Abstract
Foraging trait specialization is important for polymorphic Arctic charr and whitefish, but visual capabilities of different morphs are unexplored. Photoreceptor complements and absorbance spectra of rod visual pigments were studied by microspectrophotometry in two sympatric Arctic charr morphs and three sympatric whitefish morphs from two subarctic lakes. Four spectral classes of photoreceptor cells, rods and three types of cones, were found in all morphs of both species. Arctic charr rods had a pure A1 pigment (rhodopsin) with wavelength of maximum absorbance λ max ≈ 511–512 nm and no significant differences either between littoral and profundal morphs or sampling times (January/August). Rods of littoral and pelagic whitefish had practically pure A2 pigment (porphyropsin), whereas profundal whitefish had chromophore mixtures with A2:A1 ≈ 0.8:0.2 in June, A1 decreasing to a smaller fraction in September. λ max values of littoral and pelagic whitefish rods were similar and did not change significantly with season (539.3 ± 0.3 nm/539.3 ± 1.1 nm and 538.4 ± 0.4/539.8 ± 0.3 nm in June/September) but differed from profundal whitefish (λ max = 531.5 ± 0.8/536.7 ± 1.0 nm). Differences between Arctic charr and whitefish morphs suggest importance of local light environment determining visual pigment composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parallel divergence of teleost fishes into pelagic and benthic morphs has occurred in many distantly related species that inhabit postglacial lakes in the northern hemisphere (Skúlason & Smith, 1995; Hendry, 2009). Adaptive radiation to different niches may include morphological, physiological and life-history specializations (Schluter, 2000; Bernatchez et al., 2010). These sympatric morphs have diverged rapidly (in ca. 10,000–15,000 years) after the last Ice Age and provide excellent model systems for studies of adaptive radiation and ecological speciation over short evolutionary timescales (Schluter, 2000; Rundle & Nosil, 2005; Hendry, 2009).
Arctic charr (Salvelinus alpinus (L.)) and whitefish (Coregonus lavaretus (L.)) are highly polymorphic species across northern Scandinavia (Jonsson & Jonsson, 2001; Amundsen et al., 2004; Siwertsson et al., 2010). Population genetic studies indicate reproductive isolation among sympatric morphs of both species (Westgaard et al., 2004; Østbye et al., 2006; Præbel et al., 2013), suggesting adaptive radiation and incipient ecological speciation as a mechanisms behind their divergence. Besides the general pattern of divergence in polymorphic fish populations along the littoral-pelagic resource axis, both Arctic charr and whitefish may also diverge into the third principal niche available in subarctic lakes, the profundal habitat (Kahilainen & Østbye, 2006; Knudsen et al., 2006). One of the traits most likely to undergo niche-specific divergence along the depth gradient is the physiology of the eye and the retina, since adaptation to the light environment in the foraging habitat is critically important for visually feeding fishes such as Arctic charr and whitefish (Clarke, 1936; Langeland & Nøst, 1995; Elliott, 2011).
The intensity and spectral properties of light in an aquatic habitat depends on absorbance and scattering. Depending on the amount of primary production and dissolved organic matter, the spectral peak of transmitted light may be displaced from about 470 nm in clear water to almost 700 nm in heavily stained water (Wetzel, 1983). With increasing depth, the light intensity decreases and the spectrum narrows, and in stained waters, these changes are steep within a just few metres from the surface. For fishes living below the euphotic zone, it is particularly important that dim-light (rod) vision is spectrally adapted to utilize the available wavelengths as efficiently as possible. Closer to the surface, on the other hand, colour (cone) vision can be important both in foraging and mate choice (Seehausen et al., 2008; Muir et al., 2012). Especially, the genus Salvelinus is famous for different colouration patterns of morphs, which could be related to mate selection in addition to body size (Jonsson & Jonsson, 2001; Muir et al., 2012). In the dim and spectrally restricted illumination of the deep profundal habitat, however, colour vision is virtually useless, and this may explain why profundal Arctic charr morphs are often pale compared to their littoral relatives. Profundal whitefish morphs, on the other hand, are more brownish than their silvery counterparts in the littoral, consistent with decreased benefit of mirror camouflage at the greatest depths (Lythgoe, 1979). Polymorphic Arctic charr and whitefish in postglacial lakes provide interesting systems to study the role of vision in adaptive divergence, since the three principal niches constitute very different photic environments, particularly the dark profundal compared with the two others (littoral and pelagic).
Spectral tuning of a visual pigment molecule in fishes can be achieved in two different ways: (1) the amino acid sequence of the receptor protein, opsin, can be changed on an evolutionary time scale, i.e. within thousands of generations (e.g. Cowing et al., 2002), and (2) the chromophore (the light-absorbing prosthetic group covalently bound to the opsin) can be switched between 11-cis-retinal (A1) and 11-cis-3,4-dehydroretinal (A2) on a physiological timescale, i.e. within a single generation (e.g. Bridges, 1972; Saarinen et al., 2012). Switching from A1 to A2 shifts absorbance spectra towards longer wavelengths in a predictable manner (Dartnall & Lythgoe, 1965; Hárosi, 1994). Many fresh- and brackish-water fishes have mixtures of the two chromophores (see, e.g. Jokela-Määttä et al., 2007), and the proportions may change between seasons and different stages of life history (cf. Bridges, 1972). The wider implications of opsin- or chromophore-based spectral shifts are very different. The former concerns heritable differences in visual capabilities, which may even drive speciation (for a recent example, see Seehausen et al., 2008), whereas chromophore-based shifts confer phenotypic plasticity in response to environmental variation (Dartnall et al., 1961; Bridges, 1972; Saarinen et al., 2012). It is therefore essential to distinguish between these mechanisms for achieving spectral shifts. In the present work, we base the distinction on the fact that the two mechanisms affect the shape of absorbance spectra differently, as A2 pigments have generically wider spectra than A1 pigments (e.g. Dartnall & Lythgoe, 1965; Govardovskii et al., 2000). Varying proportions of A1 and A2 pigments have been documented for a range of both shallow- and deep-water species, suggesting no direct correlation of the pigment type with depth (Lythgoe, 1972; Jokela-Määttä et al., 2007). Temperature is the main single environmental factor affecting the A1 ↔ A2 balance, whereby raising temperature shifts the balance towards A1 (Tsin & Beatty, 1977). This makes functional sense, since the thermal stability of A2 pigments is tens of times lower than that of their A1 counterparts (Donner et al., 1990; Ala-Laurila et al., 2004a, 2004b, 2007). Shifting from A2 to A1 will therefore decrease the neural noise due to thermal pigment activations and thus increase the signal-to-noise ratio (SNR) of vision. Dim-light (rod) vision of fish in long-wavelength-shifted light environments are therefore expected to benefit from a higher proportion of A2 pigment when the water is cold, but to shift towards a higher proportion of A1 as the water temperature increases. The lake habitats utilized by fish morphs in northern postglacial lakes differ with respect to both light and temperature regimes, but their respective visual pigments have never been explored.
