for 24 h to remove any dispersed organic material; and thirdly, the shells were rinsed with ultrapure water in an ultrasonic bath and dried for 6 h at 60°C.
Since juvenile shell portions often may not be in geochemical equilibrium with ambient conditions (e.g., Schöne, 2008), the adult shells were selected for analysis. Two shells of similar shape and length were selected from each sampling site. In order to obtain a mass of 200 ± 50 μg of shell sample for δ 13C and δ 18O analysis, a portion of ca. 0.1–0.5 mm in diameter was removed from the aperture of each shell using a dental drill (Fig. 1C). Taft et al. (2012) suggested that each shell section represents a clearly defined growth period. We therefore assumed that the shell fragment from the aperture (‘shell-aperture’) would record the isotopic characteristics of the water during the last ca. sub-weekly period prior to the date on which the shells were collected. The remaining shells were homogenized with a mortar and pestle and analyzed as powdered bulk samples (‘shell-AVG’).
The δ 13C and δ 18O values of the Radix sp. shell samples were measured using a GasBench II linked to a MAT-253 ThermoFisher Scientific Isotope Ratio Mass Spectrometer at the Key Laboratory of Cenozoic Geology and Environment, Institute of Geology and Geophysics, Chinese Academy of Sciences. Data are reported using the δ‰ notation relative to V-PDB. The instrumental precisions of the isotopic measurements determined from simultaneous replicate analysis of IVA standards are <0.16‰ for δ 18O and <0.07‰ for δ 13C.
Powdered shell samples were dissolved in 5 ml of dilute ultrapure HNO3 acid (3%) for major and trace element analyses. The solutions were analyzed for Ca, Mg, and Sr using Inductively Coupled Plasma Optic Mass Spectrometry (X-7 series; Thermo Elemental). The detection limits were 0.003 ppm for Ca, 0.05 ppm for Mg, and 0.004 ppb for Sr.
Results
Physical and chemical characteristics of the lake water
The hydrochemical parameters of lakes where Radix sp. was found are shown in Table 1. pH and EC range from 7.15 to 9.89 and from 181 to 1731 μS/cm, respectively. T at the time of collection ranged from 12.5 to 19.0°C. The NPOC values ranged from 1.50 to 11.80 ppm, TN values from 0.24 and 1.26 ppm, and DIC values from 8.8 to 160.0 ppm. The Sr/Ca molar ratio (Sr/Ca) ranged from 0.0015 to 0.0156 and the Mg/Ca molar ratio (Mg/Ca) from 0.21 to 18.40. The δ 18O values ranged from −13.6 to −4.2‰ and the δD values from −117.4 to −62.8‰. The δ 13CDIC values ranged from −14.7 to 1.9‰.
The hydrochemical parameters of lakes where Radix sp. were not found are also shown in Table 1. In the case of these sites, pH and EC range from 8.68 to 10.1 and from 269 to 144,842 μS/cm, respectively. Unfortunately, EC of sample RWL130910 (Ranwu lake) was not measured. At the time of collection, T ranged from 8.4 to 16.9°C. The NPOC values range from 0.28 to 48.50 ppm and TN from 0.15 to 3.29 ppm. The DIC values range from 8.2 to 2107.0 ppm. Sr/Ca ranges from 0.0014 to 0.0083 and Mg/Ca from 0.28 to 53.60. The δ 18O values range from −15.9 to −4.0‰ and δD from −113.6 to −53.9‰.
Cation and anion concentrations (ppm) for all the sampled lake waters are listed in Table 2. Piper diagrams (Fig. 2) are used to illustrate the range of water properties of the sampled lakes. In general, the concentrations of most cations and anions exhibit significant variability. Mg2+ and/or Na+ are the dominant cations in all of the sampled lakes, except for Nongzhen Tso, Gongmo Tso, and Ranwu lake, which were Ca-dominated. In contrast, the predominant anion depends on the specific sampling locality. It is noteworthy that the lakes containing living Radix sp. have low Cl− lake water concentrations (Fig. 2A).
Sr concentration in the Radix sp. shells
The analytical data for the Radix sp. shells, and for the corresponding lake waters in which they grew, are listed in Tables 1 and 3. The Sr/Cashell plots against Sr/Cawater, EC, water T, and pH are illustrated in Fig. 3. A significant linear positive correlation is observed between Sr/Cashell and Sr/Cawater and EC (Sr/Cashell versus Sr/Cawater; R 2 = 0.78, n = 15, P < 0.001; Fig. 3A; Sr/Cashell versus EC; R 2 = 0.71, n = 14, P < 0.001; Fig. 3B), while no significant linear correlation is observed between Sr/Cashell and water T and pH (Sr/Cashell versus water T; R 2 = 0.10, n = 15, P = 0.249; Fig. 3C; Sr/Cashell versus water pH; R 2 = 0.08, n = 15, P = 0.318; Fig. 3D). The Sr concentrations in the Radix sp. shells range from 849.5 to 2383.0 ppm. The Sr/Ca values of the shells range from 0.0010 to 0.0028. The partitioning of Sr between the mollusk shell and the ambient water is usually expressed as a partition coefficient, defined as Kd Sr = (Sr/Cashell)/(Sr/Cawater). The Kd Sr values obtained in the present study range from 0.167 to 0.867 with mean Kd Sr = 0.317 (n = 15). Moreover, there is a variable sensitivity evident in the plot of Kd Sr versus Sr/Cawater (Fig. 4).
