Abstract
Here we offer an integrative review of biogeochemical cycling of carbon, nitrogen, phosphorus, and several metals in salt marshes of the Bahía Blanca Estuary, located in South America, which is a region underrepresented in the literature. The dominant species, Spartina alterniflora and Sarcocornia perennis, have low net aboveground primary productivity but play substantial and contrasting roles in the biogeochemical cycling of elements. S. perennis was more efficient at metal sequestration, whereas S. alterniflora was important in the immobilization of phosphorus. Because of the differences in net aboveground primary productivity between high and low marsh, plant position should be considered to evaluate the role of S. alterniflora on biogeochemical cycles. Some elements were also in high concentrations in belowground tissues but, based on our data, we could not accurately estimate net belowground primary productivity, a key process to evaluate elemental cycling in salt marshes. In spite of uncertainties in the estimations, the slower decomposition rates in S. alterniflora would be indicative of a higher contribution to the long-term storage of nutrients and metals within the marsh. Regardless shortcomings, our work represents a valuable tool for comparisons with salt marshes worldwide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salt marshes are recognized worldwide for the various ecosystem services they provide, including improvement of water quality and carbon sequestration (Gedan et al., 2009; Barbier et al., 2011). Salt marsh plants commonly sequester significant amounts of atmospheric carbon (Chmura et al., 2003), providing tools for mitigation of human emissions. They may also contribute to reduce eutrophication and metal pollution in coastal waters, because of their commonly high rates of nutrient uptake and metal sequestration (Weis & Weis, 2004; Sousa et al., 2008, 2010). Ecosystem services previously mentioned depend on the ecological integrity of the salt marsh (Bedford & Preston, 1988; Brinson, 1988), and different indicators are used to quantify changes in coastal ecosystems health. Estimations of biomass and net primary productivity are widely used as indicators of ecosystem condition (e.g., Day et al., 1989; Turner et al., 2004). Both above and belowground biomass have ecological implications. The production of aboveground biomass may lead to large amount of detritus that can remain and decompose in situ, but can also be exported to the adjacent waters by tides and winds, playing a significant role in estuarine food webs (Taylor & Allanson, 1995; Odum, 2000). Belowground biomass, in turn, can prevent soil erosion and enhance sediment retention, promoting the buildup of marsh elevation to keep pace with sea level rise (Turner et al., 2000).
Elemental cycling in salt marshes involves many different ecological processes, each of them affected by a particular set of environmental conditions. For instance, for biomass production and growth, plants take up nutrients from the sediments. Both essential and toxic metals can also be absorbed during plant growth (e.g., Rascio & Navari-Izzo, 2011; Tangahu et al., 2011). The rate of uptake of nutrients and metals depends mainly on the elemental concentrations in the pore water and on sediment characteristics like grain size, organic matter content, redox potential (Eh) and pH (e.g., Koretsky et al. 2008; Reboreda et al., 2008; Wang et al., 2013). After their uptake by roots, the different elements can be translocated to leaves and stems, setting a distinctive distribution of elements within aerial and belowground tissues, and linking their cycling to the different fate of above and belowground biomass. The fate of aboveground biomass is highly dependent on hydrodynamics and, particularly, on the plant position in the tidal frame (Taylor & Allanson, 1995; Bouchard & Lefeuvre, 2000). After senescence, plant tissues release nutrients and metals back to the system through decomposition, at a rate that depends not only on environmental conditions but also on detritus quality (e.g., content of lignin and nitrogen) (Enriquez et al., 1993; Rejmánková & Houdková, 2006). Hence, decomposition rates usually differ between species and between organs within a species (Bouchard & Lefeuvre, 2000; Sousa et al., 2008, 2010). These are just a few examples of the many sources of variation involved in the biogeochemical cycling of elements in salt marshes. It is worth noting, however, that most of the information on the topic comes from studies in North America, Europe, and more recently Asia. Therefore, research from poorly studied regions, based on different salt marsh species and under distinctive environmental conditions, may provide novel insights in the understanding of the functioning and role of these highly dynamics ecosystems.
