Abstract
We conducted a fish survey in 40 lakes in western and central Turkey. Fifty species (one to eleven per lake) were recorded, including eighteen endemic and seven alien species. We investigated which local geo-climatic and other environmental variables shaped the fish assemblages. Altitude and temperature turned out to be the most important factors for total species richness as well as richness of omnivorous and zooplanktivorous species and the Shannon–Wiener diversity index, with more species and higher diversity occurring in the warmer lowland lakes. Altitude may affect the fish assemblage directly through dispersal limitation or indirectly by creating a gradient in temperature with which it was strongly correlated. Cyprinidae was the most species-rich and widespread family. Atherinidae, Gobiidae, and Mugilidae (families of marine origin) were mainly found in the lowland regions, while Salmonidae exclusively appeared in the high-altitude lakes. The presence of widely distributed translocated native and alien species revealed a large human impact on the fish assemblages, potentially threatening the rich endemic fish fauna in lakes in this region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies worldwide indicate that species richness (including fish) and diversity in lakes increase with decreasing latitude [for fish: e.g., in Europe (Griffiths, 2006; Brucet et al., 2013); North America Barbour & Brown, 1974; and South America (Cussac et al., 2009)], though such gradients are weaker in freshwater than in marine or terrestrial environments (Hillebrand, 2004). Fish assemblages in warmer regions also exhibit higher abundance and richness of omnivorous species (Winemiller, 1990; Teixeira-de Mello et al., 2009) and the proportion of omnivorous species increases with decreasing latitude (González-Bergonzoni et al., 2012). Like latitude, altitude also provides gradients in environmental conditions, including temperature and precipitation, and creates geographical barriers to colonization (Drakou et al., 2009). Species richness is typically reduced with increasing altitude (Zhao et al., 2006; Volta et al., 2011), reflecting a combined effect of physical barriers (colonization restrictions) and climatic factors, such as potential evapotranspiration, with the latter likely being the most important (Zhao et al., 2006). Moreover, larger lakes typically host more fish species than smaller ones (Amarasinghe & Welcomme, 2002; Brucet et al., 2013), although the effect is sometimes less important than the altitude effect (Zhao et al., 2006).
Turkey forms a crossway between Asia, Africa, and Europe and is thus placed between the mid-latitude temperate and the subtropical climate zone, and due to its topography and orography the country exhibits large differences in local climate (Iyigun et al., 2013). There are thirteen freshwater ecoregions in Turkey (Abell et al., 2008) and the country has a rich freshwater fish fauna with 248 native species in inland waters (Fricke et al., 2007), including more than 70 endemic species (Küçük, 2006; Fricke et al., 2007) that belong, among others, to the genera Aphanius, Cobitis, Pseudophoxinus, and Squalius (Balik, 1995; Erk’akan et al., 1999; Wildekamp et al., 1999; Freyhof & Özuluğ, 2009a, b, 2010; Özuluğ & Freyhof, 2011). Actually, the number of species is most likely higher as several new species have been identified and described in recent years (e.g., Turan et al., 2009; Özuluğ & Freyhof, 2011). The Turkish freshwater fauna is, however, threatened by various anthropogenic alterations, including alteration of hydrology (e.g., irrigation and damming), eutrophication, and introduction of new species (Fricke et al., 2007; Şekercioğlu et al., 2011).
Despite the remarkable differences in climate and topography within Turkey and the country’s high biodiversity, there is a lack of studies evaluating whether the key drivers of fish assemblage composition from other parts of the world also apply to the endemic-rich fish assemblages in Turkish lakes. We investigated how fish richness and diversity in 40 lakes located in the western half of Turkey were related to geo-climatic gradients, i.e., geographical and climatic gradients such as latitude, longitude, altitude, lake area, temperature, and precipitation as well as other environmental variables. Besides total and native species richness and diversity, our study encompassed information on introduced and endemic species, and feeding functional groups of fish.
Our hypothesis was that fish species richness and diversity would be lower in highland lakes, largely because of physical barriers and low temperatures compared to the lowland lakes where connectedness and low temperature are not an obstacle to fish colonization. We further hypothesized that the number of omnivorous fish species would be higher in the warmer lowland lakes, while the density of zooplanktivorous fish species would be related to the abundance of vegetation since many of them are small-bodied and may find refuge against piscivores within macrophyte beds (Persson & Eklöv, 1995; Meerhoff et al., 2007).
Methods
Study sites
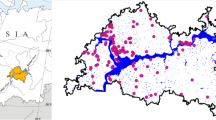
Our study included 40 lakes, widely distributed across the western half of Turkey (Fig. 1) and covering five latitudes (36° 70′ to 42° 01′), nine longitudes (27° 22′ to 36° 16′), and several climatic ecoregions. The westernmost part of the study area, including the freshwater ecoregions of Thrace, Western Anatolia, the Turkish part of Dniester-Lower Danube (Fig. 1), as well as the coastal lowland along the Mediterranean Sea, has a warm temperate climate with dry and hot summers according to the Köppen–Geiger classification system (Kottek et al., 2006). Northern Anatolia, situated along and close to the coastline of the Black Sea, with the Northern Anatolian Mountains, has a warm temperate and fully humid climate with warm summers in the highland and hot summers in the coastal lowland. South of these mountains the Anatolian Plateau stretches out, covering parts of the Northern and Central Anatolian freshwater ecoregions (Fig. 1) before it reaches the Taurus Mountains along the Mediterranean Sea in Southern Anatolia. The climate of this central part of the Anatolian Plateau is not only mostly warm temperate with dry and warm summers, but it also includes areas with an arid climate dominated by cold arid steppe (Kottek et al., 2006).