δ 13C and δ 18O values of the Radix sp. shells
The carbon and oxygen isotopic data for the shell samples are listed in Table 4.
The δ 13C and δ 18O values of both shell-aperture and shell-AVG exhibit significant variability. The δ 13C total values of shell-AVG range from −11.8 to −0.7‰, while those of shell-aperture range from -11.6 to 0.0‰. The relationships between the δ 13C values of the shells and the δ 13CDIC, DIC, and NPOC values of the lake water are illustrated in Fig. 5. A significant positive correlation is observed between both shell-AVG and shell-aperture δ 13C value and the δ 13CDIC of the lake water (δ 13Cshell-AVG versus δ 13CDIC; R 2 = 0.73, n = 10, P = 0.0017; δ 13Cshell-aperture versus δ 13CDIC; R 2 = 0.90, n = 10, P < 0.0001; Fig. 5A). There is a variable sensitivity between the shell δ 13C values and lake water DIC (Fig. 5B). Moreover, the δ 13C of shells tends to decrease with increasing NPOC in lake water (δ 13Cshell-AVG versus NPOC; R 2 = 0.37, n = 28, P < 0.001; δ 13Cshell-aperture versus NPOC; R 2 = 0.26, n = 28, P = 0.005; Fig. 5C). The δ 18O total values of shell-AVG range from −12.1 to −0.9‰, while those of shell-aperture range from −12.4 to 3.3‰. The relationships between the δ 18O values of shell-AVG and shell-aperture and the δ 18O values of the ambient lake water are illustrated in Fig. 6. In both cases, the shell δ 18O values are significantly positively correlated with the corresponding lake water values (δ 18Oshell-AVG versus δ 18Owater; R 2 = 0.90, n = 30, P < 0.0001; δ 18Oshell-aperture versus δ 18Owater; R 2 = 0.81, n = 30, P < 0.0001; Fig. 6).
The relationship between the shell δ 13C values of Radix sp. and lake water δ 13CDIC, and lake water DIC (ppm), and lake water NPOC (ppm). A δ 13Cshell versus δ 13CDIC; B δ 13Cshell versus DIC; C δ 13Cshell versus NPOC. The shell δ 13C and water δ 13CDIC values are reported using the δ‰ notation relative to V-PDB
Discussion
Living environments of Radix sp.
We noted two obvious characteristics of all of the sampling sites where Radix sp. was found: the presence of living aquatic plants in the immediate vicinity, and co-occurrence of the aquatic snail Gyraulus.
In their survey of the occurrence of Radix sp. in Tibetan Plateau lakes, Taft et al. (2013) found that lakes containing living Radix sp. were characterized by pH values ranging from 7.4 to 10.4 and EC values from 142 to 10,300 μs/cm. The EC values of most of our sampling sites lie within these ranges, and therefore, it seems that EC has only a minor influence on the survival of Radix sp. On the other hand, White et al. (2008) noted that Radix sp. inhabit extremely cold lake environments with ice cover for more than half of the year. Taft et al. (2012) concluded that there is no critical water temperature which prevents shell accretion in Radix sp. In the present study, the ranges of pH and T values for lakes with and without Radix sp. were similar, and consequently, we conclude that these two variables have only a minor influence on the survival of Radix sp. on the Tibetan Plateau.
In the case of those lakes in which Radix sp. was not found, the following observations can be made: On the basis of both the measured hydrochemical data and field observations, these lakes were often characterized by high Cl− concentrations (Fig. 2B) and/or by the absence of living aquatic plants in the lake margins. Although the geochemical characteristics of water samples ceh130826, 13# and PM-W-2 (Table 2) are favorable for the growth of Radix sp., in the case of ceh130826 (Cuoe lake), the steeply sloping lake bottom topography may have been disadvantageous, and in lake 13#, the presence of a large number of small white shrimps may indicate that in this case competition with other organisms for food was an important factor. In the case of Puma Yumco (sample PM-W-2), the very high altitude (5030 m) may have been a limiting factor; however, in sample RWL130910 (Ranwu lake), the NPOC, TN, and DIC values were the lowest of all of the sampled sites and thus may have restricted the shell growth of Radix sp.