Here, we selected two well-studied lakes with polymorphic Arctic charr and whitefish to reveal potential differences in visual pigments. In Lake Fjellfrøsvatn, Arctic charr have diverged into ecologically and genetically distinct littoral and profundal morphs (Westgaard et al., 2004; Knudsen et al., 2006). Their foraging traits are correlated to their specific niches. The profundal morph has an enclosed swim bladder for permanent dwelling in the deep habitat and large eyes, which seems to be a heritable trait (Klemetsen et al., 2002; Knudsen et al., 2011). Whitefish in Lake Muddusjärvi have ecomorphologically and genetically diverged into three specialized morphs (Harrod et al., 2010; Couton, 2012). The number of gill rakers is a heritable trait in whitefish and correlated with niche utilization (Kahilainen et al., 2011; Præbel et al., 2013). The morphs are named according to body size and gill raker traits: the small sparsely rakered (SSR) whitefish is a profundal dwelling morph with the lowest number of gill rakers, the large sparsely rakered (LSR) whitefish a littoral morph having intermediate numbers of gill rakers and the densely rakered whitefish (DR) is a small sized pelagic morph with the highest number of gill rakers (Kahilainen & Østbye, 2006; Kahilainen et al., 2011). Furthermore, SSR whitefish dwell in the coldest habitat and have the lowest respiration rate and largest eye size (Kahilainen & Østbye, 2006; Kahilainen et al., 2014). The eyes of profundal morphs are larger than those of the other morphs throughout their ontogeny (Knudsen et al., 2006; Harrod et al., 2010).
Our primary objective was to look for differential traits of dim-light vision that could be related to the different photic environments in the respective niches of the morphs in two different lakes. We hypothesized that the spectral absorbance of the photoreceptors, especially the rods, is correlated with the habitat-specific light environments utilized by each morph to enhance photon capture while keeping thermal noise low enough to maximize the signal-to-noise ratio of vision. We argue that the large eyes of the deep-water morphs of both species also enhance photon capture in the dark profundal environment. In addition, we measured non-systematically cone pigments for initial evaluation of the presence of different cone types in the morphs. Here, the tentative hypothesis was that profundal morphs permanently using the deep-water habitats may have a reduced complement of cone pigments, as the usefulness of good colour vision is limited in the dim and spectrally narrow illumination at greater depths.
Materials and methods
Study area
The study lakes are situated in subarctic Fennoscandia: Lake Fjellfrøsvatn in the northwest (69°05′N, 19°20′E) and Lake Muddusjärvi in the northeast (69°00′N, 26°50′E). At these latitudes, there are 2 months with midnight sun (from mid-May to late-July) and 2 months with polar nights (from late November to mid-January). Both Lakes Fjellfrøsvatn and Muddusjärvi are oligotrophic with surface area of 6.5 and 48 km2 and maximum depths of 88 m and 73 m, respectively. The littoral zone extends to 15 m in Lake Fjellfrøsvatn and to 7 m in Lake Muddusjärvi with corresponding Secchi depths of 13 m and 3 m, respectively (Amundsen & Knudsen, 2009; Kahilainen et al., 2009; Eloranta et al., 2013). Lakes Fjellfrøsvatn and Muddusjärvi are situated at 125 and 146 m a.s.l., respectively, and are usually ice-covered from late November to early June. Lake Fjellfrøsvatn is inhabited by two fish species: Arctic charr and brown trout (Salmo trutta L.), whereas Lake Muddusjärvi additionally has whitefish, grayling (Thymallus thymallus (L.)), perch (Perca fluviatilis L.), pike (Esox lucius L.), burbot (Lota lota (L.)), common minnow (Phoxinus phoxinus (L.)), three-spined stickleback (Gasterosteus aculeatus L.) and nine-spined stickleback (Pungitius pungitius (L.)). Arctic charr are the most numerous species in Lake Fjellfrøsvatn and whitefish in Lake Muddusjärvi.
Sampling and measurements
The sampling periods in both lakes were planned to present contrasting light and temperature conditions. Winter sampling was conducted only in Lake Fjellfrøsvatn, whereas in Lake Muddusjärvi, the ice-melting period in early June was considered to reflect cold-water conditions. Arctic charr were collected using gill nets and baited wire traps in Lake Fjellfrøsvatn in January (under the ice) and August. The littoral morph was caught from 2 to 8 m depth and the profundal morph deeper than 25 m. Whitefish were caught with gillnets (pelagic 0-3 m, profundal 15-20 m) and beach seine (littoral 0-3 m) habitats in June 2004 and September 2007. They were preliminarily classified to morph visually and later checked for gill raker counts in the laboratory (for details see Kahilainen & Østbye, 2006). The light intensity in the water column at depths 0–12 m was measured with a photometer (LI-1400, Li-Cor, Inc., USA, wavelength range 400–700 nm). In addition, the spectrum of transmitted light in the wavelength range 300–700 nm was measured at depths 0–12 m (multispectral RAMSES, VIS-ACC radiometer) in Lake Muddusjärvi. Due to technical problems, the light spectrum data (300–600 nm) from Lake Fjellfrøsvatn were available only from January.
Selected good condition fish individuals were kept alive in circular plastic tanks (70 l) at complete darkness for 1 h to ensure that most of the visual pigments were regenerated. All further procedures were carried out in dim red light in the laboratory to minimize bleaching. Fish were captured from the plastic tank with a hand net, euthanized by cerebral concussion and dispatched on ice in light-tight boxes to the University of Helsinki for microspectrophotometry (MSP). The weight and total length of each individual were measured at accuracies of 0.1 g and 1 mm, respectively. The eyes were carefully dissected, and the diameter of the isolated pair of eyes was measured with an accuracy of 0.01 mm using a dial caliper. The measured eye diameters were compared at a common fish length to correct for the allometric relationship between eye size and body size using the procedure outlined in Howland et al. (2004). Similarly, length corrected eye size measures of the two morphs from Lake Fjellfrøsvatn were extracted from Klemetsen et al. (2002) for comparative purposes (Table 1). Eye and head size show allometric growth in many fish including whitefish and Arctic charr underlining the need for length corrections for robust comparison of morphs (Klemetsen et al., 2002; Kahilainen & Østbye, 2006).
For MSP, pieces of isolated dark-adapted retina were teased apart under dim red light in fish Ringer on a microscope slide to produce either isolated photoreceptors or outer segments protruding from small pieces of retina. The preparation was covered with a coverslip and sealed at the edges with Vaseline. Absorbance spectra were recorded from the outer segments of photoreceptor cells with the measuring light laterally incident on the outer segment and polarized perpendicularly to its longitudinal axis. For a full description of the equipment and procedures, see Govardovskii et al. (2000).