More than a decade ago, we started studying ecological and biogeochemical processes in salt marshes of the Bahía Blanca estuary (hereafter BBE), South Atlantic, Argentina. We have published part of our results (Botté, 2005; Hempel et al., 2008; González Trilla et al., 2009; Botté et al., 2010; Pratolongo et al., 2010; Negrin, 2011; Negrin et al., 2011, 2012a, b, 2013, Pratolongo et al., 2013; Negrin et al., 2015), which combined would provide insight to the general patterns of ecosystem functioning in the area. Our goal is to do an integrative review of salt marshes in the BBE analyzing their role in the biogeochemical cycling of elements. Here we report biomass, decomposition, nutrient and metal dynamics in the two compartments (belowground and aboveground) of salt marshes, considering the dominant species in this estuary, Spartina alterniflora and Sarcocornia perennis. We combine this information and interpret the results in the context of the physicochemical conditions of sediments and the dominant land cover changes observed in the area. We could also identify the major shortcomings of the work performed so far and highlight the needs of further study.
The Bahía Blanca estuary
The BBE (Fig. 1a) is a mesotidal shallow system which mostly behaves as vertically homogeneous. It extends over 2,300 km2 and is formed by a series of northwest to southeast-oriented tidal channels separated by extensive tidal flats, salt marshes and islands. The northern portion of the estuary is dominated by Canal Principal, a funnel-shaped channel that has a total length of 61 km and varies in width from 200 m at the head to about 3–4 km at the mouth (Piccolo et al., 2008). Tides and winds are the main inputs of energy to the estuarine circulation. Tidal energy is provided by a quasi-stationary semidiurnal tidal wave (Perillo & Piccolo, 1991), and winds are persistent all year round (Piccolo, 2008).
The climate of the region is temperate and classified as mid-latitude dry and semiarid, with higher precipitation in summer and lower in winter. Large temporal rainfall variability has been regarded as the major climatic feature for the region (Scian & Pierini, 2013). Dry and wet periods may last decades and alternate in a cyclical oscillation, with annual precipitation values that may range from less than 300 mm year−1 during the arid phase to values exceeding 900 mm year−1 in extremely wet years (Zotelo, 2011). Rainfall patterns affect the freshwater input to the BBE, which is usually low due to the scarce discharge of the only two permanent tributaries. The Sauce Chico River and Napostá Grande Creek discharge, in average, 1.9 and 0.8 m3 s−1, respectively (Piccolo et al., 2008).
The BBE is characterized by high salinity, which usually varies between 33 and 40, but values as low as 10 have been reported (e.g., Botté et al., 2007; La Colla et al., 2015). The inner zone of the BBE is a nutrient-enriched environment that maintains high levels of organic matter and inorganic nutrients during most of the year (Freije et al., 2008). Dissolved metal concentrations are comparable with the values found in low-impacted systems (e.g., Botté et al., 2007; La Colla et al., 2015), but the particulate form of some metals are in higher concentrations than in several polluted estuaries (e.g., La Colla et al., 2015). Anthropogenic influence depends on different local pressures: cities, industries and livestock-agriculture. Sewage and industrial discharges receive poor or no treatment before reaching the estuary (Limbozzi & Leitào, 2008). In association with the industries, there is one of the largest deep water ports in the country (Ingeniero White). Due to the circulation of big ships, the Canal Principal is regularly dredged with physical and social consequences to the system (Zilio et al., 2013).
Salt marshes of the BBE
Salt marshes are distributed along all the margins of channels and on all the islands. The dominant salt marsh species are Spartina alterniflora and Sarcocornia perennis (Fig. 1b, c). S. alterniflora Loisel. (Poaceae) is a perennial grass native of the Atlantic coast of North and South America, but has been introduced worldwide for erosion control (NBBII & IUCN/SSC, 2005). S. perennis (Miller) A.J. Scott (Chenopodeaceae) is a perennial succulent subshrub that grows in well-drained sediments. It is globally distributed, occurring in Europe, Africa, and the Americas (Davy et al., 2006). In the SW Atlantic, S. perennis is a common species from the BBE to Tierra del Fuego (Isacch et al., 2006; Bortolus et al., 2009). S. alterniflora, in turn, is usually the dominant species from southern Brazil to the northern coasts of Argentina. The southernmost record of this species is 42º25′ (Bortolus et al., 2009). Therefore, in the BBE, these two species overlap, allowing for comparative studies.