Map showing Turkey and the distribution of the 40 lakes included in the study. The Freshwater Fish Ecoregions from Abell et al. (2008) are shown and the names of these freshwater ecoregions in the western part of Turkey are given as abbreviations: LD Dniester-Lower Danube, Th Thrace, NA Northern Anatolia, CA Central Anatolia, WA Western Anatolia. Lake names (1) Sarıkum, (2) Erikli, (3) Mert, (4) Pedina, (5) Hamam, (6) Saka, (7) Gici, (8) Tatli, (9) Serin, (10) Büyük, (11) Derin, (12) Ince, (13) Nazli, (14) Koca, (15) Poyrazlar, (16) Keçi, (17) Gerede, (18) Gölcük (B), (19) Çubuk, (20) Karagöl (B), (21) Kaz, (22) Eymir, (23) Mogan, (24) Seyfe Göleti, (25) Gölcuk (S), (26) Emre, (27) Gök Göl, (28) Karagol (İ), (29) Kaya, (30) Balıklı, (31) Gölcük (Ö), (32) Eğri, (33) Sarp, (34) Yayla, (35) Barutçu, (36) Gebekirse, (37) Saklı, (38) Karagöl (D), (39) Gölhisar, (40) Baldımaz
The altitudinal positions of the lakes ranged from 0.3 to 1423 m (median 982 m) with lowland lakes located in the coastal areas along the Black and the Aegean Seas and highland lakes in the mountains as well as in the mid-Anatolian Plateau. The large geographical area and large altitude gradients covered imply great differences in local climate. Climate data (30 arc-seconds resolution) obtained from WorldClim-Global Climate Data (www.worldclim.org) showed that the annual average air temperature in the lake areas ranged from 8.3 to 17.7°C (median 11.2°C). Seasonality, measured as the temperature difference between mean temperature in the coldest (January) and the warmest (July) months, ranged from 15.8 to 22.4°C (median 19.2°C); lowland and highland lakes having a seasonality of ≤19.4 and ≥18.2°C, respectively. Average annual precipitation ranged from 355 to 1017 mm with a median of 611 mm. The lakes ranged in size from 0.1 to 635 ha (median: 12 ha), only five of them were >100 ha. The lakes were mostly shallow with a median maximum depth of 3.3 m. Maximum lake depth ranged from 0.6 to 15.2 m, and only seven lakes were deeper than 6 m.
Fish survey
The lakes were sampled once in summer (July and August) during the period from 2006 to 2012. For the fish surveys, we used Nordic benthic multi-mesh-size gillnets, each containing 12 sections with different mesh sizes including 5.0, 6.25, 8.0, 10.0, 12.5, 15.5, 19.5, 24.0, 43.0, and 55.0 mm. Each section had a length of 2.5 m, yielding a total length of the survey net of 30 m with a height of 1.5 m. The survey nets were placed at the bottom in both the littoral and pelagic zones of the lakes and parallel to the shore. Half of the nets were placed in the littoral and the other half in the pelagic zone and left overnight for 12 h from dusk to dawn. The number of nets per lake depended on the lake area and was two (one littoral and one pelagic) in lakes <2 ha, four in lakes between 2 and 20 ha, six in lakes between 20 and 100 ha, and eight in the five largest lakes (>100 ha).
The fish removed from the nets were identified to species level, weighed, and the fork length measured. For fish without forked caudal fin, such as Cobitidae and Cyprinodontidae, total length was used. The number per unit effort (NPUE) and biomass (g) per unit effort (BPUE) were calculated as catch per net per night.
We divided the fish species into size classes—small (<10 cm), medium (≥10–20 cm), or large (≥20 cm)—based on fork/total length and feeding preference for each of these size classes was determined for each species based on the information found in the literature (Ünver & Erk’akan, 2011; Yalçın-Özdilek et al., 2013), the Fish-Base database (Froese & Pauly, 2013), and the Atlas of Danish Freshwater Fish (Carl & Møller, 2012). Based on the dominant feeding preferences for each of the three size classes, fish were roughly divided into feeding functional groups as omnivorous, zooplanktivorous, piscivorous, and benthivorous. If the different size classes were attributed to different feeding functional groups, they were classified to these groups, but each fish species was only counted once in each feeding functional group even if the species had the same dominant feeding preferences in two or three size classes. Omnivorous fish species were defined as those including plant or algal material as a substantial part of their diet, benthivorous species as those mainly feeding on benthic invertebrates, and zooplanktivorous species as those mainly feeding on zooplankton, i.e., many small-sized fish. The piscivorous species included species and size classes that mainly feed on fish, i.e., medium- or large-sized individuals of Perca fluviatilis L. 1758, Sander lucioperca (L. 1758), Salmo abanticus Tortonese 1954, Oncorhynchus mykiss (Walbaum 1792), and Squalius spp.
Information on the current status of each fish species was evaluated from the available literature on endemic species (Balik, 1995; Erk’akan et al., 1999; Wildekamp et al., 1999; Kuru, 2004; Özuluğ & Freyhof, 2011), and alien and translocated species (Innal & Erk’akan, 2006; Innal, 2012; Tarkan et al., 2015). How the introduced species are defined varies (Gozlan et al., 2010), but in this study “alien” refers to species whose natural distribution range is outside Turkey and “translocated” species are native species that are translocated within Turkey. For example, S. abanticus is endemic to Turkey and native only to Lake Abant but has been translocated to other lakes (Innal, 2012). For this reason, S. abanticus was considered as translocated as Lake Abant was not among the 40 study lakes. It is unclear whether Carassius carassius (L. 1758) is introduced (Innal, 2012; Tarkan et al., 2015) or translocated (Innal & Erk’akan, 2006) in Turkey, but in the current study it was considered translocated. The natural distribution range of Atherina boyeri Risso 1810, Cyprinus carpio L. 1758, Tinca tinca (L. 1758), P. fluviatilis, and S. lucioperca was roughly estimated based on the maps of Tarkan et al. (2015), and the species were considered translocated outside this range. Liza ramada (Risso 1827) and Mugil cephalus L. 1758 are also being translocated in Turkey (Innal & Erk’akan, 2006; Innal, 2012), but we considered them as native in lakes placed in close connection to the sea.