Analysis
Absorbance spectra of all visual pigments with a certain chromophore have a standard shape in the sense that all can be described by a single mathematical expression with only one variable, the wavelength of maximum absorbance (λ max). The parameters of this expression, which will be referred to as the visual pigment template, are somewhat different for A1 and A2 pigments, reflecting the fact that A1 and A2 spectra have somewhat different shapes. Therefore, both the wavelength of maximum absorbance (λ max) and the chromophore identity (A1 or A2, or the A1:A2 ratio in cases of mixed chromophore content) can be determined by fitting of pure A1 or A2 templates or sums of these. In the present work, Govardovskii et al. (2000) templates were fitted to MSP spectra averaged across cells of the same type within each individual. In cases of mixed chromophore content, A1:A2 ratios were obtained from the proportions of “paired” A1 and A2 templates (λ max values coupled by the Hárosi, 1994 relation) that gave the best fit when linearly summed.
To support the highest visual sensitivity, rod pigments should maximize the signal-to-noise ratio (SNR) by optimally absorbing the wavelengths available in the environment and at the same time be thermally stable to minimize randomly occurring spontaneous activations, i.e. pigment noise (Barlow, 1956, Baylor et al., 1980). The performance of pigments in terms of SNR at very low light levels in a given light environment was quantified by relative photon capture (“quantum catch”, QCrel) and conceptual signal-to-noise ratio (SNRdark), estimated as described by Jokela-Määttä et al. (2007): QCrel by convolution of the best-fitting visual pigment template with the spectrum of the downwelling light, and SNRdark as the ratio of QCrel to the Poisson standard deviation (i.e. √mean) of the rate of thermal pigment activations (Ala-Laurila et al., 2004b, 2007).
The total number of analysed individuals was 48 for whitefish and 21 for Arctic charr (Table 1). In each individual, recordings were made from 15–50 rods. In addition, absorbance spectra were non-systematically recorded from cone outer segments. This simply entailed recording from all morphologically good-looking cone outer segments encountered while systematically scanning the preparation for good rod outer segments. The sample sizes for each morph and season are typical or even slightly higher compared to many visual pigment studies (e.g. Jokela-Määttä et al., 2007; Saarinen et al., 2012). We acknowledge that a higher number of samples and sampling times would have been necessary to focus more on cone pigments and potentially reveal more insights to A1:A2 ratio along fish ontogeny. Despite these shortcomings, we considered our study important to promote future studies on polymorphic species.
Student’s t tests (Arctic charr) or Analysis of Variance with Tukey’s HSD pairwise tests (whitefish) were used to test differences between the morphs in photoreceptor λ max and eye size, both normally distributed, whereas λ max differences within the same morph sampled at different times were tested with Student’s t tests in both lakes. The statistical significance level was set to P < 0.05.
Results
Light measurements in the two lakes
In clear-water Lake Fjellfrøsvatn, the Secchi depth was 13 m in August and 16 m in late November prior to ice formation. The light intensity decreased gently with depth and the wavelength distribution became increasingly narrower. Even at 25 m depth as much as ~7% of the light remained over a wide wavelength band around 500–600 nm. The broad spectrum of light throughout the water column may suggest that even in the darkest season (winter) the requirements for “optimal” spectral tuning of the rod visual pigment are not likely to be very strict.
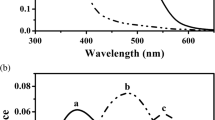
In the slightly humic Lake Muddusjärvi with Secchi depth of circa 3 metres, the euphotic zone (>1% of surface light left in water column) comprised the first seven metres (Fig. 1a, c). The light intensity was clearly higher in June than in September, but the wavelength distribution followed a similar pattern in both months (Fig. 1b, d). At about 2 m depth, the light spectrum had narrowed to a wavelength band roughly encompassing 400–700 nm. The green and yellow wavelengths (500–600 nm) penetrated deepest in the water column: at a depth of 10 m, the light spectrum peaked at 579 nm. Only about 0.2% of the light penetrated past 10 m, which was taken as the upper boundary of the profundal zone. This illustrates well how the photic environment of the profundal zone differs from the two others (littoral and pelagic) in three ways: (1) the light intensity is very low, (2) the light spectrum is narrow and (3) the peak of the light spectrum is strongly displaced towards longer wavelengths.
Proportion (%) of surface light left in the water column of Lake Muddusjärvi in June (a) and September (c). The wavelength distribution of the photon flux (µmol s−1 m−2 nm−1) at depths 0.1–12.0 m in June (b) and September (d) in Lake Muddusjärvi. The first measurements are taken just below the surface. The wavelength distribution of the photon flux (µmol s−1 m−2 nm−1) at depths 1 m and 25 m in January below the ice in Lake Fjellfrøsvatn (e). The dashed lines are hypothetical extrapolations beyond the long-wavelength limit of the measuring device
Absorbance spectra of rods
Overall, the rods of Arctic charr were maximally sensitive to significantly shorter wavelengths than those of whitefish. The rod absorbance spectra of both littoral and profundal Arctic charr morphs were well fitted by pure A1 templates peaking at 511–512 nm (Fig. 2; Table 1), and no statistical differences between morphs were observed in January (t test, t = −0.86, d.f. = 9.3, P = 0.41) or August (t test, t = 1.13, d.f. = 4.0, P = 0.32). There were no significant seasonal shifts in mean λ max in the littoral (t test, t = −1.12, d.f. = 5.0, P = 0.23) or profundal (t test, t = 0.82, d.f. = 6.4, P = 0.43) morph of Arctic charr. It may be noted that their common λ max value falls well within the spectral range where a fair proportion of the light penetrates even to 25 m depth in Lake Fjellfrøsvatn (Fig. 1e).
Examples of rod absorbance spectra of representative individuals of profundal small sparsely rakered (SSR) whitefish, littoral large sparsely rakered (LSR) whitefish and pelagic densely rakered (DR) whitefish in June (first row) and September (second row) in Lake Muddusjärvi. Similar examples for littoral and profundal Arctic charr morphs are presented in January (third row) and August (fourth row) in Lake Fjellfrøsvatn. The smooth curves are Govardovskii et al. (2000) templates. See Text for details. Note the difference in width between the A2-dominated spectra of whitefish and the A1 spectra of Arctic charr
In whitefish, a significant difference in rod absorbance spectra occurred between the SSR whitefish and the other two morphs (Fig. 2; Table 1). DR and LSR whitefish were both reasonably well fitted by pure (or nearly pure) A2 templates with no significant difference in λ max (mean ± SEM = 539.3 ± 0.3 nm for LSR whitefish and 538.4 ± 0.4 nm for DR whitefish). In contrast, the rod absorbance spectra of the SSR whitefish were shifted to shorter wavelengths and could not be fitted with pure A2 templates. Best fits were obtained with linear sums of templates corresponding to 75–80% A2 and 20–25% A1, giving mean rod λ max = 531.5 ± 0.8 nm. In June, the difference between morphs was statistically significant (ANOVA, F 2,20 = 62.4, P < 0.05), whereby the SSR whitefish differed from each of the other two morphs (Tukey’s HSD test, P < 0.05). We found a seasonal shift in the λ max of DR whitefish (t test, t = −2.79, d.f. = 15.8, P < 0.05) and SSR whitefish (t test, t = −3.99, d.f = 12.7, P < 0.05) rods towards longer wavelengths between June and September, but no differences in LSR whitefish (t test, t = 0.00, d.f. = 8.1, P = 1.00). Thus, in September, the SSR whitefish λ max approached the others (Table 1), but still there were significant differences between morphs (ANOVA, F 2,22 = 3.9, P < 0.05). Post-ANOVA pairwise testing indicated that the September λ max of SSR whitefish differed significantly from that of DR whitefish (Tukey’s HSD test, P < 0.05) but not from that of LSR whitefish.
