Abstract
The study compares the resource utilization of two sympatric Arctic charr morphs over an annual period in a subarctic lake. The two morphs are reproductively isolated in time and place of spawning, and are referred to as the littoral and profundal morphs (L-morph and P-morph) according to their spawning habitats. Fish were sampled monthly (ice-free season) or bimonthly (winter) using gillnets in the main lake habitats. The spatial range of the P-morph was restricted to the profundal zone throughout the whole annual period. The L-morph in contrast utilized all main habitats, exhibiting distinct seasonal and ontogenetic variations in habitat distribution. In the spring, the whole L-morph population was located along the bottom profile of the lake, in profundal and littoral habitats. During summer and autumn, habitat segregation occurred between different life-stages, juveniles mainly utilizing the profundal, pre-adults the pelagic and adult fishes the littoral zone. During winter the whole population was assembled in the littoral habitat. The L-morph also had large seasonal and ontogenetic variations in their feeding ecology, with littoral zoobenthos, zooplankton and surface insects being important prey. The P-morph had a narrower diet niche mainly consisting of chironomid larvae and other profundal zoobenthos. Hence, the two Arctic charr morphs exhibited a consistent resource differentiation during all annual seasons and throughout their life cycles, except for a dietary overlap between P-morph and juvenile L-morph charr in the profundal during summer. The findings are discussed in relation to resource polymorphism and incipient speciation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postglacial lakes provide good examples of the importance of resource polymorphism as a diversifying force in vertebrates (Skulason and Smith 1995; Schluter 1996; Smith and Skulason 1996). The occurrence and evolution of sympatric fish morphs in these lakes have therefore attracted much interest, particularly within the genera Gasterosteus (e.g., Schluter and McPhail 1992; McPhail 1994; Taylor and McPhail 2000; Schluter 2003), Coregonus (e.g., Svärdson 1957, 1998; Lu and Bernatchez 1999; Amundsen et al. 2004; Bernatchez 2004; Østbye et al. 2006) and Salvelinus (e.g., Sandlund et al. 1992; Adams et al. 1998: Dynes et al. 1999; Skulason et al. 1999; Alekseyev et al. 2002; Klemetsen et al. 2002, 2003a; Proulx and Magnan 2004; Sacotte and Magnan 2006). The Arctic charr, Salvelinus alpinus, is a highly polymorphic species that often may be represented by two or more sympatric living morphs within the same lake (Jonsson and Jonsson 2001; Klemetsen et al. 2003a), and polymorphism has been reported to occur throughout the circumpolar range of the species (Nyman et al. 1981; Hindar and Jonsson 1982; Klemetsen et al. 1985, 2003a; Riget et al. 1986; Sandlund et al. 1992; Reist et al. 1995; Savvaitova 1995; Adams et al. 1998; Skulason et al. 1999; Alekseyev et al. 2002).
The origin of polymorphism in Arctic charr and other freshwater fish has been explained by several hypotheses, ranging from allopatric divergence under geographical isolation during the last Ice Age, to divergent natural selection in sympatry (Nyman et al. 1981; Klemetsen 1984; Bernatchez and Dodson 1990; Lu and Bernatchez 1999; Schluter 2000; Dieckmann et al. 2004). Sympatric speciation has become increasingly accepted during the past decades, and the link between ecological differentiation and reproductive isolation seems particularly important in the evolution of postglacial fishes (Schluter 2001). Intraspecific niche specialization driven by resource competition has been suggested to facilitate reproductive isolation and counteract hybridization and gene flow between sympatric morphs, but the ecological processes driving speciation are in general poorly understood (Schluter 1996, 2000). A generalistic ancestral invader is usually assumed to have segregated into divergent groups of resource specialists; most commonly with a pelagic versus littoral segregation and subsequently a distinct morph formation (Jonsson and Jonsson 2001; Klemetsen et al. 2003a). Resource partitioning through niche specialization thus appears to be a driving force in morph formation. Resource partitioning between sympatric Arctic charr morphs has repeatedly been demonstrated, including both habitat and diet segregations (Klemetsen and Grotnes 1975, 1980; Henricson and Nyman 1976; Riget et al. 1986; Malmquist et al. 1992; Adams et al. 1998; Guiger et al. 2002; O’Connell and Dempson 2002). However, even though large seasonal variations in resource utilization may occur, most studies comparing the ecology of sympatric Arctic charr morphs have been restricted to limited periods of the summer season, and information from the ice-covered winter period of the lakes is generally absent. Ontogenetic variations in resource use have furthermore rarely been addressed in comparisons of sympatric charr morphs (but see Snorrason et al. 1994), although ontogenetic niche shifts are known commonly to occur within the species (Klemetsen et al. 1989, 2003a; L’Abée-Lund et al. 1993; Jansen et al. 2002).