Other variables
Various environmental variables (total phosphorous (TP), total nitrogen (TN), chlorophyll a, Secchi depth, submerged macrophytes, and salinity) were sampled in each lake at the same time (July and August) and year as the fish survey. Details of the sampling procedure and environmental data can be found in Çakiroğlu et al. (2014) and Levi et al. (2014). Several of the lakes are subject to anthropogenic eutrophication so the trophic state of the lakes ranged from mesotrophic with a TP concentration of 18 µg/l to hypereutrophic with a TP of 402 µg/l. Median TP was 72 µg/l. Total nitrogen ranged from 264 to 3250 µg/l (median 964 µg/l) and chlorophyll a ranged from 4.7 to 181 µg/l (median 16.5 µg/l). The clearest lake had a Secchi depth of 4.1 m. However, median Secchi depth was only 0.9 m and the most turbid lakes had Secchi depths of only 0.2–0.3 m. Macrophyte coverage and average macrophyte height were measured in the field at several points on transects across each lake (Levi et al., 2014). Based on these measurements and lake depth, the percentage of a lake’s total volume inhabited by macrophytes (PVI) was subsequently calculated (sensu Canfield et al., 1984). Submerged macrophytes were abundant in some lakes, with PVI reaching a maximum of 80%, while other lakes were without submerged macrophytes. Median PVI across all lakes was 6.9%. Six lakes had salinities ≥3.0‰, the highest being 14.5‰. The rest of the lakes had salinities <1.5‰; the lowest value measured was 0.06‰ and median salinity was 0.30‰.
Statistical analyses
From an initial set of relevant geo-climatic and other environmental variables (i.e., those mentioned above), a subset of explanatory variables was selected using Spearman correlation matrix (Table 1) and the variance inflation factor (VIF). This procedure was used to avoid including redundant (strongly correlated) variables in the models. A correlation factor >0.6 was considered strong, and from each correlation pair only the variable with the lowest VIF was used in the analyses. Each subset included latitude, longitude, lake area, max depth, Secchi ratio, salinity, PVI, and either of the correlated pairs: altitude or temperature and precipitation or seasonality. As chlorophyll a was strongly correlated with both TP and TN, each subset included either chlorophyll a or both TP and TN. Before the analysis, explanatory variables with a skewness >1 were log-transformed [i.e., lake area, max depth, salinity, TN, TP, and chlorophyll a: log10(x); PVI: log10(x + 1)]. The remaining variables had skewness values in the range from −0.57 to 0.73 and were not transformed.
Generalized linear models (GLM) (McCullagh & Nelder, 1989) were applied to assess the effect of the selected variables on fish assemblages, i.e., species richness, Shannon–Wiener diversity index (H’), and number of species belonging to each feeding functional group. The Shannon–Wiener diversity index was calculated based on data on total fish density (NPUE) using the ‘vegan’ R package (Oksanen et al., 2013) and was analyzed using GLM with a Gaussian error distribution and an identity link function. For data on species richness (i.e., the total number of species in each lake) as well as the number of species belonging to each feeding functional group, Poisson error distribution and a logarithmic link function were used in the GLMs.
For each fish assemblage variable, the full model was used and the variation explained by each GLM was given as either adjusted R 2 or adjusted pseudo R 2. For the Shannon–Wiener diversity index, which showed a Gaussian error distribution, we calculated the adjusted R 2. For models assuming Poisson error distribution, the variation explained by each GLM was estimated by calculating an adjusted pseudo R 2: 1-((residual deviance + k × φ)/null deviance), where k is the number of explanatory variables and φ is the dispersion parameter. This pseudo R 2 calculation is adjusted for potential over- or under-dispersion in accordance with Heinzl & Mittlböck (2003). The dispersion parameter, φ, can be estimated by the generalized Pearson statistic, χ 2, divided by the degrees of freedom, i.e., φ = χ 2/(n − k − 1) (Heinzl & Mittlböck, 2003). Furthermore, to evaluate the relative importance of each explanatory variable in explaining the variation in each fish assemblage variable, Akaike information criterion modified small sample size (AICc) (Burnham & Anderson, 2002) was used. AICc was calculated for each submodel derived from the full model of each fish assemblage variable. Subsequently, the relative importance of explanatory variables was estimated by summing the normalized model likelihoods (‘Akaike weights’) for each explanatory variable across all submodels in which the respective variable occurred. Thus, the larger the sum, the more important was the variable compared to other variables (Burnham & Anderson, 2002). Calculations were done by the use of the R package ‘MuMIn’ (Barton, 2015). All statistical analyses were carried out using R version 3.2.0 (R Core Team, 2015).
Results
Fifty fish species from 33 genera and 13 families were caught in the 40 lakes. NPUE ranged between 1.5 and 1425 ind./net (median 53 ind./net), and BPUE ranged between 116 and 6251 g/net (median 1119 g/net). Cyprinidae was clearly the most species-rich, comprising 28 species of which 11 were endemic, and the most widespread family, occurring in 39 lakes (Table 2). We found four species, including three endemic species, belonging to Cobitidae and three species to Gobiidae, while Cyprinodontidae, Mugilidae, Nemacheilidae, Percidae, and Salmonidae were represented by two species each. The rest of the fish families included only one species each (Table 2). Some fish families, such as Mugilidae, Gobiidae, and Cichlidae, were only found in lowland lakes, while others, for instance Salmonidae, were only found in high-altitude lakes.