Based on the foregoing evidence, we suggest that the living environment of Radix sp. is probably controlled by specific lake ecosystem attributes such as temperature, pH, altitude, nutrition (NPOC, TN and DIC values), conductivity, lake bottom topography, and competition for food. Moreover, Radix sp. tend to live in oligohaline to mesohaline water bodies, and thus, the nutrient content of the lake waters may be the main limiting factor for their survival, rather than the high pH values observed in the Tibetan Plateau lakes.
Sr/Ca and Kd Sr values of the Radix sp. shells
Several previous studies have analyzed the Sr/Ca ratio in mollusk shells and have noted significant relationships with temperature (e.g., Dodd, 1965; Surge & Walker, 2006) or salinity (e.g., Wolf et al., 1967; Eisma et al., 1976). On the other hand, many lines of evidence indicate that the Sr/Ca ratio in mollusk shells is controlled by biological factors (e.g., Gillikin et al., 2005; Poulain et al., 2015), such as growth rate (e.g., Takesue & van Geen, 2004) or metabolism (e.g., Purton et al., 1999).
In the present study, the plot of the Sr/Ca ratio of the shells of Radix sp. (Sr/Cashell) versus the corresponding value of the lake water (Sr/Cawater) reveals a significant positive correlation (Fig. 3A), implying that Sr/Cawater is a major factor controlling Sr/Cashell. Sr/Cashell is also well correlated with EC (Fig. 3B). While our results indicate that the aragonite shells formed in a range of values of water T (12.5–19°C), the relationship between Sr/Cashell and water T is unclear (Fig. 3C). Similarly, there is no significant correlation between Sr/Cashell and water pH (Fig. 3D).
Previous studies have reported a range of 0.22 to 0.31 for the partition coefficient of Sr (Kd Sr) between the shells of diverse non-marine aragonitic gastropods and bivalves and the surrounding water (Odum, 1951; Faure et al., 1967; Buchardt & Fritz, 1978; Rosenthal & Katz, 1989; Bailey & Lear, 2006; Anadón et al., 2010). In the present study, the mean Kd Sr for Radix sp. shells is 0.317 (n = 15). It is noteworthy that the MCBN130824 sampling site (Mucuobingni) has the very high Kd Sr value of 0.867 (Table 3). This anomaly probably results from the fact that lake water contains a low Sr concentration (0.034 ppm; Table 3).
Various factors may affect Kd Sr, such as temperature, salinity, and growth rate (Rosenthal & Katz 1989). Only a few workers have discussed the factors affecting Kd Sr variations in the aragonite shells of freshwater mollusks; however, no clear relationship between Kd Sr and water T has been reported for aragonitic shells (Buchardt & Fritz, 1978; Bailey & Lear, 2006; Carroll & Romanek, 2008; Anadón et al., 2010). For Radix sp. shells, our results support previous findings. Nevertheless, Bailey & Lear (2006) and Carroll & Romanek (2008) suggested that in freshwater bivalves, apart from the strong biological control of the trace element incorporation, Kd Sr decreased for values of high Sr/Cawater. In the present study, it is noteworthy that the plot of Kd Sr versus Sr/Cawater reveals a variable sensitivity. For values of Sr/Cawater less than ~0.007, Kd Sr decreases rapidly with increasing Sr/Cawater; however, in the case of Sr/Cawater values above ~0.007 (Table 3), Kd Sr exhibits only a small range of variation (Fig. 4).
δ 13C and δ 18O values of the Radix sp. shells
In view of the fact that the analyzed shell fragments (‘shell-aperture’ in Table 4) were formed prior to sampling, the isotopic characteristics of the lake water during the few weeks prior to the sampling date are clearly reflected by the shell-aperture isotopic data (Taft et al., 2012). A similar pattern is also found in other aragonitic shells (Anadón et al., 2010). In addition, each analyzed powdered bulk sample (‘shell-AVG’ in Table 4) represents the isotopic value of a Radix shell averaged over the life span of the individual.
McConnaughey et al. (1997) inferred that DIC in water can be directly used for aquatic mollusk shell accretion, and that 90% of the carbonate in the shell is derived from this source. The DIC ingested by aquatic mollusks is likely to have high δ 13C values, between −3‰ and +3‰ (Leng & Marshall, 2004). In addition, mollusks also ingest organic matter in the form of algae and plant debris which have low δ 13C values, between −16‰ and −35‰ (Talbot & Johannessen, 1992). The modern Radix sp. shells analyzed in the present study have δ 13C values ranging from −11.8 to 0.0‰. Overall, our data are consistent with those for aquatic mollusks utilizing carbon derived from both the water’s inorganic pool and dietary organic carbon (Fritz & Poplawski, 1974) but are indicative of DIC being the main source. This is further demonstrated by the significant positive correlation between both shell-AVG and shell-aperture δ 13C values and the δ 13CDIC of the lake water (Fig. 5A). This indicates that the δ 13C values of Radix sp. shells mainly reflect the δ 13CDIC of the lake water.