In the subarctic lake Fjellfrøsvatn, northern Norway, two sympatric charr morphs with a distinct divergence in the time and place of spawning have been identified (Klemetsen et al. 1997). One morph spawns in shallow water in the littoral zone in late September, whereas the other morph spawn in the profundal habitat (>25 m depth) in February–March. The two morphs thus have a strong reproductive isolation and are termed the littoral and the profundal morph (hereafter referred to as L-morph and P-morph) according to their respective spawning habitats. The two morphs are genetically differentiated by microsattelite DNA analysis (Westgaard et al. 2004; Wilson et al. 2004). They also exhibit highly different life-histories (Klemetsen et al. 1997). The L-morph is relatively fast-growing, attaining maximum sizes above 30 cm and reaching sexual maturation from the age of five years and 16 cm in length. The P-morph is in contrast slow-growing, attaining maximum lengths of 13 cm and first reach sexual maturity at the age of three years and 7 cm length (Klemetsen et al. 1997). Large ecological differences have also been documented; the L-morph has been found in all the main lake habitats whereas the P-morph appears to be restricted to the profundal habitat (Klemetsen et al. 1997; Knudsen et al. 2006). The ecological differences seem to be related to different adaptations in trophic morphology and feeding behaviour (Klemetsen et al. 2002, 2006).
The present contribution is the first comprehensive analysis that compares the food and habitat dimensions of the ecological niches of the two Fjellfrøsvatn charr morphs through a complete annual cycle and for different ontogenetic cohorts. Diet analyses have been published before from the lake, but with other questions in focus than in the present contribution. Knudsen et al. (1997) found that the parasite communities differed between the two morphs and related this to food transmission of parasites. Klemetsen et al. (2003b) found that the L-morph fed continuously during winter despite very low temperatures (0.7°C) and little light (two months of polar night) under the ice, and also demonstrated that the food assimilation was slightly above maintenance level at this time. No comparisons between the morphs were however made. By comparison with four lakes with monomorphic charr, Knudsen et al. (2006) found that the niche of the Fjellfrøsvatn P-morph has been attained by niche expansion (sensu Schluter 2000), and not by a sub-division of the ancestral niche. They also showed that the niches of the Fjellfrøsvatn morphs are stable over years.
Here, we address the aspects of ontogenetic and seasonal variation by analysing the diet of several cohorts of the L-morph charr by monthly (ice-free season) and bi-monthly (winter) sampling throughout an annual cycle. By comparing these data with seasonal data from the P-morph charr population, we explore the hypothesis that the two morphs exhibit a consistent resource partitioning over all annual seasons and throughout their ontogeny; the P-morph being a resource specialist confined to the profundal habitat, and the L-morph being an ecological generalist utilising a larger range of habitat and food resources. In addition, we perform a detailed analysis of the diets of similarly sized fishes of the two morphs when their habitats overlap during summer. By this comparison, we test the hypothesis that their resource use differ also during this crucial overlap in time and space.
Material and methods
Study site
Fjellfrøsvatn (69°05’N, 19°20’E) is a 6.5 km2, 88 m deep oligotrophic lake situated at 125 m a.s.l. in northern Norway. The lake is of a regular shape and has one main basin. The shore regions are mostly sandy or stony with little emergent vegetation. The catchment area is about 90 km2, and consists of treeless mountains and woodland with birch, Betula pubescens, and pine, Pinus silvestris, and with a few small farms and some cabins located around the lake. The lake is dimictic with ice-cover for 6–7 months (usually November to May). At this latitude, there are nearly two months of polar night around winter solstice, and nearly two months midnight sun around summer solstice. Fish species in the lake are Arctic charr, S. alpinus, and brown trout, Salmo trutta.