Eighteen (without S. abanticus) of the 50 species recorded are endemic to Turkey (Table 3). Some of these species [Cobitis simplicispina Hankó 1925, Cobitis turcica Hankó 1925, Capoeta baliki Turan, Kottelat, Ekmekçi & Imamoglu 2006, Chondrostoma meandrense Elvira 1987, Gobio gymnostethus Ladiges 1960, Gobio sakaryaensis Turan, Ekmekçi, Luskova & Mandel 2012, Oxynoemacheilus kosswigi (Erk’akan & Kuru 1986) and Squalius fellowesii (Günther 1868)] were only found in one lake each, and none of the endemic species occurred in more than four of the lakes (Table 3). A total of six translocated native species were found, but—apart from C. carpio, and T. tinca—only in relatively few lakes (Table 3). The species found in the largest number of lakes (24 lakes) was C. carpio, while T. tinca was found in 12 lakes. Atherina boyeri was found in 11 lakes but was considered native in eight of them. Another seven species were alien. Among these, Carassius gibelio Bloch 1782 was found in the largest number of lakes (16 lakes), while the remaining species, including Carassius auratus (L. 1758), Pseudorasbora parva (Temminck & Schlegel 1846), Gambusia holbrooki Girard 1859, Lepomis gibbosus (L. 1758), Tilapia zillii (Gervais 1848), and O. mykiss, were found in one to five lakes each (Table 3).
Total species richness ranged from one to eleven species per lake (median 3.5). Two lakes supported eleven species. These two lakes were situated in the lowland coastal area (Northern Anatolia) (Fig. 2) and held four alien species, while the rest of the lakes had zero to two alien species. Three lakes supporting seven species each, including two alien species, were situated near the Black Sea in the lowland coastal area in the Dniester-Lower Danube ecoregion, while one lake in the lowland coastal area in the south (Western Anatolia) supported eight species, including one alien (Fig. 2). The rest of the lakes each held six or fewer species (Fig. 2). The richness of native species ranged from zero to seven (median 1) species per lake.
The bars and the numbers show the total number of species found in each lake. For each bar, the colors show: white number of endemic species, light gray number of native species (non-endemics and non-translocated), dark gray number of translocated species, black number of alien species. Abbreviations for freshwater ecoregions are as in Fig. 1: LD Dniester-Lower Danube, Th Thrace, NA Northern Anatolia, CA Central Anatolia, WA Western Anatolia
In five of the seven models, either altitude or temperature was selected as the most important variable. The Shannon–Wiener diversity index ranged from 0 to 1.9 (median 0.62), altitude being the most important explanatory variable with a negative effect, while precipitation, TP, and longitude had a positive effect as the second, third, and fourth most important variable, respectively (Table 4). Likewise, altitude was clearly the most important variable and PVI the second most important variable in explaining the number of zooplanktivorous species with a negative and positive effect, respectively (Table 4). Temperature was the most important explanatory variable for species richness followed by latitude, lake area, and TP in that order. All four variables had a positive effect on the number of species (Table 4). For native species richness, the GLM analysis showed that temperature again had a positive effect followed by TP, latitude, and PVI, all having a positive effect as well. Also, temperature was clearly the most important variable in explaining the number of omnivorous species and, again, with a positive effect (Table 4). For the number of piscivorous fish species, chlorophyll a and salinity were the two most important variables in the GLM, both having a negative effect on the number of species (Table 4). Latitude and longitude were the most important variables explaining the number of benthivorous species, both having a positive, though weak, effect (Table 4). All variables included in each model had VIF values <4.
Adjusted R 2 revealed a 28% explanation of the variation in the Shannon–Wiener diversity index (Table 4). The adjusted pseudo R 2 values for the GLMs assuming Poisson error distribution ranged from 0.03 for a number of benthivorous species to 0.70 for the species richness of native species (Table 4). Altitude and temperature were the strongest correlated explanatory variables (r s: −0.79, Table 1). Exchanging them in the final models changed the pseudo R 2 value by less than 1% (results not shown), exceptions being richness of native species and number of omnivorous species where pseudo R 2 values increased from 0.70 to 0.75 and from 0.38 to 0.42, respectively, when including altitude instead of temperature (results not shown). The pseudo R 2 value for the number of omnivorous species increased from 0.38 to 0.45 when excluding the most widespread species, C. carpio, from the data (results not shown).
Discussion
Including 40 lakes located from north to south in the western and central parts of Turkey, the current study is the most comprehensive analysis of the role of geo-climatic and other environmental factors affecting fish assemblages in Turkish lakes. Fifty fish species were caught, comprising 18 endemic and 13 alien or translocated species. It is possible that the number and biomass of species might be underestimated, especially in the deep and large lakes, as we only used benthic nets and also a limited number of nets compared to the recommendations by CEN for such lakes (CEN, 2005). Moreover, gillnets may not be fully effective in catching all species present (e.g., Menezes et al., 2013).
The models explained a substantial part of the variation in total and native species richness as indicated by high pseudo R 2 scores. Especially temperature and/or altitude (being highly correlated) were important explanatory variables for these two models with the highest species richness being found in the warmest/lowland lakes. Altitude was also the most important variable in the model for the Shannon–Wiener diversity index, although this model only explained a smaller proportion of the total variation as indicated by the adj. R 2 of 0.28. Other studies from various parts of the world have also identified altitude as a primary predictor of fish species richness, for instance Argentina (Amarasinghe & Welcomme, 2002) and China (Zhao et al., 2006), while lake area emerged as the primary predictor in studies of lakes in North America and Africa (Barbour & Brown, 1974; Amarasinghe & Welcomme, 2002), as well as Asian and American tropical lakes (Amarasinghe & Welcomme, 2002). For European lakes, most of which were lowland lakes, lake area and temperature were the best predictors of both fish species richness and diversity (Brucet et al., 2013). Due to the strong correlation between altitude and temperature, it was, however, not possible to clearly disentangle the relative effect of altitude and temperature in our study.
Altitude may have a direct effect on the fish assemblage by setting dispersal limitations (Hesthagen & Sandlund, 2004; Drakou et al., 2009; Volta et al., 2011), leading to a low natural richness of fish (Hesthagen & Sandlund, 2004) or, as we found, lower diversity in high-altitude lakes. Fish assemblages in Turkish lakes may, however, also be influenced by past dispersal limitation caused by the rise of the Anatolian Plateau and the barrier effect of the mountains, which may have promoted speciation and thus endemism in the lakes (Kosswig, 1955). Indeed, we found many endemic species, although not restricted only to the highland lakes but in all ecoregions. Each of these endemic species was restricted to one or a few lakes, indicating current dispersal limitation [e.g., C. meandrense restricted to one river system (Özcan, 2008) and Pseudophoxinus elizavetae Bogutskaya, Küçük & Atalay 2006 restricted to the Central Anatolian marshes (Bogutskaya et al., 2007)]. The high number of endemic species recorded in our study is consistent with results from the nearby Balkan Peninsula (Oikonomou et al., 2014), with Cyprinidae contributing most of the endemic species pool in both places.