Pure stands of S. alterniflora, which cover an area of 196 km2, are commonly restricted to marshes in the middle zone of the BBE, but rarely appear in the inner area (Piovan et al., 2014). In the middle zone of the BBE, S. alterniflora marshes form monospecific associations with variable plant heights and densities, from sparse stands of plants, up to 20 cm tall (González Trilla et al., 2013) to dense stands taller than 1 m in places subject to high sedimentation rates (Pratolongo et al., 2010). In the inner section of BBE, seasonally hypersaline conditions commonly develop, and salt marshes in this zone are usually restricted to elevations close to the mean high-tide level, with S. perennis as the dominant species (Pratolongo et al., 2010). Intertidal marshes of S. perennis are less represented (72 km2), and this species commonly forms circular mounds, sometimes in association, at higher elevations, with Heterostachys ritteriana or Spartina densiflora. Vegetation cover in S. perennis marshes is highly uneven, from a few isolated patches in a matrix of bare soil to an almost continuous carpet at some locations (Piovan et al., 2014).
The erosion of S. perennis marshes is the dominant landscape process in the BBE. Pratolongo et al. (2013) estimated that salt marsh loss would have been about 267 ha year−1 between 1967 and 2005, mainly driven by lateral erosion at the edge of the marsh, as well as land replacement by human uses. The current rate of relative sea level rise, estimated from tidal records in the area, is 1.6 mm year−1 (Lanfredi et al., 1988). The coastal platform presently occupied by S. perennis marshes formed under the higher relative sea level during the Holocene transgressive phase (González, 1989). At present, these low lying coastal landforms became relict and their erosion may be accelerated by sea level rise. On the other hand, S. alterniflora marshes are expanding their cover in a process that involves sediment accretion and mudflat colonization by plants (Pratolongo et al., 2010, 2013). While the observed erosion of soft sediments is in agreement with the rising relative sea level, the loss of S. perennis marshes supplies large amounts of suspended solids to the water column and may contribute to the sediment deposition that allows the expansion of S. alterniflora marshes. In the harbor area, land cover changes are clearly dominated by human activities. In the northern shore of Canal Principal, in front of major cities, 33% of the area originally covered by S. perennis marshes was replaced by coastal development infrastructure, and the expansion of S. alterniflora was related to sedimentation after dredging activities (Pratolongo et al., 2013).
Salt marshes of the BBE: belowground compartment
Belowground biomass, nutrient and metal contents, and decomposition rates have been evaluated in two different salt marshes from the BBE: Villa del Mar (VM), dominated by S. alterniflora, and Puerto Cuatreros (PC), dominated by S. perennis. In VM, S. alterniflora grows within a broad range of elevations within the tidal frame, with usually conspicuous differences in size between plants in the lower (smaller plants) and higher (taller plants) elevations within the marsh (hereafter, LM and HM, for lower marsh and higher marsh, respectively). Both zones (LM and HM) were studied separately in all cases because of the recognized effects of flooding in biogeochemical cycles. In the S. perennis marsh (PC), no distinction was made between LM and HM because of the restricted range of elevations and the uniform appearance of the marsh. Belowground biomass of both species was evaluated by destructive methods, in a one-and-a-half-year study for S. alterniflora (Negrin et al., 2012a) and in one-year study for S. perennis (Negrin et al. 2015). We have also studied the elemental concentration of carbon (C), nitrogen (N) and phosphorus (P) in tissues of S. alterniflora (Negrin et al., 2012a) and S. perennis (Negrin et al., 2015). Decomposition rate and elemental dynamics of C, N, and P during decomposition were evaluated in S. alterniflora (VM) (Negrin et al., 2012b) and S. perennis (PC) (Negrin et al., 2015). In the same marshes, metal concentrations in sediments and in belowground tissues of S. alterniflora (Hempel et al., 2008) and S. perennis (Botté, 2005) were also evaluated. Values presented are mean ± standard deviation.
Belowground biomass
Belowground biomass of S. alterniflora was sampled in VM from October 2006 to April 2008 in LM and HM (Negrin et al., 2012a). Sampling of S. perennis biomass in PC was performed from November 2007 to November 2008 (Negrin et al., 2015). Samples were collected using a corer of 11 cm in diameter for sampling the first 15 cm which contains the majority of belowground biomass of the studied species (Gross et al., 1991; Palomo & Niell, 2009). The plant material was washed free of sediment in a 500-m sieve, dried at 60°C for 72 h and weighed to the nearest 0.01 g. Live and dead plant material could not be separated.
The maximum belowground biomass of S. alterniflora in HM and LM was 935 ± 760 and 862 ± 281 g m−2, respectively (Negrin et al., 2012a), whereas maximum belowground biomass of S. perennis was 350 ± 134 g m−2 (Negrin et al., 2015). Differences between zones and species were not compared in the cited works, which is an important shortcoming of this work, but reanalyzing the original data we found that maximum biomass of S. perennis was significantly lower than maximum biomass of LM S. alterniflora (Student’s t test: P < 0.01). Differences between S. perennis and HM S. alterniflora and between HM and LM were not statistically significant (Student’s t test: P > 0.05 and P > 0.5, respectively).