Optical factors affecting visual sensitivity
The efficiency of vision in utilizing light also depends on optical factors of the eye and the photoreceptors. Potential optical differences to consider when comparing quantum catch between morphs are eye size, and (for dim-light vision) the dimensions and retinal mosaic of rods. No systematic differences in rod outer segment length or cell morphology were observed between whitefish morphs at the light microscopic level (mean length of outer segments 37 μm, mean width 5 μm). However, the eye diameters were significantly larger in the SSR whitefish than in the other two morphs both in June (ANOVA, F 2,20 = 49.1, P < 0.05) and September (ANOVA, F 2,22 = 16.8, P < 0.05) (Table 1). Assuming that the amount of light entering the eye is proportional to eye diameter squared, the eyes of SSR whitefish in our material would catch ca. 20% more light than DR whitefish eyes and ca. 50% more than LSR whitefish eyes. Admittedly, isometric upscaling of an eye does not in itself increase the brightness of the retinal image of an extended source. In Arctic charr, too, differences in length-corrected eye size have been observed between the morphs (Table 1).
As the restricted illumination spectrum below 10 m depth in Lake Muddusjärvi peaks at ca 580 nm, it may seem paradoxical that SSR whitefish rods (λ max ≈ 531 nm in June) should be blue-shifted compared with LSR and DR whitefish rods (λ max ≈ 539 nm), i.e. even further away from the peak of the light distribution. However, the relative loss in quantum catch due to this is rather modest as shown in Fig. 3, where Lake Muddusjärvi light spectrum (thin line) is reproduced together with estimated quantum catches for two rod absorbance spectra: (1) a pure A2 spectrum with λ max = 539 nm, representative of LSR and DR whitefish rods (hatched); (2) a mixed spectrum with the same A2 pigment (539 nm) and its A1 pair (509 nm) in the ratio 0.8:0.2, peaking at 531 nm and representative of SSR whitefish rods (bold line). The integrals of the curves (1)–(2) (i.e. the areas under the curves) give relative measures of total quantum catch. If the quantum catch for the A2 (539 nm) pigment is normalized to unity, that of the mixed pigment is 0.92. Thus, the 8 nm blue-shift of the SSR whitefish rod spectrum will reduce the quantum catch by only ca. 8% compared with the LSR and DR whitefish.
The spectrum of light at a depth of 10 m (thin line) in Lake Muddusjärvi and estimated photon capture spectra for littoral large sparsely rakered (LSR) whitefish (hatched line) and for profundal small sparsely rakered (SSR) whitefish (bold solid line) in June. The latter have been obtained by multiplication of the light spectrum with Govardovskii et al. (2000) templates for 100% A2(539) and 80%:20% A2(539):A1(509), respectively. The total photon capture is obtained as the integral of the respective spectrum (i.e. the area under the curve)
Absorbance spectra of cones
While the main objective of this study was to compare dim-light (rod) vision between niches and species, some non-systematic observations on the absorbance spectra of cones were also made. In all morphs of both Arctic charr and whitefish, three distinct spectral classes of cone outer segments were found, corresponding to short-wavelength sensitive (SWS, “blue”), middle-wavelength sensitive (MWS, “green”) and long-wavelength sensitive (LWS, “red”) cones. We did not find UV cones in any Arctic charr or whitefish individual.
All cone spectra from Arctic charr were well fitted by pure A1 templates with mean λ max ≈ 430, 504 and 560 nm for SWS, MWS and LWS cones, respectively. There were no differences in the λ max between littoral and profundal Arctic charr morphs (SWS n = 2 and 2; MWS n = 6 and 3; LWS n = 2 and 2 for littoral and pelagic morphs, respectively).
In DR and LSR whitefish, all cone spectra were well fitted by pure A2 templates with λ max ≈ 450–452 nm (SWS), 536–539 nm (MWS) and 610–614 nm (LWS). In the SSR whitefish (June n = 3; September n = 6), the shapes of cone spectra were roughly consistent with mixed chromophore content in proportions similar to those found in the rods (A2:A1 ≈ 0.8:0.2), and the LWS cone λ max (604 nm) was correspondingly lower than those in DR whitefish (June n = 4; September n = 6) and LSR whitefish (June n = 4; September n = 3). Somewhat surprisingly, however, the λ max values of SWS and MWS cones in the SSR whitefish did not differ from those of the “pure A2 morphs” DR and LSR whitefish. At least for the MWS cone, this cannot be reconciled with the idea that the SSR whitefish has the expected admixture of A1 chromophore in the same MWS cone opsin. With respect to cell morphology, single and double cones were observed, but not the triple cones. Such a cone type may easily escape notice in MSP preparations, however. The single cones studied by MSP had either SWS or MWS pigments. At least some of the double cones had outer segments with different pigments (LWS and MWS).
Discussion
The different visual specialisations of Arctic charr and whitefish morphs from the same latitudinal area in analogous lake habitats suggested that local light environment and thermal conditions are important. In Arctic charr, both morphs had similar rod spectral sensitivities, which did not differ between sampling times, and both used only A1 chromophore in the visual pigments. By contrast, in whitefish the rod absorbance spectra of the profundal SSR whitefish differed significantly from those of the shallow-water LSR and DR whitefish. In LSR and DR whitefish sampled in June and September, practically only A2 chromophore was detected in the rods, whereas the profundal SSR whitefish had seasonally varying A2:A1 proportions with up to 20% of A1. Moreover, the profundal morphs of both species had relatively larger eyes than the sympatric shallow-water adapted morphs. All morphsof both species also had three spectral cone types, suggesting good colour vision.