The altitude effect may be further enforced by the fact that the coastal ecoregions generally include more fish species from families of marine origin than does Central Anatolia (Abell et al., 2008). In accordance with this, we found species belonging to the marine fish families Mugilidae and Gobiidae only in the lowland lakes, while the euryhaline species A. boyeri (Atherinidae) was found also in highland lakes, likely due to translocation.
Temperature differences along the altitude gradient may also be of importance as temperature differences like altitude potentially have strong effects on species assemblages (Magnuson et al., 1979). We found cold-water salmonids only in the highland, i.e., lakes in the Northern Anatolian Mountains (though influenced by stocking), while cyprinids dominated by warm-water fish species mainly occurred in warmer areas, e.g., in lowland lakes. Cyprinidae is the most species-rich fish family in the Thrace region and in Northern, Western, and Central Anatolia (Abell et al., 2008) as well as in the nearby Balkan Peninsula (Oikonomou et al., 2014) and other parts of Europe (Reyjol et al., 2007) where this fish family accounts for more than 50% of the total number of species. Many cyprinids are omnivorous and omnivory is expected to increase with increasing temperature (González-Bergonzoni et al., 2012). Accordingly, omnivorous fish species richness increased with temperature in our study lakes, although the pseudo R 2 value of the model was low. The weak relationship may in part reflect that omnivorous C. carpio, one of the first translocated fish species in Turkey (Innal & Erk’akan, 2006), is spread throughout the country and is one of the most important fish in Turkish inland fisheries (Harlioğlu, 2011). Removing this species from the analyses increased pseudo R 2 by 7%.
The observed positive effect of lake area on species richness is well established (MacArthur & Wilson, 1967) and reflects that larger lakes provide higher habitat heterogeneity and therefore more available niches. A comparative analysis of European lakes showed species richness and diversity to be the highest in large and deep lakes in warmer areas (Brucet et al., 2013). Furthermore, Brucet et al. (2013) found that both high TP concentration and agriculture in the catchment contributed significantly to an increase in fish species richness and diversity when taking into account the most important geographical, climatic, and morphometric differences between the lakes. Accordingly, we found that TP had a positive effect on Shannon–Wiener diversity and species richness. However, a study of Danish lakes covering a wider gradient in TP revealed a unimodal relationship of fish species richness and diversity with TP (Jeppesen et al., 2000).
The number of zooplanktivorous species was strongly negatively related to altitude and positively to PVI, the latter likely reflecting that the plants may act as a refuge against predation by piscivores (Persson & Eklöv, 1995) as well as a foraging area. Aggregation of small-bodied fish species among submerged plants has been observed in other studies of warm lakes (Teixeira-de Mello et al., 2009). Our model failed to reveal any pattern in the distribution of benthivores (very low pseudo R 2), while we found chlorophyll a to be the most important variable for piscivorous species whose abundance declined with increasing chlorophyll a, as also recorded in other studies (Jeppesen et al., 2000), though with low pseudo R 2, perhaps because of translocation or stocking of alien species. Such stockings may have drastic effects on the otherwise rich endemic fish fauna of Turkish lakes. By way of example, the introduction of S. lucioperca has led to a strong population decline of endemic and non-endemic species in the Lakes Eğirdir and Beyşehir (Innal & Erk’akan, 2006; Küçük et al., 2009) as well as to the extinction of at least two endemic species (Küçük, 2012). Not only piscivorous species are stocked, but our study also clearly revealed that the present-day fish assemblages in Turkish lakes are strongly exposed to human-induced introduction of both native (translocated) and alien species. The majority of alien (C. auratus, C. gibelio, G. holbrooki, O. mykiss, and L. gibbosus) and translocated (C. carpio and S. lucioperca) species observed in our study are among the most frequently introduced species in the Mediterranean region (Ribeiro & Leunda, 2012) and in European freshwaters (Savini et al., 2010). Such non-native species may have a negative impact on the endemic fish species through competition for habitat and food, predation on fish, larvae, and eggs, hybridization, habitat alterations, and spread of parasites and diseases (Crivelli, 1995; Ribeiro & Leunda, 2012). The most widespread of the alien species in our study, C. gibelio, has invaded freshwaters in several Mediterranean countries (Ribeiro & Leunda, 2012) and should be a species of concern due to its various impacts and evident eurytopy (Savini et al., 2010). Both C. gibelio (Aydin et al., 2011) and P. parva (Ekmekçi & Kirankaya, 2006) are considered to be invasive in Turkey.
In many of the lakes, C. carpio was found in domesticated forms as mirror carp or tall-bodied forms (pers. obs.) and thus constitutes a threat to the genetically pure populations of wild carp (Memiş & Kohlmann, 2006), which generally show greater genetic variability than the domesticated forms (Kohlmann et al., 2005). Furthermore, C. carpio is known to cause resuspension of sediment and thus influence water turbidity, nutrient mobilization, and algae growth (e.g., Fischer et al., 2013) and to reduce macrophyte abundances (Miller & Crowl, 2006), with major ecosystem impacts (Matsuzaki et al., 2009). Thus, translocation of native fish species to other lakes may also threaten endemic species through habitat destruction (Gozlan et al., 2010). The impact of translocated species such as C. carpio, seems critical considering that our results showed plant PVI to be one of the most important predictors of the richness of native fish species in Turkish lakes.