δ 13CDIC in a lake is controlled by photosynthesis, mineralogical substrate, decomposition of organic matter, the isotopic composition of inflowing waters, and exchange with atmospheric CO2 (Yan et al., 2009; Li et al., 2012). Our results indicate that Nongzhen Tso and Gongmo Tso have significantly low δ 13C values in shells and lake water. In addition, both lakes, especially Nongzhen Tso are characterized by an abundance of aquatic vegetation, shallow water depth, and small lake area. The low δ 13C values in lake water DIC probably reflect an abundance of aquatic vegetation which through aerobic decay or respiration adds isotopically light CO2 to the lake water (Fritz et al., 1975).
It is noteworthy that overall the plot of shell δ 13C against water DIC reveals a variable sensitivity. Shell δ 13C changes rapidly during low water DIC at the early stages of the reaction; however, in the case of water DIC values above 33 ppm, the corresponding shell δ 13C values exhibit only a small range of variation (Fig. 5B). In addition, the δ 13C of both shell-AVG and shell-aperture tends to decrease with increasing NPOC in the lake water (Fig. 5C). Therefore, a more reasonable explanation is that the δ 13C of the Radix sp. shells is probably limited by the lake water DIC which is derived from the decomposition of organic matter when the water total DIC content is low (<33 ppm; Fritz & Poplawski, 1974; Fritz et al., 1975; Yan et al., 2009). We suggest, however, that more work is needed to understand the mechanisms by which carbon is incorporated into the Radix sp. shells.
In general, the δ 18O values of freshwater mollusk shells depend primarily on the temperature and oxygen isotopic composition of the water in which they grew (e.g., Fritz & Poplawski, 1974; Leng et al., 1999). Leng & Marshall (2004) have systematically reviewed the controls on the oxygen isotope composition of lacustrine skeletal deposits and illustrate how oxygen isotope studies contribute to an understanding of changes in temperature, precipitation patterns, and evaporation. Over the Tibetan Plateau, the δ 18O value of precipitation reflects information about the interaction between the westerlies and Indian monsoon, combined with local recycling which is characterized by evaporation, convection, and droplet re-evaporation (Yao et al., 2013). The lake water δ 18O is controlled by distinct climatic parameters such as precipitation, evaporation, temperature, humidity, and particular lake system parameters such as size, depth, water residence time, and water composition of inflows (Taft et al., 2012). Monsoon-influenced exoreic lakes display a characteristic monsoonal isotopic pattern which is marked by a negative correlation between shell δ 18O and the amount of rainfall, resembling the ‘amount effect’ in precipitation (Lee & Fung, 2007; Taft et al., 2012). Taft et al. (2013) indicated that the δ 18O values of Radix sp. shells from lakes on the eastern and central Tibetan Plateau reflect monsoon signals.
In the present study, the fractionation factor between δ 18Oshell-AVG and δ 18Owater (α shell-AVG-water) ranges from 1.031 to 1.034 with a mean of 1.033, while the fractionation factor between δ 18Oshell-aperture and δ 18Owater (α shell-aperture-water) ranges from 1.032 to 1.040 with a mean of 1.034. These values are close to the observations on natural samples of Patterson et al. (1993) and to the calculated results for the aragonite–water system by Zheng (1999).
The temperature dependence of the oxygen isotopic fractionation between aragonite (natural biogenic and synthesized aragonite) and water has also been reported (Grossman & Ku, 1986; Patterson et al., 1993; Zhou & Zheng, 2000). With regard to the Radix sp. on the Tibetan Plateau, however, our data suggest that there is no significant relationship between either the α shell-AVG-water or the α shell-aperture-water with the measured ambient water T (103lnα shell-AVG-water = 10.329 × 103/T–3.1357, R 2 = 0.088, n = 30, P = 0.111; 103lnα shell-aperture-water = –16.246 × 103/T + 89.918, R 2 = 0.072, n = 30, P = 0.152). In the present study, lake water conditions during Radix sp. growth were not monitored continuously, and despite the fact that we measured the lake water temperature, these temperature values are unlikely to represent seasonal and/or inter-seasonal average Radix sp. growth conditions. Moreover, we speculate that the effect of temperature on fractionation may be relatively weak for contemporary Radix sp. (Tyler et al., 2008). We suggest that the temperature dependence of the oxygen isotopic fractionation in the aragonite-water system for Radix sp. needs to be further studied.