Arctic charr and whitefish: different adaptations for high visual sensitivity in profundal morphs
In the rods of Arctic charr from Lake Fjellfrøsvatn, there were no differences between morphs refuting the hypothesis of different visual pigments in littoral and profundal habitats in this clear-water lake. Rod spectra of both littoral and profundal fish caught in both January and August were well fitted by Govardovskii et al. (2000) A1 templates with λ max ≈ 511–513 nm. Lack of seasonal differences in rod spectra may suggest stability of temperature and light conditions in this lake. The profundal zone of this clear-water lake with its fairly broad spectral light distribution may not present any strong selection pressure for a different λ max, and the evolutionary time scale for differential opsin adaptation in our study systems is short anyway compared with, for example, Lake Baikal cottoids (Cowing et al., 2002). On the other hand, we do not at present know whether Arctic charr have even retained the option of spectral tuning by chromophore exchange, now known to be associated with the expression of Cyp27c1, a cytochrome P450 family member that converts vitamin A1 into vitamin A2 (Enright et al., 2015). In the Arctic charr of Lake Saimaa, a large lake with variable optic conditions in southern Finland, only A1 has been found in fish ranging in total length of 5-25 cm (T. Smura, K.K. Kahilainen and K. Donner, unpublished). Older studies based on difference spectra of pigment extracts have given variable results on the presence of A2 in the genus Salvelinus, including unconvincing evidence for a small proportion of A2 in one population of S. alpinus (Bridges & Yoshikami, 1970). A functional A1 ↔ A2 system is present at least in Salvelinus willughbii of Lake Windermere (England), where A2 is dominant during spawning regardless of season (Bridges, 1967). These questions merit further study. A larger sample size within the lake would share light on potential ontogenetic shifts in rod absorbance spectra that could potentially be important if, e.g. juvenile and adult habitats are different. We know that profundal Arctic charr dwell permanently in deep water (Klemetsen et al., 2002; Knudsen et al., 2006), but littoral Arctic charr may show microhabitat shifts, e.g. in respect to distance to surface that could have relevance for putative spectral tuning.
In whitefish, the spectral absorbance of the dim-light photoreceptors, rods, was significantly different in the profundal morph (SSR) compared with the littoral and pelagic morphs (LSR and DR) supporting the hypothesis of habitat-specific visual pigments of morphs. Judging by template fitting to absorbance spectra, the difference depended on the plastic A2 ↔ A1 chromophore system known to underlie differential spectral tuning for different stages of life history in salmonids and eels (Anguilla spp.) and for different seasons also in several other families of fish (see Bridges, 1972). The rods of the DR and LSR whitefish had (nearly) pure A2 pigments, but those of the SSR whitefish had A2:A1 mixtures in the approximate proportion 0.8:0.2 (in June). The admixture of A1 shifted λ max from about 539 nm to about 531 nm. Somewhat surprisingly, this is a shift away from the peak of the profundal light distribution (ca 580 nm) and its functionality will be considered further below. This seasonal tuning of chromophore system and rod absorbance spectra would require a follow-up study using an annual sampling design. Whitefish is prone for some ontogenetic habitat shifts as at least pelagic and littoral morphs use near surface habitats during their first year of life and thus should be exposed to the widest range of underwater wavelength distribution compared to older individuals inhabiting deeper and colder water layers in their respective habitats (Kahilainen et al., 2014). Overall, the sample sizes (numbers of individuals) for determining rod spectra for each condition (e.g. time point) in the present study are typical and sufficient for visual pigment studies (Jokela-Määttä et al., 2007; Saarinen et al., 2012), as suggested also by the relatively small standard deviations.
Neither in Arctic charr nor in whitefish, could we observe any differences in rod morphology between the different morphs. While we did not investigate the retinal rod mosaics, it may be noted that Reckel et al. (1999) found a preferentially dorsal rod distribution in Lake Constance whitefish, suggesting that rod vision primarily looks downwards, into the water or bottom below the fish. Such asymmetry may be functional in fish dwelling not too far below the surface, where the light from above during much of the day is strong enough for cone vision, whereas a more homogeneous rod distribution might be expected in morphs inhabiting the permanently dark profundal. A comparison between morphs in this respect might be rewarding.
Seasonal differences
In the profundal whitefish morph (SSR), mean rod λ max shifted from ca. 531 nm to ca. 537 nm between spring/early summer (June) and late summer/early autumn (September), probably reflecting a decrease in the proportion of A1 chromophore. The observed rod λ max shifts of whitefish refuted our hypothesis on seasonal stability suggesting a need for seasonal adjustment to varying light and thermal conditions in this lake. SSR whitefish rod λ max in September approaches those of DR and LSR whitefish and suggests that A1 has dropped near zero. The fact that the A2 (%) estimates do not reveal this clearly as a change in means, only as greatly increased SEMs, highlights the variability as well as the uncertainties in estimating chromophore proportions just from the shapes of recorded spectra, unless the recording is of very high quality (the spectrum is “smooth” with little noise and no drift) (Saarinen et al., 2012). In the DR whitefish, too, there was a slight red-shift in rod λ max between June and September, suggesting replacement of the (final) small proportion of A1 by A2.
The usual pattern of seasonal A1/A2 variation in fishes is that A2 increases in winter and A1 during the summer months (Dartnall et al., 1961; Muntz & Mouat, 1984). The signal-to-noise ratio of photoreceptors sets an ultimate limit to visual sensitivity in a certain light environment. The signal is proportional to the rate of photon capture and thus to the “match” between the visual pigment’s absorbance spectrum and the illumination spectrum in the environment (see Materials and Methods). Noise means random variability, and the crucial noise of a rod photoreceptor in darkness or at very low light levels largely originates in thermal (spontaneous) activations of visual pigment molecules, which cause randomly occurring electrical signals that are identical to responses to real photons (Baylor et al., 1980). To achieve high visual sensitivity, it is often equally important to minimize noise as to maximize the signal. The seasonal variation in the A1:A2 ratio is consistent with the idea that the thermally more stable A1 chromophore should be favoured during the warm season in order to avoid excessive noise from A2 pigment. Both light and temperature have been found to affect the A1:A2 ratio, but laboratory experiments have shown that at least in rainbow trout (Oncorhynchus mykiss) temperature overrides light regimes (Tsin & Beatty, 1977).