In conclusion, our study emphasized the importance of altitude and temperature in shaping fish assemblages in Turkish lakes, which is in agreement with key drivers detected to shape lake fish assemblages in other parts of the world (e.g., Zhao et al., 2006; Brucet et al., 2013). The warm lowland lakes supported higher species richness and diversity as well as more omnivorous and zooplanktivorous species than the highland lakes. Several endemic fish species were recorded in our study, but, unfortunately, the study also revealed a wide distribution of translocated and alien species, some of which are also invasive, in the western and central parts of Turkey. The potential threat of translocated native species and introduced alien species should be a subject of concern when preparing plans for the management and conservation of the rich endemic fish fauna in the Turkish lakes.
References
Abell, R., M. L. Thieme, C. Revenga, M. Bryer, M. Kottelat, N. Bogutskaya, B. Coad, N. Mandrak, S. C. Balderas, W. Bussing, L. J. S. Melanie, P. Skelton, G. R. Allen, P. Unmack, A. Naseka, R. Ng, N. Sindorf, J. Robertson, E. Armijo, J. V. Higgins, T. J. Heibel, E. Wikramanayake, D. Olson, H. L. López, R. E. Reis, J. G. Lundberg, M. H. Sabaj Pérez & P. Petry, 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience 58: 403.
Amarasinghe, U. S. & R. L. Welcomme, 2002. An analysis of fish species richness in natural lakes. Environmental Biology of Fishes 65: 327–339.
Aydin, H., Ö. Gaygusuz, A. S. Tarkan, N. Top, Ö. Emiroğlu & Ç. Gürsoy Gaygusuz, 2011. Invasion of freshwater bodies in the Marmara region (northwestern Turkey) by nonnative gibel carp, Carassius gibelio (Bloch, 1782). Turkish Journal of Zoology 35: 829–836.
Balik, S., 1995. Freshwater fish in Anatolia, Turkey. Biological Conservation 72: 213–223.
Barbour, C. D. & J. H. Brown, 1974. Fish species diversity in lakes. The American Naturalist 108: 473–489.
Barton, K., 2015. MuMIn: multi-model inference. R package version 1.13.4. http://cran.r-project.org/package=MuMIn.
Bogutskaya, N. G., F. Küçük & M. A. Atalay, 2007. A description of three new species of the genus Pseudophoxinus from Turkey (Teleostei: Cyprinidae: Leuciscinae). Zoosystematica Rossica 15: 335–341.
Brucet, S., S. Pédron, T. Mehner, T. L. Lauridsen, C. Argillier, I. J. Winfield, P. Volta, M. Emmrich, T. Hesthagen, K. Holmgren, L. Benejam, F. Kelly, T. Krause, A. Palm, M. Rask & E. Jeppesen, 2013. Fish diversity in European lakes: geographical factors dominate over anthropogenic pressures. Freshwater Biology 58: 1779–1793.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer, New York.
Çakıroğlu, Aİ., Ü. Nihan Tavşanoğlu, E. E. Levi, T. A. Davidson, T. Bucak, A. Özen, G. K. Akyıldız, E. Jeppesen & M. Beklioğlu, 2014. Relatedness between contemporary and subfossil cladoceran assemblages in Turkish lakes. Journal of Paleolimnology 52: 367–383.
Canfield, D. E., J. V. Shireman, D. E. Colle, W. T. Hailer, C. E. Watkins & M. J. Maceina, 1984. Prediction of chlorophyll a concentrations in Florida lakes: importance of aquatic macrophytes. Canadian Journal of Fisheries and Aquatic Sciences 41: 497–507.
Carl, H. & P. R. Møller (eds.), 2012. Atlas Over Danske Ferskvandsfisk (in Danish, Atlas of Danish Freshwater Fish). Natural History Museum of Denmark, Copenhagen.
CEN, 2005. Water quality — sampling of fish with multi-mesh gill nets. European Committee for Standardization. Ref. No. EN 14757:2005.
Crivelli, A. J., 1995. Are fish introductions a threat to endemic freshwater fishes in the northern Mediterranean region? Biological Conservation 72: 311–319.
Cussac, V. E., D. A. Fernández, S. E. Gómez & H. L. López, 2009. Fishes of southern South America: a story driven by temperature. Fish Physiology and Biochemistry 35: 29–42.
Drakou, E. G., D. C. Bobori, A. S. Kallimanis, A. D. Mazaris, S. P. Sgardelis & J. D. Pantis, 2009. Freshwater fish community structured more by dispersal limitation than by environmental heterogeneity. Ecology of Freshwater Fish 18: 369–379.
Ekmekçi, F. G. & G. Ş. Kirankaya, 2006. Distribution of an invasive fish species, Pseudorasbora parva (Temminck & Schlegel, 1846) in Turkey. Turkish Journal of Zoology 30: 329–334.
Erk’akan, f, F. G. Atalay-Ekmekçi & T. T. Nalbant, 1999. A review of the genus Cobitis in Turkey (Pisces: Ostariophysi: Cobitidae). Hydrobiologia 403: 13–26.
Fischer, J. R., R. M. Krogman & M. C. Quist, 2013. Influences of native and non-native benthivorous fishes on aquatic ecosystem degradation. Hydrobiologia 711: 187–199.
Freyhof, J. & M. Özuluğ, 2009a. Pseudophoxinus fahrettini, a new species of spring minnow from Central Anatolia (Teleostei: Cyprinidae). Ichthyological Exploration of Freshwaters 20: 325–332.
Freyhof, J. & M. Özuluğ, 2009b. Pseudophoxinus evliyae, a new species of spring minnow from Western Anatolia with remarks on the distribution of P. ninae and the systematic position of P. fahirae (Teleostei: Cyprinidae). Ichthyological Exploration of Freshwaters 20: 309–318.
Freyhof, J. & M. Özuluğ, 2010. Pseudophoxinus hittitorum, a new species of spring minnow from Central Anatolia (Teleostei: Cyprinidae). Ichthyological Exploration of Freshwaters 21: 239–245.
Fricke, R., M. Bilecenoğlu & H. M. Sarı, 2007. Annotated checklist of fish and lamprey species (Gnathostomata and Petromyzontomorphi) of Turkey, including red list of threatened and declining species. Stuttgarter Beiträge zur Naturkunde Serie A (Biologie) 706: 1–169.