When δ 18Oshell is plotted against δ 18Owater (Fig. 6), the slopes of the regression line are ≈1. This implies that the changes in δ 18Oshell may predominantly reflect changes in lake water δ 18Owater, for example, due to evaporation or the changing δ 18O of precipitation (Tyler et al., 2008). Thus, our data suggest that the δ 18O values of Radix sp. shells can be used to reflect climatic and hydrologic variability at the large regional scale of the Tibetan Plateau. In addition, the good correlation between shell-aperture δ 18O and the δ 18O of the lake water (Fig. 6) to some degree confirms that the Radix sp. shells can be used as high-resolution archive for analysis of sclerochronological δ 13C and δ 18O patterns in lakes across the Tibetan Plateau.
Conclusions
Our results lead to the following conclusions:
-
1.
Radix sp. tend to live in oligohaline to mesohaline water bodies. The nutritional status of the lake water is possibly the main factor limiting the survival of Radix sp, rather than the high pH values typical of the lakes of the Tibetan Plateau.
-
2.
The observed strong correlation between the Sr/Ca ratio of the Radix sp. shells and that of the ambient waters, as well as with lake water conductivity, makes the Sr/Ca ratio a valuable tool for paleohydrological studies. However, pH and water T have only a minor influence on the Sr/Ca ratio of the Radix sp. shells. The mean Kd Sr value obtained in the present study (0.317, n = 15) is close to the mean values obtained for diverse non-marine aragonitic gastropods and bivalves. In addition, the Kd Sr decreases rapidly when Sr/Cawater is low; however, in the case of Sr/Cawater values above 0.0076, Kd Sr exhibits only a small range of variation.
-
3.
The shell δ 13C of Radix sp. depends on the δ 13C of lake water DIC. The δ 13C values of lake water DIC are primarily controlled by that of the aqueous carbonate species. Our results suggest that the contribution of DIC originated from the degradation of organic matter to the Radix sp. shells increases when the water is deficient in DIC of inorganic origin.
-
4.
The oxygen isotope ratios of the shells of Radix sp. provide information on the isotopic composition of the water in which the shells were formed, which in turn relates to the climatic conditions prevailing during the life span of individuals of Radix sp.
-
5.
Overall, the fossil shells of the gastropod Radix sp. in the terraces of the lakes on the Tibetan Plateau are a potentially valuable archive for reconstructing the paleohydrology and paleoclimatology of the region.
References
Abell, P. I. & M. A. J. Williams, 1989. Oxygen and carbon isotope ratios in gastropod shells as indicators of paleoenvironments in the Afar region of Ethiopia. Palaeogeography, Palaeoclimatology, Palaeoecology 74: 265–278.
Anadón, P., A. Moscariello, J. Rodríguez-Lázaro & M. L. Filippi, 2006. Holocene environmental changes of Lake Geneva (Lac Léman) from stable isotopes (δ 13C, δ 18O) and trace element records of ostracod and gastropod carbonates. Journal of Paleolimnology 35: 593–616.
Anadón, P., M. Martín-Rubio, F. Robles, J. Rodriguez-Lázaro, R. Utrilla & A. Vázquez, 2010. Variation in Sr uptake in the shell of the freshwater gastropod Bithynia tentaculata from Lake Arreo (northern Spain) and culture experiments. Palaeogeography, Palaeoclimatology, Palaeoecology 288: 24–34.
Bailey, T. R. & C. H. Lear, 2006. Testing the effect of carbonate saturation on the Sr/Ca of biogenic aragonite: a case study from the River Ehen, Cumbria, UK. Geochemistry, Geophysics, Geosystems 7: 1–6.
Baroni, C., G. Zanchetta, A. E. Fallick & A. Longinelli, 2006. Mollusca stable isotope record of a core from Lake Frassino, northern Italy: hydrological and climatic changes during the last 14 ka. The Holocene 16: 827–837.
Buchardt, B. & P. Fritz, 1978. Strontium uptake in shell aragonite from the freshwater gastropod Lymnaea stagnalis. Science 199: 191–192.
Carroll, M. & C. S. Romanek, 2008. Shell layer variation in trace element concentration for the freshwater bivalve Elliptio complanata. Geo-Marine Letters 28: 369–381.
Curry, G. B. & A. E. Fallick, 2002. Use of stable oxygen isotope determinations from brachiopod shells in palaeoenvironmental reconstruction. Palaeogeography, Palaeoclimatology, Palaeoecology 182: 133–143.
Dettman, D. L., A. K. Reische & K. C. Lohmann, 1999. Controls on the stable isotope composition of seasonal growth bands in aragonitic fresh-water bivalves (Unionidae). Geochimica et Cosmochimica Acta 63: 1049–1057.
Dodd, J. R., 1965. Environmental control of strontium and magnesium in Mytilus. Geochimica et Cosmochimica Acta 29: 385–398.
Dodd, J. R. & E. L. Crisp, 1982. Non-linear variation with salinity of Sr/Ca and Mg/Ca ratios in water and aragonitic bivalve shells and implications for paleosalinity studies. Palaeogeography, Palaeoclimatology, Palaeoecology 38: 45–56.