Sampling and analyses
Samples were collected monthly during the ice-free period (June–November) and three times under ice during winter (December, March, May) in 1992–1993. In the ice-free season, fish were sampled in littoral, profundal and pelagic habitats, whereas sampling under the ice was restricted to littoral and profundal habitats. Sampling in the pelagic zone was omitted during wintertime as studies in the nearby lake Takvatn have shown that Arctic charr rarely use this habitat during the ice-covered period (Klemetsen et al. 2003b). The ice thickness was about 30 cm in December, 80 cm in March and 60 cm in May. There was clear ice and practically no snow in December, about half a meter snow on top of the ice in March and opaque ice and little snow in May. We used 1.5 × 40 m survey gillnets composed by eight randomly distributed 5 m panels of 10, 12.5, 15, 18, 22, 26, 35 and 45 mm bar mesh sizes and additional 8, 10 and 12.5 mm 1.5 × 30 m nets all placed on the bottom in the benthic habitats, and 6 × 40 m survey nets of the same mesh sizes placed at the lake surface in the pelagic habitat. The littoral nets were set down to 15 m depths, whereas the profundal sampling was done at 25–40 m depths. The gill nets were randomly positioned within the different habitats. The two charr morphs were separated by external morphology and colour in the field. P-morph charr are faint yellow–brown with a brass tinge, with deep bodies and large eyes (Knudsen et al. 2006). There are no, or very faint, parr marks (Klemetsen et al. 1997). Juvenile L-morph charr are in contrast silvery and slender, with small eyes and often with parr marks present (see Knudsen et al. 2006 for further details). DNA analyses performed by Westgaard et al. (2004) confirmed that the criteria used in the field separation are accurate. The habitat use of the two morphs was compared by estimating the % habitat distribution of each morph at each sampling occasion from area-adjusted catch per unit effort data (number of fish per survey gillnet per night) from the littoral, profundal and pelagic habitats, respectively. The fish were measured (fork length) and weighed, and stomach contents and otholiths were sampled and preserved in 96% ethanol until analyzed in the laboratory. Ageing of fish was performed by surface reading of otoliths under a binocular microscope. The stomachs were opened and the total fullness visually determined on a percentage scale ranging from empty (0%) to full (100%). The prey items were identified and their contribution to the total fullness estimated (Amundsen 1995). The proportion of each diet category was expressed as percent prey abundance (A i ):
where S i is the stomach fullness composed by prey i and S t the total stomach fullness of all prey categories (Amundsen et al. 1996). The prey items were mainly identified to the species, genus or family level, and a total of 32 taxa were recorded. In the graphical presentation of the results the prey taxa have been sorted in eight functional diet categories, including a) zooplankton (planktonic cladocerans and copepods), b) surface insects (adult terrestrial and aquatic insects), c) chironomid pupae, d) littoral chironomid larvae, e) other littoral zoobenthos (mainly Lymnaea peregra, Gammarus lacustris, and Plecoptera, Ephemeroptera and Tricoptera larvae, f) the chydorid Eurycercus lamellatus, g) profundal chironomid larvae (mainly Heterotrissocladius subpilosus), and h) other profundal prey (the pea mussel Pisidium sp. and the benthic copepod Acanthocyclops gigas).
Results
Habitat distribution
During the ice-free summer season the L-morph of all age groups combined utilized the littoral, profundal and pelagic habitats, whereas the littoral was the principal habitat during wintertime (Fig. 1a). In June just after ice-break, most L-morph charr were found in the profundal habitat, but some fish also occupied the littoral. The fraction of the population utilizing the profundal habitat gradually decreased during the summer season, and from July to October all three habitats were occupied by the L-morph. By November, the main bulk of the L-morph population was located in the littoral zone, and during the ice-covered period from December to May, more or less all L-morph charr were located in this habitat. For the P-morph, the whole population across all ages was in contrast confined to the profundal habitat during the whole annual period (Fig. 1b), and no fish were caught at depths shallower than 25 m.
The L-morph charr exhibited distinct ontogenetic differences in their habitat use, especially during the ice-free season (Fig. 2). In June, the profundal was the principal habitat for 2 to 5-yr old L-morph charr. A few 1-yr old fish that were caught later in the summer season were also mainly found in this habitat. The use of the profundal habitat did however in general decrease from June and throughout the ice-free season with a subsequent increase in the use of the littoral and pelagic habitats, especially for the oldest age-classes. Younger age-classes of the L-morph occupied the profundal habitat for a longer part of the summer season than older age-classes. For 2-yr old fish, this was the main habitat throughout the whole ice-free season up to November, whereas 3-yr old fish used the profundal as the dominant habitat up to September, 4-yr old up to August, and 5-yr old only in June. The use of the pelagic zone increased with increasing age from 3 yr to 6 yr, and for the 5- and 6-yr old L-morph fish, the pelagic was the dominant habitat during August and September, and was also important in July and October. Also ≥7-yr fish utilized the pelagic habitat, especially in August, but the littoral zone was the most important habitat for the oldest age-classes. From December and all through the ice-covered winter period, all age-classes of the L-morph charr were consistently found in the littoral habitat except for a few 2- and 3-year old fish that were caught in the profundal habitat in December and in May (Fig. 2).
During the winter there was almost no overlap in habitat use between the L-morph and the P-morph (Fig. 1). During the ice-free period there was some overlap in habitat use, especially during the early summer when the youngest age-classes of the L-morph to a large extent utilized the profundal habitat (Fig. 2). For adult fish, in contrast, there was little overlap in habitat use between the two morphs also during summertime.
Diet
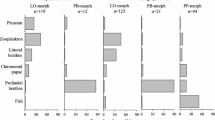
The sampled P-morph fish had a narrow size distribution, ranging in fork length from 7.3 cm to 13.1 cm. Similarly, there were small variations in mean length-at-age between the different age classes represented in the total sample of the P-morph population, ranging from 8.0 cm at age 2-yr to 10.9 cm for 8-yr old fish. A comparison of the diet between 2-4-year old and >4-yr old P-morph charr revealed a high degree of similarity in prey composition, and ontogenetic diet shifts are therefore considered to be of insignificant importance within this morph. For the L-morph in contrast, there were larger size differences between different age-classes with an annual increment in mean length of 3–4 cm and a total size span from 7.5 cm to 36.0 cm among the sampled fish. Accordingly, there were also large ontogenetic variations in the diet of the L-morph charr (Fig. 3). In particular, the youngest age classes of the L-morph had been feeding more on zooplankton, E. lamellatus and chironomid larvae than the oldest age classes, and these prey categories gradually decrease in importance with increasing age of the L-morph charr. In contrast, the contribution of littoral zoobenthos and surface insects in the diet increased with increasing age of the fish.