Froese, R., & D. Pauly (eds), 2013. FishBase.World Wide Web electronic publication, version (10/2013). www.fishbase.org.
González-Bergonzoni, I., M. Meerhoff, T. A. Davidson, F. Teixeira-de Mello, A. Baattrup-Pedersen & E. Jeppesen, 2012. Meta-analysis shows a consistent and strong latitudinal pattern in fish omnivory across ecosystems. Ecosystems 15: 492–503.
Gozlan, R. E., J. R. Britton, I. Cowx & G. H. Copp, 2010. Current knowledge on non-native freshwater fish introductions. Journal of Fish Biology 76: 751–786.
Griffiths, D., 2006. Pattern and process in the ecological biogeography of European freshwater fish. Journal of Animal Ecology 75: 734–751.
Harlioğlu, A. G., 2011. Present status of fisheries in Turkey. Reviews in Fish Biology and Fisheries 21: 667–680.
Heinzl, H. & M. Mittlböck, 2003. Pseudo R-squared measures for Poisson regression models with over- or underdispersion. Computational Statistics and Data Analysis 44: 253–271.
Hesthagen, T. & O. T. Sandlund, 2004. Fish distribution in a mountain area in south-eastern Norway: human introductions overrule natural immigration. Hydrobiologia 521: 49–59.
Hillebrand, H., 2004. On the generality of the latitudinal diversity gradient. The American Naturalist 163: 192–211.
Innal, D., 2012. Alien fish species in reservoir systems in Turkey: a review. Management of Biological Invasions 3: 115–119.
Innal, D. & F. Erk’akan, 2006. Effects of exotic and translocated fish species in the inland waters of Turkey. Reviews in Fish Biology and Fisheries 16: 39–50.
Iyigun, C., M. Türkeş, İ. Batmaz, C. Yozgatligil, V. Purutçuoğlu, E. K. Koç & M. Z. Öztürk, 2013. Clustering current climate regions of Turkey by using a multivariate statistical method. Theoretical and Applied Climatology 114: 95–106.
Jeppesen, E., J. P. Jensen, M. Søndergaard & T. L. Lauridsen, 2000. Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. Freshwater Biology 45: 201–218.
Kohlmann, K., P. Kersten & M. Flajšhans, 2005. Microsatellite-based genetic variability and differentiation of domesticated, wild and feral common carp (Cyprinus carpio L.) populations. Aquaculture 247: 253–266.
Kosswig, C., 1955. Zoogeography of the near east. Systematic Zoology 4: 49–73.
Kottek, M., J. Grieser, C. Beck, B. Rudolf & F. Rubel, 2006. World Map of the Köppen–Geiger climate classification updated. Meteorologische Zeitschrift 15: 259–263.
Kuru, M., 2004. Recent systematic status of inland water fishes of Turkey (in Turkish, with English abstract). Gazi Eğitim Fakültesi Dergisi Gazi Üniversitesi Gazi Eğitim Fakültesi Dergisi, Cilt 24, Sayı (3) 24: 1–21.
Küçük, F., 2006. Türkiye’deki bazı endemik içsu balıklarının Dünya Doğayı Koruma Birliği (IUCN) Ölçütlerine göre değerlendirilmesi 1. Ulusal Balıklandırma ve Rezervuar Yönetimi Sempozyumu Bildiriler Kitabı: 151–160.
Küçük, F., 2012. Extinct endemic fishes of Turkey: Alburnus akili (Gövce) and Pseudophoxinus handlirschi (Kavinne) (Pisces: Cyprinidae). Turkish Journal of Fisheries and Aquatic Sciences 12: 345–347.
Küçük, F., H. M. Sari, O. Demir & İ. Gülle, 2009. Review of the ichthyofaunal changes in Lake Eğirdir between 1915 and 2007. Turkish Journal of Zoology 33: 277–286.
Levi, E. E., Aİ. Çakıroğlu, T. Bucak, B. V. Odgaard, T. A. Davidson, E. Jeppesen & M. Beklioğlu, 2014. Similarity between contemporary vegetation and plant remains in the surface sediment in Mediterranean lakes. Freshwater Biology 59: 724–736.
MacArthur, R. H. & E. O. Wilson, 1967. The Theory of Island Biogeography. Princeton University Press, Princeton, NJ.
Magnuson, J. J., L. B. Crowder & P. A. Medvick, 1979. Temperature as an ecological resource. American Zoologist 19: 331–343.
Matsuzaki, S.-I. S., N. Usio, N. Takamura & I. Washitani, 2009. Contrasting impacts of invasive engineers on freshwater ecosystems: an experiment and meta-analysis. Oecologia 158: 673–686.
McCullagh, P. & J. A. Nelder, 1989. Generalized Linear Models. Chapman and Hall/CRC, London.
Meerhoff, M., J. M. Clemente, F. Teixeira-de Mello, C. Iglesias, A. R. Pedersen & E. Jeppesen, 2007. Can warm climate-related structure of littoral predator assemblies weaken the clear water state in shallow lakes? Global Change Biology 13: 1888–1897.
Memiş, D. & K. Kohlmann, 2006. Genetic characterization of wild common carp (Cyprinus carpio L.) from Turkey. Aquaculture 258: 257–262.
Menezes, R. F., F. Borchsenius, J.-C. Svenning, M. Søndergaard, T. L. Lauridsen, F. Landkildehus & E. Jeppesen, 2013. Variation in fish community structure, richness, and diversity in 56 Danish lakes with contrasting depth, size, and trophic state: does the method matter? Hydrobiologia 710: 47–59.
Miller, S. A. & T. A. Crowl, 2006. Effects of common carp (Cyprinus carpio) on macrophytes and invertebrate communities in a shallow lake. Freshwater Biology 51: 85–94.