C, N and P
The elemental concentration of C, N and P was estimated in S. alterniflora (Negrin et al., 2012a) and S. perennis (Negrin et al., 2015) in the same samples used for biomass estimations. Dried belowground biomass from all samples at each sampling date was pooled and ground in one representative composite sample. C was estimated by dry combustion (carbon elemental analyzer LECO model CR12), N with the Kjeldahl technique, and P by the Watanabe & Olsen (1965) method. Element pools were calculated multiplying the element concentration by the mean biomass at each sampling date.
Concentrations of C, N, and P in belowground tissues (%, Table 1, made based on Negrin et al., 2012a, b, 2015) were in the range of those reported for the same species in other salt marshes in the world (see references in Negrin et al. 2012a, 2015). C, N, and P pools (g m−2) of both species varied along the study period (Fig. 2, made based on Negrin et al., 2012a and 2015), mainly reflecting biomass patterns.
Decomposition
Decomposition rates of belowground biomass of S. alterniflora (Negrin et al., 2012b) and S. perennis (Negrin et al., 2015) were estimated by measuring the disappearance of material from litter bags (Bocock & Gilbert, 1957), a method widely used in salt marshes (e.g., Blum, 1993; Menéndez & Sanmartí, 2007; Simões et al., 2011). Plastic bags (20 × 20 cm, 2 mm of mesh size) were filled with plant tissues collected from the study site, which were previously rinsed and dried at 60°C for 72 h. Bags were labeled and their content was individually weighed (approximately 10 g of biomass). At the beginning of the sampling period, bags (N = 21 for S. alterniflora and N = 12 for S. perennis) were buried at a depth of 10 cm in its corresponding salt marsh. Every 2 months, during a year, between two and four bags were removed and taken to the laboratory. The content of each bag was washed, dried, and weighed. The amount of biomass retrieved from litterbags represents the net balance between decomposition of the original material and the appearance of new tissues due to root ingrowth. Fresh belowground tissues and new growth were never observed inside litterbags, so it was assumed that root ingrowth was negligible. Under the assumption of no net primary production inside bags, the disappearance of material during the experiment was entirely considered as loss of biomass due to decomposition. For the S. alterniflora marsh only HM was evaluated, given that tides and winds made impossible to keep the litterbags in the field in LM. Two bags from each sampling date were analyzed for the C, N and P contents (%) using the same analytical procedures described for biomass. C/N and C/P molar ratios were calculated.
Decomposition rates (% of plant material lost after a year) of belowground tissues of both species are shown in Table 1. Although the decomposition rates of both species were not compared statistically in the cited papers, reanalyzing here the original data, we found that S. perennis decomposed at a rate significantly faster than S. alterniflora (Student’s t test: P < 0.01). These results suggest that S. perennis was more efficient in the recycling of elements, while S. alterniflora would play a major role in the long-term storage of elements and the building up of elevation, through the accumulation of undecomposed plant material. Lower decomposition rates are usually associated with higher C/N and C/P ratios (Enriquez et al., 1993; Rejmánková & Houdková, 2006), and both ratios showed a trend of higher values in belowground tissues of S. alterniflora than in S. perennis at the beginning of the experiment (Table 1). Comparing with other salt marshes through the world, we found lower values than those reported for S. alterniflora and higher rates that those published for S. perennis or related species (see references in Negrin et al., 2012b, 2015). Although compared values always referred to estimations obtained through the litterbag technique, methodological considerations, like assuming a negligible root ingrowth, may largely bias results. Estimations of belowground decomposition in marshes are scarce, especially for S. perennis; to the best of our knowledge, Palomo & Niell (2009) is the only reference in the international literature, besides Negrin et al., (2015). Hence, our results, taken carefully after considering methodological limitations, may provide valuable information about this species.
During decomposition, both species showed fluctuations in their contents of C, N, and P and in molar ratios (C/N and C/P) (Negrin et al., 2012b, 2015). This might be related with the variations of environmental conditions and/or variations in the composition of decomposer communities through time (e.g., Menéndez & Sanmartí, 2007; Simões et al., 2011). Despite fluctuations, there was a trend of higher C/P at the end than at the beginning of the experiment for both species, and the opposite was true for C/N (Table 1). This suggests that there would be a net release of P, but not of N, through decomposition of belowground tissues. Nitrogen may be immobilized, probably because of the bounding of exogenous N to plant constituents, mediated by microbial activity (Pozo & Colino, 1992; Simões et al., 2011). The different patterns of N and P release could be due to the fact that N remineralization is strictly microbially mediated, whereas phosphorus may be remineralized autolytically (Pozo & Colino, 1992).