The temperatures in Lake Muddusjärvi in the respective niches in the two seasons were (June/September, °C) 9/7 (littoral), 9/7 (pelagic) and 4/9.5 (profundal). Thus, the slight A2 increase in the pelagic DR whitefish from June (9°C) to September (7°C) is consistent with the idea that at lower water temperature a higher proportion of A2 can be allowed. By contrast, the SSR whitefish chromophore usage again appears somewhat puzzling. As such, the ca. 5°C temperature rise is fairly modest, expected to increase thermal activation rates by no more than ca. twofold (Baylor et al., 1980). But more importantly, thermal activations of visual pigment cease to be a crucial noise source at higher light levels: when the rate of photoactivation becomes significantly higher than the rate of spontaneous thermal activations, the random distribution of photon arrivals (often referred to as quantal fluctuations) becomes the dominant source of random variability (noise). A calculation based on (1) the light measurements, (2) pigment quantum catch and (3) rod dimensions and pupil size suggests that at 20 m depth in Lake Muddusjärvi, each SSR whitefish rod receives 40–100 (in June) or 5–20 (in September) photoisomerization per second from the downwelling light in daytime. This is 3–4 orders of magnitude more than the estimated rate of thermal activations per rod. Obviously, light reflected from the bottom is much dimmer, and low-light conditions all the way to practically absolute darkness may be encountered, e.g. during 24 h depending on season. The amount of light during the midnight sun period in June is very high and SSR whitefish are feeding very actively (Kahilainen et al., 2003, 2009), which may suggests that light is important in foraging success of this morph too. However, in wintertime, the amount of light is limited to the two first metres of water, but despite the darkness, both whitefish and Arctic charr are feeding, although at lower levels than in the open-water season (Amundsen & Knudsen, 2009; Hayden et al., 2015). Here, we only wish to emphasize that rod vision in SSR whitefish may also have to deal with not very low light intensities even in the profundal habitat. The increase in A2 from June to September might then be interpreted as a phase-lagged response to the favourable light conditions of the summer months (starting with the ice-break in early June), when quantum catch may be more important than thermal noise control. It should also be noted that the measured profundal temperature of 9.5°C represents the highest annual temperature in this habitat, briefly obtained in late September during the vertical mixing of the water column (Kahilainen et al., 2014).
Quantum catch and signal-to-noise ratio (SNR) of SSR whitefish rods
Visual discrimination is always a question of discriminating a light signal from random variation, i.e. noise. At the absolute dark-adapted threshold, the limiting noise seems to be that produced by the rods themselves (Barlow, 1956; Baylor et al., 1980; Aho et al., 1988). For a visual pigment, the “signal” is proportional to the quantum catch (photoactivations), whereas the “noise” arises from the randomly occurring thermal activations and is proportional to the square root of the mean rate (Poisson standard deviation). Thus, a relevant signal-to noise ratio (SNR) measure for a visual pigment is photon catch divided by the square root of the thermal activation rate (Jokela et al., 2003; Jokela-Määttä et al., 2007; Saarinen et al., 2012). The rate of thermal pigment activations (k) increases with increasing λ max (red-shifting the pigment) and can be predicted by linear regression of log k on 1/λ max. Such a relation summarizing all empirical data on vertebrate rods (Ala-Laurila et al., 2004b; Luo et al., 2011) predicts that shifting λ max from 539 nm (as in LSR and DR whitefish) to 531 nm (as in SSR whitefish) would decrease the rate of thermal events by the factor 1.6 and the associated Poisson noise by the factor √1.6 = 1.27. Given that quantum catch decreases only by 8%, the net effect would be a 17% SNR increase. An independent estimation purely based on chromophore properties, i.e. the “noisiness” of A2 pigments compared with their A1 pairs (Donner et al., 1990; Ala-Laurila et al., 2007) similarly indicates that replacing 20% of A2 pigment by A1 will decrease noise by more than 8%. Thus, the blue-shift of SSR whitefish rods appears as adaptive if the goal is to maximize dark-adapted visual sensitivity in the profundal.
Cone complements
All three whitefish morphs and both Arctic charr morphs had three spectrally different cone pigments, in principle supporting trichromatic vision and thus refuting our last hypothesis. This may indicate either that the postglacial time has been too short for evolutionary reduction of the cone complement in the profundal morphs, or that even they spend enough time closer to the surface for trichromacy to remain useful. Arguing against the latter explanation are studies suggesting that SSR whitefish in Lake Muddusjärvi seem to stay in the profundal habitat consistently across seasons throughout the year (Kahilainen et al., 2004; K.K. Kahilainen, unpublished). Likewise, the profundal morph in Lake Fjellfrøsvatn has a stable deep-water habitat preference throughout the year (Amundsen et al., 2008), yet even short-term excursions to shallower layers may make good colour vision worthwhile. Indeed, the apparently selective spectral tuning of the MWS cone in the SSR whitefish (inconsistent with passive reproduction of the chromophore ratios of rods and LWS cones) suggests that the spacing of cone spectra is functionally important rather than just a “relict”. This observation, here based on measurements from only three SSR whitefish individuals, would merit further study, as would the wider question where and for what purposes the profundal morphs (of both species) use wavelength discrimination (colour vision).
How important is vision for feeding in the highly specialized profundal morphs?
The profundal niche is the least productive of the three principal habitat types in lakes (Kahilainen et al., 2003, 2005), and benthic prey densities in the profundal in the study region are 3–8 times lower than in the littoral (Hayden et al., 2013). The most important prey resources for profundal Arctic charr and SSR whitefish, chironomids and Pisidium sp., are partly buried in very fine-grained mud in the profundal (Kahilainen et al., 2003; Knudsen et al., 2006) and effective foraging may depend more on traits other than visual sensitivity, such as the blunt snout and subterminal mouth position shared by the profundal morphs of both species (Klemetsen et al., 2002; Harrod et al., 2010; Knudsen et al., 2011) and the low number of gill rakers of SSR whitefish (Kahilainen et al., 2011). In feeding experiments, SSR whitefish ingest considerable amounts of sand while foraging on chironomids (K. Kahilainen, unpublished). Sand was ejected between gill rakers and gill arches before entering the oesophagus. This foraging tactic could be one explanation for the morphological divergence of the gill raker apparatus, where SSR whitefish has the lowest number of short gill rakers with the widest spacing (Kahilainen & Østbye, 2006; Kahilainen et al., 2011). Additionally, the profundal morph of Arctic charr is a more effective predator on chironomids than the littoral morph, suggesting heritable behavioural traits (Klemetsen et al., 2006). Physiological traits related to profundal feeding include higher food conversion efficiency of the profundal Arctic charr morph in cold-water temperatures (Knudsen et al., 2015) and generally low metabolism of the profundal SSR whitefish (Kahilainen et al., 2014). Traits for permanent dwelling in the profundal habitat seem to be numerous in both Arctic charr and whitefish and thus may not be purely dependent on visual capabilities.
References
Aho, A. C., K. Donner, C. Hydén, L. O. Larsen & T. Reuter, 1988. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature 334: 348–350.
Ala-Laurila, P., J. Pahlberg, A. Koskelainen & K. Donner, 2004a. On the relation between the photoactivation energy and the absorbance spectrum of visual pigments. Vision Research 44: 2153–2158.
Ala-Laurila, P., K. Donner & A. Koskelainen, 2004b. Thermal activation and photoactivation of visual pigments. Biophysical Journal 86: 3653–3662.
Ala-Laurila, P., K. Donner, R. K. Crouch & M. C. Cornwall, 2007. Chromophore switch from 11-cis-dehydroretinal (A2) to 11-cis-retinal (A1) decreases dark noise in salamander red rods. Journal of Physiology 585: 57–74.