Large seasonal variations could also be seen in the diet of the L-morph charr (Fig. 3). Chironomid pupae were only important in spring and early summer, whereas E. lamellatus was mainly consumed during summer. Surface insects were consumed by the older L-morph charr during summer and early autumn, whereas zooplankton was an important prey for young L-morph charr in late summer and autumn. Some zooplankton was even consumed after ice-cover, but the main prey of the L-morph charr during winter was littoral chironomid larvae and in particular other littoral zoobenthos such as G. lacustris, snails and other insect larvae (in particular Trichoptera, Plecoptera and Sialis sp.).
The P-morph charr also exhibited distinct seasonal variations in their feeding ecology (Fig. 3). In June, chironomid larvae and, quite surprisingly, littoral zoobenthos dominated the diet. The littoral zoobenthos consumed by the profundal dwelling P-morph consisted mainly of Ephemeroptera nymphs. The June samples from the profundal habitat were taken from a site located in the vicinity of the main inlet river. Apparently, the Ephemeroptera nymphs had been flushed into the profundal habitat during the spring flood in the inlet river, as they were also found in Ekman grab samples from the profundal habitat in June. During summer and early autumn (July to September), E. lamellatus was an important prey for the P-morph together with profundal chironomid larvae and other profundal zoobenthos. In late autumn and during the winter, the P-morph diet was completely dominated by profundal chironomid larvae and other profundal zoobenthos (mainly Pisidium sp. and A. gigas).
For most of the year, there were large differences in the diets of the L-morph and P-morph charr, the former mainly feeding on zooplankton, surface insects and littoral zoobenthos and the latter on soft-bottom profundal prey like chronomids and Pisidium sp. (Fig. 3). The diet differences were particularly large between the older age classes of the L-morph charr and the P-morph charr, which showed very small overlap in their prey utilization. Some dietary similarities could however be observed between the two morphs, but these were restricted to spring and early summer and only included the younger age-classes of the L-morph. At this time of the year, the main bulk of the 2-yr and 3-yr old L-morph charr were located in the profundal habitat, exhibiting a large habitat overlap with the P-morph (Fig. 2). A comparison of the profundal diet of the two morphs during the summer season (Fig. 4), revealed quite large diet overlaps between the P-morph and the 2-yr and 3-yr L-morph charr from June to August, with E. lamellatus as the principal common prey. In the autumn (September to November), in contrast, profundal dwelling 2- and 3-yr old L-morph mainly fed on zooplankton whereas the P-morph predominantly consumed profundal chironomid larvae and other zoobenthos (Fig. 4).
Discussion
The two sympatric Arctic charr morphs in Fjellfrøsvatn have large differences in their life-histories, including parameters such as sexual maturation, somatic growth and age and size distribution, as well as time and place of spawning (Klemetsen et al. 1997). The present study demonstrates that these differences also are accompanied by large and consistent differences in niche and resource use of the two morphs. The P-morph appeared to have a specialized niche solely utilizing the profundal habitat, whereas the L-morph had a broader niche both along the spatial and dietary axes. The ecological divergence of the two morphs was chiefly maintained both over their ontogeny and during the different seasons of the year. The P-morph was apparently confined to the profundal habitat throughout all seasons and the whole life cycle and did not reveal any significant ontogenetic niche shifts in their spatial or trophic resource utilization. The L-morph in contrast, exhibited distinct ontogenetic niche shifts. In terms of habitat utilization during the ice-free season, the profundal was the main habitat for the youngest age-classes of the L-morph charr, whereas intermediate age-classes to a large extent resided in the pelagic, and the oldest fish mainly in the littoral habitat. This is in accordance with previous findings of ontogenetic habitat shifts in monomorphic Arctic charr populations (Klemetsen et al. 1989; Bjøru and Sandlund 1995). The L-morph charr in Fjellfrøsvatn also exhibited characteristic ontogenetic diet shifts, which in particular were manifested during the ice-free season in the dominance of crustacean zooplankton in the diet of juvenile fish, and surface insects and littoral zoobenthos in older fish. Also during wintertime some ontogenetic diet shifts could be observed within the L-morph population, particularly with respect to a larger inclusion of chironomid larvae among the younger fish and more G. lacustris, snails and large-sized insect larvae among the older fish. The niche shifts appeared to a large extent to be related to an increase in the size of ingested prey with increasing predator size, which is a typical feature of ontogenetic diet shifts in fish (Werner and Gilliam 1984; Werner 1986; Hjelm et al. 2000).