Eisma, D., W. G. Mook & H. A. Das, 1976. Shell characteristics, isotopic composition and trace-element contents of some euryhaline molluscs as indicators of salinity. Palaeogeography, Palaeoclimatology, Palaeoecology 19: 39–62.
Faure, G., J. H. Crocket & P. M. Hueley, 1967. Some aspects of the geochemistry of strontium and calcium in the Hudson Bay and the Great Lakes. Geochimica et Cosmochimica Acta 31: 451–461.
Fritz, P. & S. Poplawski, 1974. 18O and 13C in the shells of freshwater molluscs and their environments. Earth and Planetary Science Letters 24: 91–98.
Fritz, P., T. W. Anderson & C. F. M. Lewis, 1975. Late Quaternary climatic trends in history of Lake Erie from stable isotope studies. Science 190: 267–269.
Gaten, E., 1986. Life cycle of Lymnaea peregra (Gastropoda: Pulmonata) in the Leicester canal, U.K., with an estimate of annual production. Hydrobiologia 135: 45–54.
Gillikin, D. P., A. Lorrain, J. Navez, J. W. Taylor, L. André, E. Keppens, W. Baeyens & F. Dehairs, 2005. Strong biological controls on Sr/Ca ratios in aragonitic marine bivalve shells. Geochemistry, Geophysics, Geosystems 6: 1–16.
Gittenberger, E., A. W. Janssen, W. J. Kuijper, J. G. J. Kuiper, T. Meijer, G. van der Velde & J. N. de Vries, 1998. De Nederlandse zoetwatermollusken: recente en fossiele weekdieren uit zoet en brak water. In: Nederlandse Fauna 2. Nationaal Natuurhistorisch Museum Naturalis, KNNV Uitgeverij & EIS-Nederland, Leiden.
Glöer, P., 2002. Süsswassergastropoden Nord-und Mitteleuropas: Bestimmungsschlüssel, Lebensweise, Verbreitung. ConchBooks, Hackenheim.
Grossman, E. L. & T.-L. Ku, 1986. Oxygen and carbon isotope fractionation in biogenic aragonite: temperature effects. Chemical Geology 59: 59–74.
Jones, M. D., M. J. Leng, W. J. Eastwood, D. H. Keen & C. S. M. Turney, 2002. Interpreting stable-isotope records from freshwater snail-shell carbonate: a Holocene case study from Lake Gölhisar, Turkey. The Holocene 12: 629–634.
Knecht, V. A. & J. E. Walter, 1977. Vergleichende Untersuchung der Diäten von Lymnaea auricularia und L. peregra (Gastropoda: Basommatophora) im Zürichsee. Schweizerische Zeitschrift für Hydrologie 39: 299–305.
Lee, J. E. & I. Fung, 2007. “Amount effect” of water isotopes and quantitative analysis of post-condensation processes. Hydrological Processes 22: 1–8.
Lee, J., S. H. Li & J. C. Aitchison, 2009. OSL dating of paleoshorelines at Lagkor Tso, western Tibet. Quaternary Geochronology 4: 335–343.
Leng, M. J. & J. D. Marshall, 2004. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quaternary Science Reviews 23: 811–831.
Leng, M. J., A. L. Lamb, H. F. Lamb & R. J. Telford, 1999. Palaeoclimatic implications of isotopic data from modern and early Holocene shells of the freshwater snail Melanoides tuberculata, from lakes in the Ethiopian Rift Valley. Journal of Paleolimnology 21: 97–106.
Li, B. Y., 2000. The last greatest lakes on the Xizang (Tibetan) Plateau. Acta Geographica Sinica 55: 182–188 (in Chinese with English abstract).
Li, X., W. Liu & L. Xu, 2012. Carbon isotopes in surface-sediment carbonates of modern Lake Qinghai (Qinghai–Tibet Plateau): implications for lake evolution in arid areas. Chemical Geology 300–301: 88–96.
Linz, E. & G. Müller, 1981. Isotopen-geochemische Untersuchungen an Mollusken-Schalen verschiedener Seen Mitteleuropas. Tschermaks mineralogische und petrographische Mitteilungen 29: 55–65.
Liu, Y.-Y., 1963. On the freshwater pulmonata from shigatze and gyangtse regions in Tibet, China. Acta Zoologica Sinica 1: 71–78 (in Chinese with English abstract).
Long, H., Z. P. Lai, P. Frenzel, M. Fuchs & T. Haberzettl, 2012. Holocene moist period recorded by the chronostratigraphy of a lake sedimentary sequence from Lake Tangra Yumco on the south Tibetan Plateau. Quaternary Geochronology 10: 136–142.