Amundsen, P.-A. & R. Knudsen, 2009. Winter ecology of Arctic charr (Salvelinus alpinus) and brown trout (Salmo trutta) in a subarctic lake, Norway. Aquatic Ecology 43: 765–775.
Amundsen, P.-A., R. Knudsen, A. Klemetsen & R. Kristoffersen, 2004. Resource competition and interactive segregation between sympatric whitefish morphs. Annales Zoologici Fennici 41: 301–307.
Amundsen, P.-A., R. Knudsen & A. Klemetsen, 2008. Seasonal and ontogenetic variations in resource use by two sympatric Arctic charr morphs. Environmental Biology of Fishes 83: 45–55.
Barlow, H. B., 1956. Retinal noise and absolute threshold. Journal of the Optical Society of America 46: 634–639.
Baylor, D. A., G. Matthews & K. W. Yau, 1980. Two components of electrical dark noise in toad retinal rod outer segments. Journal of Physiology 309: 591–621.
Bernatchez, L., S. Renaut, A. R. Whiteley, N. Derome, J. Jeukens, L. Landry, G. Lu, A. W. Nolte, K. Østbye, S. M. Rogers & J. St-Cyr, 2010. On the origin of species: insights from the ecological genomics of lake whitefish. Philosophical Transactions of the Royal Society B 365: 1783–1800.
Bridges, C. D. B., 1967. Photopigments in the char of Lake Windermere (Salvelinus willughbii, form autumnalis and form vernalis). Nature 214: 205–206.
Bridges, C. D. B., 1972. The rhodopsin-porphyropsin visual system. In Dartnall, H. J. A. (ed.), Handbook of Sensory Physiology VII/1. Springer, Berlin: 417–479.
Bridges, C. D. B. & S. Yoshikami, 1970. Distribution and evolution of visual pigments in salmonid fishes. Vision Research 10: 609–626.
Clarke, G. L., 1936. On the depth at which fish can see. Ecology 17: 452–456.
Couton, M., 2012. Adaptive radiation of the European whitefish, Coregonus lavaretus (L.), in the Pasvik watercourse: the genetic description of a new morph. MSc thesis, University of Tromsø, Tromsø.
Cowing, J. A., S. Poopalasundaram, S. E. Wilkie, J. K. Bowmaker & D. M. Hunt, 2002. Spectral tuning and evolution of short-wave-sensitive cone pigments in cottoid fish from Lake Baikal. Biochemistry 41: 6019–6025.
Dartnall, H. J. A., M. R. Lander & F. W. Munz, 1961. Periodic changes in the visual pigment of a fish. In Christensen, B. & B. Buchmann (eds), Progress in Photobiology. Elsevier, Amsterdam: 203–213.
Dartnall, H. J. A. & J. N. Lythgoe, 1965. The spectral clustering of visual pigments. Vision Research 5: 81–100.
Donner, K., M. L. Firsov & V. I. Govardovskii, 1990. The frequency of isomerization-like “dark” events in rhodopsin and porphyropsin rods of the bullfrog retina. Journal of Physiology 428: 673–692.
Elliott, J. M., 2011. A comparative study of the relationship between light intensity and feeding ability in brown trout (Salmo trutta) and Arctic charr (Salvelinus alpinus). Freshwater Biology 56: 1962–1972.
Eloranta, A. P., R. Knudsen & P.-A. Amundsen, 2013. Niche segregation of coexisting Arctic charr (Salvelinus alpinus) and brown trout (Salmo trutta) constrains food web coupling in subarctic lakes. Freshwater Biology 58: 207–221.
Enright, J.M., M.B. Toomey, S. Sato, S.E. Temple, J.E. Allen, R. Fujiwara, V.M. Kramlinger, L.D. Nagy, K.M. Johnson, Y. Xiao, M.J. How, S.L. Johnson, N.W. Roberts, V.J. Kefalov, F.P. Guengerich & J.C. Corbo, 2015. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2. Current Biology 25: 3048–3057.
Govardovskii, V. I., N. Fyhrquist, T. Reuter, D. G. Kusmin & K. Donner, 2000. In search of the visual pigment template. Visual Neuroscience 17: 509–528.
Hárosi, F. I., 1994. An analysis of two spectral properties of vertebrate visual pigments. Vision Research 34: 509–528.
Harrod, C., J. Mallela & K. K. Kahilainen, 2010. Phenotype-environment correlations in a putative whitefish adaptive radiation. Journal of Animal Ecology 79: 1057–1068.
Hayden, B., T. Holopainen, P.-A. Amundsen, A. P. Eloranta, R. Knudsen, K. Præbel & K. K. Kahilainen, 2013. Interactions between invading benthivorous fish and native whitefish in subarctic lakes. Freshwater Biology 58: 1234–1250.
Hayden, B., C. Harrod, E. Sonninen & K. K. Kahilainen, 2015. Seasonal depletion of resources intensifies trophic interactions in subarctic freshwater fish communities. Freshwater Biology 60: 1000–1015.
Hendry, A. P., 2009. Ecological speciation! Or the lack thereof? Canadian Journal of Fisheries and Aquatic Sciences 66: 1383–1398.
Howland, H. C., S. Merola & J. R. Basarab, 2004. The allometry and scaling of the size of vertebrate eyes. Vision Research 44: 2043–2065.
Jokela, M., A. Vartio, L. Paulin, N. Fyhrquist-Vanni & K. Donner, 2003. Polymorphism of visual pigment between allopatric populations of the sand goby (Pomatoschistus minutus): a microspectrophotometric study. Journal of Experimental Biology 206: 2611–2617.
Jokela-Määttä, M., T. Smura, A. Aaltonen, P. Ala-Laurila & K. Donner, 2007. Visual pigments of Baltic Sea fishes of marine and limnic origin. Visual Neuroscience 24: 389–398.
Jonsson, B. & N. Jonsson, 2001. Polymorphism and speciation in Arctic charr. Journal of Fish Biology 58: 605–638.
Kahilainen, K. & K. Østbye, 2006. Morphological differentiation and resource polymorphism in three sympatric whitefish Coregonus lavaretus (L.) forms in a subarctic lake. Journal of Fish Biology 68: 63–79.
Kahilainen, K., H. Lehtonen & K. Könönen, 2003. Consequence of habitat segregation to growth rate of two sparsely rakered whitefish (Coregonus lavaretus (L.)) forms in a subarctic lake. Ecology of Freshwater Fish 12: 275–285.
Kahilainen, K., T. Malinen, A. Tuomaala & H. Lehtonen, 2004. Diel and seasonal habitat and food segregation of three sympatric Coregonus lavaretus forms in a subarctic lake. Journal of Fish Biology 64: 418–434.
Kahilainen, K., E. Alajärvi & H. Lehtonen, 2005. Planktivory and diet-overlap of densely rakered whitefish (Coregonus lavaretus (L.)) in a subarctic lake. Ecology of Freshwater Fish 14: 50–58.