Whereas significant ontogenetic habitat and diet shifts only could be seen in the L-morph charr, both morphs showed distinct seasonal variations in their feeding ecology. For the profundal-dwelling P-morph charr, the diet was throughout most of the year dominated by typical soft-bottom profundal invertebrates, although from July to September the semi-benthic chydorid E. lamellatus was the dominant prey. For the L-morph charr, zooplankton and surface insects were the most important prey types during the ice-free period, whereas littoral chironomid larvae and other zoobenthos dominated the diet during wintertime. There was thus in general a large niche differentiation between the two morphs throughout the whole annual period. The observed differences in the feeding ecology of the two morphs are in accordance with observations from experimental studies, where naïve offspring of the two morphs were found to differ in their ability to catch and ingest different prey types (Klemetsen et al. 2002, 2006). The P-morph offspring were most successful in acquiring typical profundal prey (chironomid larvae; Klemetsen et al. 2002), whereas the L-morph offspring were the most effective predators on Gammarus, Daphnia and Gerris, representing littoral benthos, zooplankton and surface prey, respectively (Klemetsen et al. 2006). Genetically based differences in trophic morphology and behaviour have also been found between the two morphs (Klemetsen et al. 2002; Westgaard et al. 2004), and the present in situ study confirms that the inter-morph segregation in resource use is maintained both over the whole annual period and the ontogeny of the fish. The only exception from this pattern was related to the youngest age-classes of the L-morph charr which during the summer months mainly resided in the profundal habitat and from June to September exhibited a quite large resemblance in diet choice with the P-morph charr. In particular, E. lamellatus was an important prey for both the P-morph and the 2+ and 3+ L-morph charr during these months, most likely as a result of a high abundance of this prey type at this time of the year. However, as the dietary importance of E. lamellatus declined throughout the ice-free season, the two morphs developed a characteristic divergence in their feeding ecology in the profundal habitat with the P-morph feeding on profundal zoobenthos and the juvenile L-morph mainly on zooplankton. The ontogenetic habitat and diet shifts found in the generalistic L-morph are similar to ontogenetic niche shifts observed in allopatric Arctic charr populations (Klemetsen et al. 1989, 2003a; L’Abée-Lund et al. 1993; Jansen et al. 2002; Knudsen et al. 2006), and the feeding ecology of the L-morph thus seem not to be influenced by the specialized resource utilization of the co-occurring P-morph.
According to the observed niche divergence between the two morphs, the P-morph appears to be strongly orientated towards the soft bottom substrate of the profundal habitat, mainly feeding on prey items that are submerged in the sediments. The L-morph diet in contrast comprises pleuston, plankton, nekton and littoral benthos, and the fish appear to utilize both the bottom gradient from the shallow littoral to the deep profundal habitat as well as different parts of the water column in their feeding. The Arctic charr is a physostomous fish species, having an open connection (the pneumatic duct) between the swim bladder and the esophagus. This means that fish that are emerging in the water column are able to regulate their buoyancy by releasing gases from the swim bladder as these expand due to the decreasing atmospheric pressure. When L-morph charr have been caught by traps in deep water in Fjellfrøsvatn, the gases released from the swim bladder can be observed as emerging gas bubbles when the traps are hauled to the lake surface. However, according to our observations the P-morph charr are in contrast not able to release gases from their swim bladder as they are hauled from deep water. They therefore arrive at the lake surface with highly inflated gas bladders and even after several hours of decompression at about 5 m depth, their bladders are still expanded. Apparently, their pneumatic duct is not open and they can therefore not regulate the volume of their swim bladder. Consequently, a P-morph charr that starts to emerge in the water column will rapidly get a positive buoyancy due to an increasing gas volume in the bladder. The fish may eventually be unable to swim downwards again with its increasingly inflated swim bladder, resulting in an uncontrolled and likely fatal ascent to the lake surface. The strong orientation towards the bottom in the profundal habitat of the P-morph charr may thus be related to the apparent dysfunction of the pneumatic duct of their swim bladders, and this may tentatively have played a significant role in the evolutionary divergence of the two morphs.