Oikonomou, A., F. Leprieur & I. D. Leonardos, 2014. Biogeography of freshwater fishes of the Balkan Peninsula. Hydrobiologia 738: 205–225.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. Henry, H. Stevens, & H. Wagner, 2013. Vegan: community ecology package. R package version 2.0-8.
Özcan, G., 2008. Threatened fishes of the world: Chondrostoma meandrense Elvira, 1987 (Cyprinidae). Environmental Biology of Fishes 83: 297–298.
Özuluğ, M. & J. Freyhof, 2011. Revision of the genus Squalius in Western and Central Anatolia, with description of four new species (Teleostei: Cyprinidae). Ichthyological Exploration of Freshwaters 22: 107–148.
Persson, L. & P. Eklöv, 1995. Refuges affecting interactions between piscivorous perch and juvenile perch and roach. Ecology 76: 70–81.
R Core Team, 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, URL. http://www.R-project.org.
Reyjol, Y., B. Hugueny, D. Pont, P. G. Bianco, U. Beier, N. Caiola, F. Casals, I. Cowx, T. Ferreira, G. Haidvogl, R. Noble, A. de Sostoa, T. Vigneron & T. Virbickas, 2007. Patterns in species richness and endemism of European freshwater fish. Global Ecology and Biogeography 16: 65–75.
Ribeiro, F. & P. M. Leunda, 2012. Non-native fish impacts on Mediterranean freshwater ecosystems: current knowledge and research needs. Fisheries Management and Ecology 19: 142–156.
Savini, D., A. Occhipinti-Ambrogi, A. Marchini, E. Tricarico, F. Gherardi, S. Olenin & S. Gollasch, 2010. The top 27 animal alien species introduced into Europe for aquaculture and related activities. Applied Ichthyology 26: 1–7.
Şekercioğlu, Ç. H., S. Anderson, E. Akçay, R. Bilgin, Ö. E. Can, G. Semiz, Ç. Tavşanoğlu, M. B. Yokeş, A. Soyumert, K. İpekdal, İ. K. Sağlam, M. Yücel & H. Nüzhet Dalfes, 2011. Turkey’s globally important biodiversity in crisis. Biological Conservation 144: 2752–2769.
Tarkan, A. S., S. M. Marr & F. G. Ekmekç, 2015. Non-native and translocated freshwater fish species in Turkey. FiSHMED Fishes in Mediterranean Environments 2015(003): 28p.
Teixeira-de Mello, F., M. Meerhoff, Z. Pekcan-Hekim & E. Jeppesen, 2009. Substantial differences in littoral fish community structure and dynamics in subtropical and temperate shallow lakes. Freshwater Biology 54: 1202–1215.
Turan, D., M. Kottelat & S. Engin, 2009. Two new species of trouts, resident and migratory, sympatric in streams of northern Anatolia (Salmoniformes: Salmonidae). Ichthyological Exploration of Freshwaters 20: 333–364.
Ünver, B. & F. Erk’akan, 2011. Diet composition of chub, Squalius cephalus (Teleostei: Cyprinidae), in Lake Tödürge, Sivas, Turkey. Journal of Applied Ichthyology 27: 1350–1355.
Volta, P., A. Oggioni, R. Bettinetti & E. Jeppesen, 2011. Assessing lake typologies and indicator fish species for Italian natural lakes using past fish richness and assemblages. Hydrobiologia 671: 227–240.
Wildekamp, R. H., F. Küçük, M. Ünlüsayın & W. van Neer, 1999. Species and subspecies of the genus Aphanius Nardo 1897 (Pisces: Cyprinodontidae) in Turkey. Turkish Journal of Zoology 23: 23–44.
Winemiller, K. O., 1990. Spatial and temporal variation in tropical fish trophic networks. Ecological Monographs 60: 331–367.
Yalçın-Özdilek, Ş., Ş. G. Kırankaya & F. G. Ekmekçi, 2013. Feeding ecology of the topmouth gudgeon Pseudorasbora parva (Temminck and Schlegel, 1846) in the Gelingüllü Reservoir, Turkey. Turkish Journal of Fisheries and Aquatic Sciences 13: 87–94.
Zhao, S., J. Fang, C. Peng, Z. Tang & S. Piao, 2006. Patterns of fish species richness in China’s lakes. Global Ecology and Biogeography 15: 386–394.
Acknowledgments
This study was supported by TÜBİTAK- ÇAYDAG (Projects Numbers: 105Y332 and 110Y125), the Middle East Technical University (METU)-BAP program of Turkey (BAP.07.02.2007-2012), FP-7 REFRESH (Adaptive strategies to Mitigate the Impacts of Climate Change on European Freshwater Ecosystems, Contract No.: 244121), and the MARS project (Managing Aquatic ecosystems and water Resources under multiple Stress) funded under the 7th EU Framework Programme, Theme 6 (Environment including Climate Change), Contract No.: 603378 (http://www.mars-project.eu). TB was supported by TÜBİTAK program 2216—Research Fellowship Program for Foreign Citizens (Ref: B.14.2.TBT.0.06.01.03-216.01-24962). EEL, GB, UNT, and AİÇ were also supported by TÜBİTAK (Project Nos.: 105Y332 and 110Y125). SB’s contribution was supported by the TÜBITAK 2221—Visiting Scientist Fellowship Program and by the Marie Curie Intra European Fellowship no. 330249 (CLIMBING). EJ was further supported by CIRCE, CRES, and CLEAR. We are pleased to thank Gülşah Saç, Çiğdem Kaptan, Gürçay Kıvanç Akyıldız, and Ümmühan Aslan for assistance in the field and Anne Mette Poulsen for valuable editing of the manuscript. We also thank three anonymous reviewers for most valuable comments that helped shaping this paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling editor: Odd Terje Sandlund
Rights and permissions
About this article
Cite this article
Boll, T., Levi, E.E., Bezirci, G. et al. Fish assemblage and diversity in lakes of western and central Turkey: role of geo-climatic and other environmental variables. Hydrobiologia 771, 31–44 (2016). https://doi.org/10.1007/s10750-015-2608-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2608-3