Metals
Metal concentrations were evaluated in VM (S. alterniflora marsh; Hempel et al., 2008) and in PC (S. perennis marsh; Botté, 2005). In both cases, belowground tissues and sediments (between roots of each species and in bare mudflats close to the marsh) were analyzed. Sampling was carried out once in April 2006 for S. alterniflora and for S. perennis from June 2000 to October 2001, in a monthly basis. Although the same marshes were evaluated for biomass, decomposition and content of C, N, and P and for metals, it should be considered that the sampling was not simultaneous. Plant tissues were washed and dried. Sediments were cleaned free of roots and shell fragments with tweezers and dried. Both tissues and sediments were ground and metal concentrations were determined following the methodology described by Ferrer et al. (2000). Subsamples of approximately 0.5 g were separated into two test tubes, spiked with 3 ml of concentrated nitric acid (65%) and 1 ml of concentrated perchloric acid (HClO4; 70–72%), and taken to a glycerine bath at 120 ± 5°C. After digestion, 0.7% nitric acid was added to the residue up to 10 ml into centrifuge tubes. Metal concentrations (chromium (Cr), cadmium (Cd), lead (Pb), nickel (Ni), cooper (Cu), and zinc (Zn)) of these solutions were measured by Atomic Absorption Spectroscopy (AAS) with air-acetylene flame using a Perkin-Elmer AA-2380 spectrophotometer. Analytical grade reagents, reagent blanks, and certified reference materials (CRM) were used to ensure analytical quality control. Pond sediments (R.M. N º2) were used as CRM for sediments and pepperbush (R.M. N º1) for plants. CRM was provided by The National Institute for Environmental Studies (NIES), from Tsukuba, Japan. The recovery percentages for all metals in its corresponding CRM were between 80 and 110%, indicating a high efficiency of extraction and of measurement. In addition, all samples were analyzed by duplicate.
Compared with different salt marshes throughout the world (e.g., Reboreda & Caçador, 2007; Caetano et al., 2008; Wang et al., 2013), metal concentrations in sediments from the BBE were low (Fig. 3, made based on Botté, 2005; Hempel et al., 2008), even lower than those in estuaries as Mondego, which is considered to have low levels of contamination (Couto et al., 2013). Our low values are in agreement with the concentrations found in pore water in the BBE (Botté et al., 2007). Considering bare and vegetated sediments some trends were observed. Most metals showed higher concentrations in sediments between roots of LM S. alterniflora than in adjacent bare sediments; in the case of HM S. alterniflora, this pattern was not observed (Fig. 3). Sediments between the roots of S. perennis also showed a slightly higher concentration of metals than bare sediments (Fig. 3). Despite these are only trends, these results might suggest that LM S. alterniflora and S. perennis would be efficient in the accumulation of metals in sediments. Deeper analysis of the data is desirable, especially for S. alterniflora (both HM and LM) since the available information was obtained from a punctual sampling date.
In both salt marshes, and for most metals, concentrations were higher in sediments than in roots (Fig. 3). Zn, however, was more concentrated in belowground tissues of HM S. alteniflora than in surrounding sediments, whereas Cu showed a trend to higher concentration in tissues in LM S. alterniflora (Fig. 3). In S. perennis, even though the concentrations of Zn and Cu were lower in belowground tissues than in sediments, the difference between both matrixes was lower than for the other metals (Fig. 3). Zn and Cu are essential elements for plant growth and that could explain these patterns of concentration. The accumulation of only a few metals, including Zn, in belowground tissues was also observed in a nonimpacted Spartina densiflora salt marsh in Argentina (Idaszkin et al., 2014). Similar observations were made by Couto et al. (2013) in a low-impacted estuary. Caetano et al. (2008) evaluated the concentration of several metals (Fe, Mn, Zn, Cr, Ni, Cu, As, and Cd) in plant tissues and vegetated sediments in a highly human-impacted estuary (Tagus Estuary) and found that the most of them had higher concentrations in belowground tissues than in associated sediments. In the BBE, only two metals were slightly more concentrated in plant tissues than in sediments, which is probably due to the abovementioned low levels of metals in sediments. Despite these are only trends, the study of metal cycling in the BBE should not be dismissed, especially considering of the observed tendency to increasing metal levels in waters in the last years (La Colla et al., 2015).