Knudsen et al. (2006) suggested that the L-morph represents the ancestral Arctic charr form that immigrated to Fjellfrøsvatn after the last glacial period, and that the P-morph has evolved in sympatry from this ancestral form to become an effective soft-bottom prey feeder by invading a novel profundal food niche. This was supported by a comparison of the ecology of the Fjellfrøsvatn charr morphs with monomorphic charr populations from four other lakes, which showed that the P-morph charr utilize a food resource that is not utilized by neither the L-morph nor comparable monomorphic populations (Knudsen et al. 2006). It was therefore concluded that the P-morph in Fjellfrøsvatn likely has evolved as a result of a niche expansion, where high intraspecific competition with in the ancestral Arctic charr population may have promoted part of the population to specialize and utilize untapped food resources in the profundal habitat. Expansions towards the utilization of new resources are important in respect to adaptive radiation, and divergence and incipient speciation may develop rapidly if an unoccupied niche is invaded (Schluter 2000). Heterochrony, i.e. perturbations in the onset or timing of ontogenetic developments, has been suggested as a potential driving mechanism for the development of polymorphism in Arctic charr (Eiriksson et al. 1999; Skulason et al. 1999; Klemetsen et al. 2003a). Heterochronous adaptations that occur early in the charr ontogeny may be particularly important (Eiriksson et al. 1999; Jonsson and Skulason 2000; Adams and Huntingford 2002). Small-sized morphotypes like the P-morph in Fjellfrøsvatn are often considered to be paedomorphs that retain juvenile characters in the adult phenotype (Klemetsen et al. 2003a). Ontogenetic niche shifts in the ancestral charr morph in Fjellfrøsvatn may in this respect have been important for the evolution of the P-morph through a niche expansion in the profundal habitat. Hypothetically, the observed profundal habitat use of the juvenile L-morph charr may have been a starting point for the present polymorphism, with heterochronic paedomorphosis as the driving evolutionary force. The polymorphic charr in Fjellfrøsvatn may thus be an example of evolutionary branching through ontogenetic niche shifts as suggested by Claessen and Dieckmann (2002), but further studies are needed for a critical test of these theoretical considerations.
In conclusion, the two Arctic charr morphs in Fjellfrøsvatn exhibited distinct resource differentiation during all annual seasons and throughout their life cycles, except for a dietary overlap between P-morph and juvenile L-morph charr in the profundal during summer. The P-morph was restricted to the profundal habitat, whereas the L-morph used all main habitats and exhibited characteristic ontogenetic shifts in habitat and diet utilization. The L-morph is likely representative for the ancestral charr form that invaded the lake after the last glacial period, and the documented ontogenetic niche shifts, in particular the juvenile profundal-dwelling stage, may have been important in the evolution of polymorphism in the lake through niche expansion. An apparent dysfunction of the pneumatic duct of the swim bladder of P-morph charr may furthermore contribute to the highly restricted habitat and diet utilization of this morph, and potentially have had a significant role in the resource differentiation and evolutionary divergence of the two morphs.
References
Adams CE, Huntingford FA, Fraser D, Greer RG, Askew CM, Walker AF (1998) Trophic polymorphism amongst Arctic charr from Loch Rannoch, Scotland. J Fish Biol 52:1259–1271
Adams CE, Huntingford FA (2002) The functional significance of inherited differences in feeding morphology in sympatric polymorphic population of Arctic charr. Evol Ecol 16:15–25
Alekseyev SS, Samusenok VP, Matveev AN, Pichugin MY (2002) Diversification, sympatric speciation, and trophic polymorphism of Arctic charr, Salvelinus alpinus complex, in Transbaikalia. Environ Biol Fish 64:97–114
Amundsen P-A (1995) Feeding strategy of Arctic charr: general opportunist, but individual specialist. Nordic J Freshw Res 71:150–156
Amundsen P-A, Gabler H-M, Staldvik FJ (1996) A new method for graphical analysis of feeding strategy from stomach contents data. J Fish Biol 48:607–614
Amundsen P-A, Knudsen R, Klemetsen A, Kristoffersen R (2004) Resource partitioning and interactive segregation between sympatric whitefish morphs. Ann Zool Fennici 41:301–307
Bernatchez L (2004) Ecological theory of adaptive radiation. An empirical assessment from Coregonine fishes (Salmoniformes) In: Hendry AP, Stearns SC (eds) Evolution illuminated: salmon and their relatives. Oxford University Press, Oxford, p 175