Ma, R. H., G. S. Yang, H. T. Duan, J. H. Jiang, S. M. Wang, X. Z. Feng, A. N. Li, F. X. Kong, B. Xue & J. L. Wu, 2011. China’s lakes at present: number, area and spatial distribution. Science China Earth Sciences 54: 283–289.
McConnaughey, T. A., J. Burdett, J. F. Whelan & C. K. Paull, 1997. Carbon isotopes in biological carbonates: respiration and photosynthesis. Geochimica et Cosmochimica Acta 61: 611–622.
Mii, H. S., G. R. Shi, C. J. Cheng & Y. Y. Chen, 2012. Permian Gondwanaland paleoenvironment inferred from carbon and oxygen isotope records of brachiopod fossils from Sydney Basin, southeast Australia. Chemical Geology 291: 87–103.
Montfort, P. D. D., 1810. Conchyliologie systématique, et classification méthodique des coquilles; offrant leurs figures, leur arrangement générique, leurs descriptions caractéristiques, leurs noms; ainsi que leur synonymie en plusieurs langues. F. Schoell, Paris.
Odum, H., 1951. The uptake of Sr by freshwater snail (Physa sp.). Science 144: 407–411.
Økland, J., 1990. Lakes and Snails: Environment and Gastropoda in 1,500 Norwegian Lakes, Ponds and Rivers. Universal Book Services/Dr. W. Backhuys, Oegstgeest.
Pan, B. L., C. L. Yi, T. Jiang, G. C. Dong, G. Hu & Y. Jin, 2012. Holocene lake-level changes of Linggo Co in central Tibet. Quaternary Geochronology 10: 117–122.
Patterson, W. P., G. R. Smith & K. C. Lohmann, 1993. Continental paleothermometry and seasonality using the isotopic composition of aragonitic otoliths of freshwater fishes. In: Swart, P. K., C. K. Lohmann, J. Mckenzic, S. Savin (eds), Climate Change in Continental Isotopic Records. American Geophysical Union, 78:191–202.
Poulain, C., D. P. Gillikin, J. Thebault, J.-M. Munaron, M. Bohn, R. Robert, Y.-M. Paulet & A. Lorrain, 2015. An evaluation of Mg/Ca, Sr/Ca, and Ba/Ca ratios as environmental proxies in aragonite bivalve shells. Chemical Geology 396: 42–50.
Purton, L. M. A., G. A. Shields, M. D. Brasier & G. W. Grime, 1999. Metabolism controls Sr/Ca ratios in fossil aragonitic mollusks. Geology 27: 1083–1086.
Rosenthal, Y. & A. Katz, 1989. The applicability of trace elements in freshwater shells for paleogeochemical studies. Chemical Geology 78: 65–76.
Royden, L. H., B. C. Burchfiel & R. D. van der Hilst, 2008. The geological evolution of the Tibetan Plateau. Science 321: 1054–1058.
Schöne, B., 2008. The curse of physiology—challenges and opportunities in the interpretation of geochemical data from mollusk shells. Geo-Marine Letters 28: 269–285.
Stauch, G., S. Pötsch, H. Zhao & F. Lehmkuhl, 2014. Interaction of geomorphological processes on the north-eastern Tibetan Plateau during the Holocene, an example from a sub-catchment of Lake Donggi Cona. Geomorphology 210: 23–35.
Surge, D. & K. J. Walker, 2006. Geochemical variation in microstructural shell layers of the southern quahog (Mercenaria campechiensis): implications for reconstructing seasonality. Palaeogeography, Palaeoclimatology, Palaeoecology 237: 182–190.
Tütken, T., T. W. Vennemann, H. Janz & E. P. J. Heizmann, 2006. Palaeoenvironment and palaeoclimate of the Middle Miocene lake in the Steinheim basin, SW Germany: a reconstruction from C, O, and Sr isotopes of fossil remains. Palaeogeography, Palaeoclimatology, Palaeoecology 241: 457–491.
Taft, L., U. Wiechert, F. Riedel, M. Weynell & H. C. Zhang, 2012. Sub-seasonal oxygen and carbon isotope variations in shells of modern Radix sp. (Gastropoda) from the Tibetan Plateau: potential of a new archive for palaeoclimatic studies. Quaternary Science Reviews 34: 44–56.
Taft, L., U. Wiechert, H. C. Zhang, G. L. Lei, S. Mischke, B. Plessen, M. Weynell, A. Winkler & F. Riedel, 2013. Oxygen and carbon isotope patterns archived in shells of the aquatic gastropod Radix: hydrologic and climatic signals across the Tibetan Plateau in sub-monthly resolution. Quaternary International 290: 282–298.
Takesue, R. K. & A. van Geen, 2004. Mg/Ca, Sr/Ca, and stable isotopes in modern and Holocene Protothaca staminea shells from a northern California coastal upwelling region. Geochimica et Cosmochimica Acta 68: 3845–3861.