The ability of salt marsh plants to uptake metals and, hence, the distribution of these elements within sediments or plant tissues, depends on several factors, including plant species and the metal considered (e.g., Weis & Weis, 2004; Reboreda & Caçador, 2007; Couto et al., 2013). Differential uptake related to the metal evaluated has already been discussed in the previous paragraph. Regarding plant species, unfortunately we did not observed clear patterns between S. perennis and S. alterniflora in the BBE, mainly because of the limited amount of information available for the later. Sediment condition is another important factor to be evaluated (Weis & Weis, 2004; Reboreda et al., 2008; Wang et al., 2013). Changes in Eh and pH can produce changes in metal speciation and solubility and, therefore, differential fluxes from sediments to pore water and uptake rates. In salt marshes, plants can oxidize the sediments in the root zone through the movement of oxygen downwards through aerenchyma tissue, and this oxidation can remobilize the metals (e.g., Weis & Weis, 2004). Differences in Eh values were observed between the studied marshes and zones, which followed this trend: LM S. alterniflora < HM S. alterniflora < S. perennis marsh (Negrin et al., 2011; 2013). This would imply a greater availability of metals in sediments following the same trend, which is consistent with the concentration in sediments of some metals (Cr, Cd) but not others (Fig. 3). The sampling we are carrying out at the moment considers more samples for determination of metal concentration and the measurement of sediment conditions simultaneously, which would allow a better analysis of metal dynamics in salt marshes.
Salt marshes of the BBE: aboveground compartment
In VM and PC, the same marshes considered in the previous sections, we evaluated aboveground biomass, productivity, and decomposition rates, as well as nutrient and metal concentrations in aboveground tissues. In the case of S. alterniflora, biomass and productivity were evaluated by destructive (one-and-a-half-year study; Negrin et al., 2012a) and nondestructive methods (two-year study; González Trilla et al., 2009). Aboveground biomass and productivity of S. perennis were evaluated only by destructive methods during one year (Negrin, 2011; Negrin et al., 2015). Elemental concentrations of C, N and P in S. alterniflora (Negrin et al., 2012a) and S. perennis (Negrin et al., 2015) were also estimated. Decomposition rates and changes in the elemental concentrations were evaluated in both species (Negrin et al., 2012b, 2015). Metal concentrations were also evaluated in aboveground tissues of S. alterniflora (Hempel et al., 2008) and S. perennis (Botté, 2005). Values presented are mean ± standard deviation.
Biomass and productivity
By destructive methods, biomass of S. alterniflora was sampled in VM from October 2006 to April 2008 in LM and HM (Negrin et al., 2012a). The aboveground tissues were harvested by clipping vegetation at the sediment surface in randomly established 25 × 25 cm plots (n = 5 on each sampling date and zone, LM and HM). All standing live and dead tissues were removed and placed in plastic bags. Samples were washed, and the dead and senescent tissues (hereafter dead) were separated from live biomass. The live and dead plant material were dried and weighed as described for belowground tissues. Net aboveground primary productivity (NAPP) was estimated using the Smalley method (Linthurst & Reimold, 1978). In the same marsh, S. alterniflora biomass and productivity were also evaluated by nondestructive methods from October 2005 to October 2007 (González Trilla et al., 2009). All tillers in ten 10 × 10 cm permanent plots (5 in each zone, LM and HM) were tagged and measured from their bases to the tip of their longest leaf. The biomass of each individual tiller was estimated by a weight:height relationship developed for the live and dead stems and each zone separately. The live and dead biomass of each plot were estimated as the summed mass of all standing green and dead tillers, respectively. The difference of tiller biomass between two consecutive sampling periods was calculated, and positive values of live tillers were interpreted as increases of biomass (production). NAPP was calculated by summing the positive growth of each individual tiller and was estimated for the first (October 2005–October 2006) and second (October 2006–October 2007) year. S. perennis biomass and productivity were evaluated only by destructive methods (Negrin, 2011; Negrin et al., 2015), in the same way as described for S. alterniflora, but, because of the patchy distribution of S. perennis, clip plots were located within randomly selected plants. Biomass and productivity estimations were accordingly corrected considering the mean plant cover.