Bernatchez L, Dodson JJ (1990) Allopatric origins of sympatric populations of lake whitefish (Coregonus clupeaformis) as revealed by mitochondrial DNA restriction analysis. Evolution 44:1263–1271
Bjøru B, Sandlund OT (1995) Differences in morphology and Ecology within a stunted Arctic charr population. Nordic J Freshw Res 71:163–172
Claessen D, Dieckmann U (2002) Ontogenetic niche shifts and evolutionary branching in size-structured populations. Evol Ecol Res 4:189–217
Dieckmann U, Doebeli M, Metz JAJ, Tautz D (eds) (2004) Adaptive speciation. Cambridge University Press, Cambridge
Dynes J, Magnan P, Bernatchez L, Rodriguez MA (1999) Genetic and morphological variation between two forms of lacustrine brook charr. J Fish Biol 54:955–972
Eiriksson G, Skulason S, Snorrason SS (1999) Heterochrony in skeletal development and body size in progeny of two morphs of Arctic charr from Thingvallavatn, Iceland. J Fish Biol 55:175–185
Guiger KRRA, Reist JD, Power M, Babaluk JA (2002) Using stable isotopes to confirm the trophic ecology of Arctic charr morphotypes from Lake Hazen, Nunavut, Canada. J Fish Biol 60:348–362
Hjelm J, Persson L, Christensen B (2000) Growth, morphological variation and ontogenetic niche shifts in perch (Perca fluviatilis) in relation to resource availability. Oecologia 122:190–199
Henricson J, Nyman L (1976) The ecological and genetical segregation of two sympatric species of dwarfed char (Salvelinus alpinus (L.) species complex). Rep Inst Freshw Res, Drottninghom 55:15–37
Hindar K, Jonsson B (1982) Habitat and food segregation of dwarf and normal Arctic charr (Salvelinus alpinus) from Vangsvatnet lake, western Norway. Can J Fish Aquat Sci 39:1030–1045
Jansen PA, Finstad AG, Langeland A (2002) The relevance of individual size to management of Arctic charr, Salvelinus alpinus, populations. Env Biol Fish 64:313–320
Jonsson B, Jonsson N (2001) Polymorphism and speciation in Arctic charr. J Fish Biol 58:605–638
Jonsson B, Skulason S (2000) Polymorphic segregation in Arctic charr Salvelinus alpinus (L.) from Vatnshlidarvatn, a shallow Icelandic lake. Biol J Linnean Soc 69:55–74
Klemetsen A (1984) The Arctic charr speciation problem as seen from Northern Norway. In: Johnson L, Burns B (eds) Biology of the Arctic charr. Proceedings of the international symposium on Arctic charr. University of Manitoba Press, Winnipeg, p 65
Klemetsen A, Grotnes PE (1975) Food and habitat segregation of two sympatric Arctic char populations. Verh Internat Verein Limnol 19:2521–2528
Klemetsen A, Grotnes PE (1980) Coexistence and immigration of two sympatric Arctic charr populations. In: Balon EK (ed) Charrs, salmonid fishes of the genus Salvelinus. Junk, The Hague, p 757
Klemetsen A, Grotnes PE, Holthe H, Kristoffersen K (1985) Bear Island charr. Rep Inst Freshw Res Drottningholm 62:98–119
Klemetsen A, Amundsen P-A, Muladal H, Rubach S, Solbakken JI (1989) Habitat shifts in a dense, resident Arctic charr Salvelinus alpinus population. Physiol Ecol Japan, Special volume 1:187–200
Klemetsen A, Amundsen P-A, Knudsen R, Hermansen B (1997) A profundal, winter-spawning morph of Arctic charr Salvelinus alpinus (L.) in lake Fjellfrøsvatn, northern Norway. Nordic J Freshw Res 73:13–23
Klemetsen A, Elliott JM, Knudsen R, Sørensen P (2002) Evidence for genetic differences in the offspring of two sympatric morphs of Arctic charr. J Fish Biol 60:933–950
Klemetsen A, Amundsen P-A, Dempson B, Jonsson B, Jonsson N, O’Connell MF, Mortensen E (2003a) Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol Freshw Fish 12:1–59
Klemetsen A, Staldvik FJ, Knudsen R, Amundsen P-A (2003b) Habitat, diet and food assimilation of Arcitic charr under the winter ice in two subarctic lakes. J Fish Biol 62:1082–1098
Klemetsen A, Knudsen R, Primicerio R, Amundsen P-A (2006) Divergent, genetically based feeding behaviour of two sympatric Arctic charr, Salvelinus alpinus (L.), morphs. Ecol Freshw Fish 15:350–355
Knudsen R, Kristoffersen R, Amundsen P-A (1997) Parasite communities in two sympatric morphs of Arctic charr Salvelinus alpinus (L.) in northern Norway. Can J Zool 75:2003–2009
Knudsen R, Klemetsen A, Amundsen P-A, Hermansen B (2006) Incipient speciation through niche expansion: an example from the Arctic charr in a subarctic lake. Proc Roy Soc B 273:2291–2298
L’Abée-Lund JH, Langeland A, Jonsson B, Ugedal O (1993) Spatial segregation by age and size in Arctic charr: a trade-off between feeding possibility and risk of predation. J Anim Ecol 62:160–168
Lu G, Bernatchez L (1999) Correlated trophic specialization and genetic divergence in sympatric lake whitefish ecotypes (Coregonus clupeaformis): support for the ecological speciation hypothesis. Evolution 53:1491–1505
Malmquist H J, Snorrason SS, Skulason S, Jonsson B, Sandlund OT, Jonasson PM (1992) Diet differentiation in polymorphic Arctic charr in Thingvallavatn, Iceland. J Anim Ecol 61:21–35