Talbot, M. R. & T. Johannessen, 1992. A high resolution palaeoclimatic record for the last 27,500 years in tropical West Africa from the carbon and nitrogen isotopic composition of lacustrine organic matter. Earth and Planetary Science Letters 110: 23–37.
Tyler, J. J., M. J. Leng, H. J. Sloane, D. Sachse & G. Gleixner, 2008. Oxygen isotope ratios of sedimentary biogenic silica reflect the European transcontinental climate gradient. Journal of Quaternary Science 23: 341–350.
von Oheimb, P. V., C. Albrecht, F. Riedel, L. Du, J. X. Yang, D. C. Aldridge, U. Bossneck, H. C. Zhang & T. Wilke, 2011. Freshwater biogeography and limnological evolution of the Tibetan Plateau–insights from a plateau-wide distributed gastropod taxon (Radix spp.). PloS one 6: e26307.
Wachniew, P. & K. Różański, 1997. Carbon budget of a mid-latitude, groundwater-controlled lake: isotopic evidence for the importance of dissolved inorganic carbon recycling. Geochimica et Cosmochimica Acta 61: 2453–2465.
White, D., R. C. Preece, A. A. Shchetnikov, S. A. Parfitt & K. G. Dlussky, 2008. A Holocene molluscan succession from floodplain sediments of the upper Lena River (Lake Baikal region), Siberia. Quaternary Science Reviews 27: 962–987.
Wolf, K. H., G. V. Chilingar & F. W. Beales, 1967. Elemental composition of carbonate skeletons, minerals, and sediments. Developments in Sedimentology 9: 23–149.
Yan, H., X. Q. Lee, H. Zhou, H. G. Cheng, Y. Peng & Z. H. Zhou, 2009. Stable isotope composition of the modern freshwater bivalve Corbicula fluminea. Geochemical Journal 43: 379–387.
Yao, T. D., V. Masson-Delmotte, J. Gao, W. S. Yu, X. X. Yang, C. Risi, C. Sturm, M. Werner, H. B. Zhao, Y. He, W. Ren, L. D. Tian, C. Shi & S. G. Hou, 2013. A review of climatic controls on δ 18O in precipitation over the Tibetan Plateau: observations and simulations. Reviews of Geophysics 51: 525–548.
Young, M. R., 1975. The life cycles of six species of freshwater molluscs in the Worcester-Birmingham canal. Journal of Molluscan Studies 41: 533–548.
Zanchetta, G., F. P. Bonadonna & G. Leone, 1999. A 37-meter record of paleoclimatological events from stable isotope data on continental molluscs in Valle di Castiglione, Near Rome, Italy. Quaternary Research 52: 293–299.
Zhao, X. T., D. G. Zhu, F. H. Yan, Z. H. Wu, Z. B. Ma & X. S. Mai, 2003. Climate change and lake-level variation of Nam Co, Xizang since the last interglacial stage. Quaternary Sciences 23: 41–52 (in Chinese with English abstract).
Zhao, W., M. P. Zheng, X. Z. Xu, X. F. Liu, G. L. Guo & Z. H. He, 2005. Biological and ecological features of saline lakes in northern Tibet, China. Hydrobiologia 541: 189–203.
Zheng, M. P., H. R. Yuan, X. T. Zhao & X. F. Liu, 2006. The Quaternary pan-lake (overflow) period and paleoclimate on the Qinghai-Tibet Plateau. Acta Geologica Sinica 79: 821–834.
Zheng, Y. F., 1999. Oxygen isotope fractionation in carbonate and sulfate minerals. Geochemical Journal 33: 109–126.
Zhou, G. T. & Y. F. Zheng, 2000. Experimental studies of oxygen isotope fractionation factors between aragonite and water at low temperatures. Geological Journal of China Universities 6: 89–105 (in Chinese with English abstract).
Acknowledgments
We wish to express our thanks to Yue-Ying Liu, Zhi-Guo Su, Shao-Peng Gao, Dong-Mei Qu, and Xiao-Ming Liu for their help with the laboratory analyses; Junbo Wang and Lei Huang for their part assistance during field sampling; and Ruimin Yang for her help in plotting the location graph. We thank Dr. Jan Bloemendal for linguistic improvements. We also thank two anonymous reviewers for their critical comments. This study was supported by the National Basic Research Program of China (973 Program, Grant No. 2012CB956101) and the Knowledge Innovation Program of the Chinese Academy of Sciences (Grant No. KZCX2-EW-113).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Jasmine Saros
Rights and permissions
About this article
Cite this article
Chen, F., Feng, JL. & Hu, HP. Relationship between the shell geochemistry of the modern aquatic gastropod Radix and water chemistry of lakes of the Tibetan Plateau. Hydrobiologia 771, 239–254 (2016). https://doi.org/10.1007/s10750-015-2634-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2634-1