Standing live and dead aboveground biomass of S. alterniflora was present all year round. The estimation of maximum total (live + dead) standing biomass was highly dependent on the sampling technique, but significantly higher in HM than LM. Using the destructive technique, Negrin et al. (2012a) found a maximum biomass of 548 ± 204 g m−2 in HM, which was significantly higher (Student’s t test: P < 0.05) than the corresponding value in LM (152 ± 43 g m−2). Considering the nondestructive technique (González Trilla et al., 2009), maximum biomass in the first year was 1450 ± 618 g m−2 in the HM and 906 ± 506 g m−2 in the LM; in the second year, the values were 677 ± 219 and 553 ± 293 g m−2, in HM and LM, respectively; for both years, the differences between HM and LM were statistically significant (ANOVA: P < 0.05). Considering live and dead biomass separately, we found interesting patterns. González Trilla et al. (2009) reported maximum values of live aboveground biomass, during the first year, of 506 ± 152 and 813 ± 396 g m−2 in HM and LM, respectively; for the second year, the values were lower: 311 ± 255 and 539 ± 289 g m−2 for HM and LM, respectively. For dead aboveground biomass, in the first year, they found maximum values of 545 ± 318 and 93 ± 112 g m−2, for HM and LM, respectively; during the second year, values decreased: 283 ± 199 and 14 ± 17 g m−2 in HM and LM, respectively. Both years, dead aboveground biomass in HM was higher than in LM (ANOVA: P< 0.05; González Trilla et al., 2009). Negrin et al. (2012a) reported values of maximum live aboveground biomass of 326 ± 189 and 109 ± 71 g m−2 for HM and LM, respectively, and values of maximum dead biomass of 282 ± 179 and 44 ± 17 g m−2 for HM and LM, respectively. Values of dead aboveground biomass obtained by the destructive technique were also significantly different (Student’s t test: P < 0.05; Negrin et al., 2012a). In spite of differences between years and methodologies in absolute values, dead biomass was always higher in HM than in LM. These results are in agreement with the differential exposure of each zone to physical events. In low zones, the mechanical action of tides breaks and removes dead tillers, favoring the export and redistribution of detritus within the estuary. In HM, on the contrary, hydrodynamic forces are attenuated, preventing tillers removal.
Similar to biomass, NAPP of S. alterniflora varied greatly with the position within the marsh and the method used for the estimation. Negrin et al. (2012a) obtained NAPP values of 439 ± 262 and 106 ± 84 g m−2 year−1 for HM and LM, respectively. González Trilla et al. (2009), through the allometric technique, reported NAPP values for HM of 599 ± 182 and 495 ± 231 g m−2 year−1, for the first and second year of study, respectively, while for LM reported values were of 936 ± 719 and 482 ± 191 g m−2 year−1 (for first and second year), without significant differences between sites and years (ANOVA: P ≥ 0.05). Despite variability of estimations, NAPP of S. alterniflora in the BBE were in the range registered for this species in the world, which varies between 101 and 4400 g m−2 year−1 (see references in González Trilla et al., 2009).
Standing live and dead aboveground biomass of S. perennis was present all year round. Maximum total aboveground biomass was 518 ± 277 g m−2, and maximum live and dead aboveground biomass were 446 ± 272 and 203 ± 76 g m−2, respectively (Negrin et al., 2015). NAPP, after correction for estimated plant cover, was 511 ± 288 g m−2 year−1, which is slightly lower than the range (581–2973 g m−2 year−1) of the reported values for this and related species at different geographic locations through the world (Negrin, 2011). Nevertheless, unlike S. alterniflora, reports on productivity and biomass of S. perennis are scarce, and the low values registered in the BBE could be in agreement with yet unexplored patterns.
To evaluate the relative importance of above and belowground biomass between species and zones, the ratio of belowground/aboveground biomass was estimated in each sampling date (using data only of destructive methods) (Negrin et al., 2012a, 2015). The ratios for S. alterniflora were higher than 1, reaching up to 12 in LM and up to 5 in HM and being significantly higher in LM than in HM in most sampling dates (Student’s t test: P < 0.05) (Negrin et al., 2012a). This suggests a higher allocation of biomass in belowground tissues in LM than in HM. For S. perennis, the ratios were always below 1, except for a single date, suggesting a lower investment in belowground tissues for this species (Negrin et al., 2015). It has to be reminded at this point that samples for belowground biomass estimations were collected only up to a depth of 15 cm; however, most of the tissues are found there, and the differences between species and zones in the biomass of fine roots growing deeper in the sediment profile may not change the observed trends in biomass allocation. A greater investment in belowground tissues is commonly related with unfavorable sediment conditions (Schubauer & Hopkinson, 1984; Scarton et al., 2002). As already mentioned, Eh in sediments, evaluated in the same sites under study, were as follows: LM S. alterniflora < HM S. alterniflora < S. perennis marsh (Negrin et al., 2011, 2013). This is in agreement with a lower investment in belowground biomass under more oxidized conditions.