McPhail JD (1994) Speciation and the evolution of reproductive isolation in the sticklebacks (Gasterosteus) of southwestern British Columbia. In: Bell MA, Foster SA (eds) Evolutionary biology of the three-spine stickleback. Oxford University Press, Oxford, p 399
Nyman L, Hammar J, Gydemo R (1981) The systematics and biology of landlocked populations of Arctic charr from northern Europe. Rep Inst Freshw Res Drottningholm 59:128–141
O’Connell MF, Dempson JB (2002) The biology of Arctic charr, Salvelinus alpinus, of Gander Lake, a large, deep, oligotrophic lake in Newfoundland, Canada. Env Biol Fish 64:115–126
Østbye K, Amundsen P-A, Bernatchez L, Klemetsen A, Knudsen R, Kristoffersen R, Næsje T, Hindar K (2006) Parallel evolution of eco-morphological traits in European whitefish Coregonus lavaretus (L.) during postglacial times. Mol Ecol 15:3083–4001
Proulx R, Magnan P (2004) Contribution of phenotypic plasticity and heredity to the trophic polymorphism of lacustrine brook charr (Salvelinus fontinalis M.). Evol Ecol Res 6:503–522
Reist JD, Gyselman E, Babaluk JA, Johnson JD, Wissink R (1995) Evidence for two morphotypes of Arctic charr Salvelinus alpinus (L.) from Lake Hazen, Ellesmere Island, Northwest Territories, Canada. Nordic J Freshw Res 71:396–410
Riget FF, Nygaard KH, Christensen B (1986) Population structure, ecological segregation, and reproduction in a population of arctic char (Salvelinus alpinus) from lake Tasersuaq, Greenland. Can J Fish Aquat Sci 43:985–992
Sacotte S, Magnan P (2006) Inherited differences in foraging behaviour in the offspring of two forms of lacustrine brook charr. Evol Ecol Res 8:843–857
Sandlund OT, Gunnarson K, Jonasson PM, Jonsson B, Lindem T, Magnusson KP, Malmquist HJ, Sigurjonsdottir H, Skulason S, Snorrason SS (1992) The arctic charr Salvelinus alpinus in Thingvallavatn. Oikos 64:305–351
Savvaitova K (1995) Patterns of diversity and processes of speciation in Arctic charr. Nord J Freshw Res 71:81–91
Schluter D (1996) Ecological speciation in postglacial fishes. Phil Trans R Soc Lond B 351:807–814
Schluter D (2000) The ecology of adaptive radiation. Oxford Univ Press, Oxford
Schluter D (2001) Ecology and the origin of species. Trends Ecol Evol 16:372–380
Schluter D (2003) Frequency dependent natural selection during character displacement in sticklebacks. Evolution 57:1142–1150
Schluter D, MaPhail JD (1992) Ecological character displacement and speciation in sticklebacks. Am Nat 140:85–108
Skulason S, Smith TB (1995) Resource polymorphism in vertebrates. Trends Ecol Evol 10:366–370
Skulason S, Snorrason SS, Jonsson B (1999) Sympatric morphs, populations and speciation in freshwater fish with emphasis on Arctic charr In: Magurran AE, May RM (eds) Evolution of biological diversity. Oxford University Press, Oxford, p 70
Smith HD, Skulason S (1996) Evolutioninary significance of resource polymorphisms in fishes amphibians and birds. Ann Rev Ecol Syst 27:111–133
Snorrason SS, Skulason S, Jonsson B, Sandlund OT, Malmquist HJ, Jonasson PM (1994) Trophic specialisation in Arctic charr Salvelinus alpinus (Pisces: Salmonidae): morphological divergence and ontogenetic niche shifts. Biol J Linnean Soc 52:1–18
Svärdson G (1957) The coregonid problem. VI. The palearctic species and their intergrades. Rep Inst Freshw Res Drottningholm 38:267–356
Svärdson G (1998) Postglacial dispersal and reticulate evolution of Nordic coregonids. Nordic J Freshw Res 74:3–32
Taylor EB, McPhail JD (2000) Historical contingency and ecological determinism interact to prime speciation in sticklebacks, Gasterosteus. Proc Roy Soc Lond B 267:2375–2384
Westgaard JI, Klemetsen A, Knudsen R (2004) Genetic differences between two sympatric morphs of Arctic charr Salvelinus alpinus (L.) confirmed by microsatellite DNA. J Fish Biol 65:1185–1191
Wilson AJ, Gíslason D, Skúlason S, Snorrason SS, Adams CE, Alexander G, Danzmann RG, Ferguson MM (2004) Population genetic structure of Arctic charr, Salvelinus alpinus, from northwest Europe on large and small spatial scales. Mol Ecol 13:1129–1142
Werner EE (1986) Species interactions in freshwater communities. In: Diamond J, Case T (eds) Community ecology. Harper and Row, New York, p 344
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Ann Rev Ecol Syst 15:393–425
Acknowledgements
Many people have contributed to this study, but we especially like to thank Laina Dalsbø, Jan Evjen and Bjørn Hermansen for their skilful assistance in the field and laboratory work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amundsen, PA., Knudsen, R. & Klemetsen, A. Seasonal and ontogenetic variations in resource use by two sympatric Arctic charr morphs. Environ Biol Fish 83, 45–55 (2008). https://doi.org/10.1007/s10641-007-9262-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-007-9262-1