Kahilainen, K. K., T. Malinen & H. Lehtonen, 2009. Polar light regime and piscivory govern diel vertical migrations of planktivorous fish and zooplankton in a subarctic lake. Ecology of Freshwater Fish 18: 481–490.
Kahilainen, K. K., A. Siwertsson, K. Ø. Gjelland, R. Knudsen, T. Bøhn & P.-A. Amundsen, 2011. The role of gill raker number variability in adaptive radiation of coregonid fish. Evolutionary Ecology 25: 573–588.
Kahilainen, K. K., W. P. Patterson, E. Sonninen, C. Harrod & M. Kiljunen, 2014. Adaptive radiation along a thermal gradient: preliminary results of habitat use and respiration rate divergence among whitefish morphs. PloS One 9: e112085.
Klemetsen, A., J. M. Elliott, R. Knudsen & P. Sørensen, 2002. Evidence for genetic differences in the offspring of two sympatric morphs of Arctic charr. Journal of Fish Biology 60: 933–950.
Klemetsen, A., R. Knudsen, R. Primicerio & P.-A. Amundsen, 2006. Divergent, genetically based feeding behaviour of two sympatric Arctic charr, Salvelinus alpinus (L.), morphs. Ecology of Freshwater Fish 15: 350–355.
Knudsen, R., A. Klemetsen, P.-A. Amundsen & B. Hermansen, 2006. Incipient speciation through niche expansion: an example from the Arctic charr in a subarctic lake. Proceedings of the Royal Society B: Biological Sciences 273: 2291–2298.
Knudsen, R., A. Siwertsson, C. E. Adams, M. Garduno-Paz, J. Newton & P.-A. Amundsen, 2011. Temporal stability of niche use exposes sympatric Arctic charr to alternative selection pressures. Evolutionary Ecology 25: 589–604.
Knudsen, R., H. Johnsen, B.-S. Sæther & S. I. Siikavuopio, 2015. Divergent growth patterns between juveniles of two sympatric Arctic charr morphs with contrasting depth gradient niche preferences. Aquatic Ecology 49: 33–42.
Langeland, A. & T. Nøst, 1995. Gill raker structure and selective predation on zooplankton by particulate feeding fish. Journal of Fish Biology 47: 719–732.
Luo, D.-G., W. W. S. Yue, P. Ala-Laurila & K. W. Yau, 2011. Activation of visual pigments by light and heat. Science 332: 1307–1312.
Lythgoe, J. N., 1972. List of vertebrate visual pigments. In Dartnall, H. J. A. (ed.), Handbook of Sensory Physiology VII/1: Photochemistry of Vision. Springer, Berlin: 604–624.
Lythgoe, J. N., 1979. The Ecology of Vision. Clarendon Press, Oxford.
Muir, A. M., C. T. Blackie, J. E. Marsden & C. C. Krueger, 2012. Lake charr Salvelinus namaycush spawning behaviour: new field observations and a review of current knowledge. Reviews in Fish Biology and Fisheries 22: 575–593.
Muntz, W. R. A. & G. S. V. Mouat, 1984. Annual variations in the visual pigments of brown trout inhibiting lochs providing different light environments. Vision Research 24: 1575–1580.
Østbye, K., P.-A. Amundsen, L. Bernatchez, A. Klemetsen, R. Knudsen, R. Kristoffersen, T. F. Næsje & K. Hindar, 2006. Parallel evolution of ecomorphological traits in the European whitefish Coregonus lavaretus (L.) species complex during postglacial times. Molecular Ecology 15: 3983–4001.
Præbel, K., R. Knudsen, A. Siwertsson, M. Karhunen, K. K. Kahilainen, O. Ovaskainen, K. Østbye, S. Peruzzi, S.-E. Fevolden & P.-A. Amundsen, 2013. Ecological speciation in postglacial European whitefish: rapid adaptive radiations into the littoral, pelagic and profundal lake habitats. Ecology and Evolution 3: 4970–4986.
Reckel, F., R. R. Melzer & U. Smola, 1999. Ultrastructure of the retina of two subspecies of Coregonus lavaretus (Teleostei) from Lake Constance (Germany). Acta Zoologica 80: 153–162.
Rundle, H. D. & P. Nosil, 2005. Ecological speciation. Ecology Letters 8: 336–352.
Saarinen, P., J. Pahlberg, G. Herczeg, M. Viljanen, M. Karjalainen, T. Shikano, J. Merilä & K. Donner, 2012. Spectral tuning by selective chromophore uptake in rods and cones of eight populations of nine-spined stickleback (Pungitius pungitius). Journal of Experimental Biology 215: 2760–2773.
Schluter, D., 2000. The ecology of adaptive radiation. Oxford University Press, New York.
Seehausen, O., Y. Terai, I. S. Magalhaes, K. L. Carleton, H. D. J. Mrosso, R. Miyagi, I. van der Sluijs, M. V. Schneider, M. E. Maan, H. Tachida, H. Imai & N. Okada, 2008. Speciation through sensory drive in cichlid fish. Nature 455: 620–627.
Siwertsson, A., R. Knudsen, K. K. Kahilainen, K. Præbel, R. Primicerio & P.-A. Amundsen, 2010. Sympatric diversification as influenced by ecological opportunity and historical contingency in a young species lineage of whitefish. Evolutionary Ecology Research 12: 929–947.
Skúlason, S. & T. B. Smith, 1995. Resource polymorphism in vertebrates. Trends in Ecology and Evolution 10: 366–370.
Tsin, A. T. C. & D. D. Beatty, 1977. Visual pigment changes in rainbow trout in response to temperature. Science 195: 1358–1360.
Westgaard, J. I., R. Knudsen & A. Klemetsen, 2004. Genetic differences between two sympatric morphs of Arctic charr confirmed by microsatellite DNA. Journal of Fish Biology 65: 1185–1191.
Wetzel, R. G., 1983. Limnology. Saunders College Publishing, New York.
Acknowledgments
The authors wish to thank O. Aikio, A. Eloranta, C. Lien, J. Marttila, K. Mäenpää and N. Kangas for help in the field work. The financial support of the Finnish Cultural Foundation, the Lapland Cultural Foundation, the Otto A. Malm Foundation, Societas Scientiarum Fennica and the Academy of Finland (Grants 206221 and 1140903, 1268566) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Power, R. Knudsen, C. Adams, M. J. Hansen, J. B. Dempson, M. Jobling & M. Ferguson / Advances in Charr Ecology and Evolution
Rights and permissions
About this article
Cite this article
Kahilainen, K.K., Smura, T., Knudsen, R. et al. Visual pigments of Arctic charr (Salvelinus alpinus (L.)) and whitefish (Coregonus lavaretus (L.)) morphs in subarctic lakes. Hydrobiologia 783, 223–237 (2016). https://doi.org/10.1007/s10750-015-2588-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2588-3