C, N and P
Concentrations of C, N and P were estimated in aboveground tissues of S. alterniflora (Negrin et al., 2012a) and S. perennis (Negrin et al., 2015). As described for belowground biomass, all dried samples at each sampling date were pooled and ground in one composite sample. After grinding, C, N and P were determined with the chemical methods described previously. Element pools were also calculated.
Concentrations of C, N and P in aboveground tissues (%, Table 2, made based on Negrin et al., 2012a, b, 2015) were in the range of those reported for the same species in other salt marshes through the world (see references in Negrin et al. 2012a, 2015). Since element pools (g m−2) reflect biomass patterns, for S. alterniflora, HM aboveground pools were higher than LM ones (Fig. 4, made based on Negrin et al., 2012a, 2015).
Decomposition
Decomposition rates of aboveground biomass of S. alterniflora (Negrin et al., 2012b) and S. perennis (Negrin et al., 2015) were estimated by the litterbag technique. Plastic bags (20 × 20 cm, 2 mm of mesh size) were filled with approximately 20 g of plant tissues, previously washed and dried. At the beginning of the sampling period, the bags (N = 32 for S. alterniflora and N = 21 for S. perennis) were placed on the sediment surface. It has to be noted that litter in non-abscising plants, like S. alterniflora, decompose under a particular set of conditions that are not necessarily reflected within litter bags, mainly due to the physical and chemical environment for decomposers (Newell et at., 1989). However, in the study area, standing dead leaves and stems are only a small portion of the total decomposing biomass. Winds and tides commonly break and remove dead stems, favoring the redistribution of plant litter within the high marsh and large amounts of senescent stems and leaves is commonly observed lying on the marsh surface. Therefore, the litterbag technique may not reflect the actual conditions under which all biomass decompose, but provides standardized estimations for comparisons. Every 2 months, during a year, between two and four bags were removed and taken to the laboratory. The content of each bag was washed, dried and weighed. As for belowground tissues, only HM S. alterniflora was evaluated. Two bags from each sampling date were analyzed for the C, N and P contents (%) using the methods mentioned previously. C/N and C/P molar ratios were calculated.
Decomposition rates (% of plant material lost after a year) of aboveground tissues of both species are shown in Table 2. Decomposition rate of aboveground tissues of S. perennis (mean of 64%) is higher than those of S. alterniflora (mean of 47%), although the differences were not significant (Student’s t test: P > 0.10; novel statistical analysis performed based on published data). Decomposition rates of both species are in the range of those reported in the literature (see references in Negrin et al., 2012b, 2015) for the corresponding species and/or related ones in the case of S. perennis. As already mentioned, Palomo & Niell (2009) is the only reference for decomposition of S. perennis, beside ours, highlighting the importance of our results despite the methodological limitations. As noted for belowground tissues, the fluctuation of the content of C, N, and P and molar ratios during decomposition might be associated with environmental conditions and the action of different types of decomposers. Both C/N and C/P showed a trend of higher values at the end than at the beginning of the experiment for both species, mainly for S. perennis (Table 2), suggesting a net release of both N and P by aboveground tissues.
Metals
As described for belowground tissues, metal concentrations (Cr, Cd, Pb, Ni, Cu and Zn) in aboveground biomass of S. alterniflora was evaluated in April 2006 in LM and HM in VM (Hempel et al., 2008), while monthly sampling of tissues of S. perennis was performed from June 2000 to October 2001 in PC (Botté, 2005). For both species, all aboveground tissues (live + dead) were pooled. Samples were washed and dried. Determinations were performed in the same way described for sediments and belowground tissues. Using the metal concentrations in above and belowground tissues, we estimated translocation factors (metal concentration in aboveground tissues/metal concentration in belowground tissues).
Translocation from belowground to aboveground tissues defines the ultimate fate of metals accumulated in plant biomass. According to the literature, salt marsh species, including S. alterniflora and S. perennis, commonly concentrate most metals in belowground biomass (e.g., Whidman et al., 2003; Caçador et al., 2009; Duarte et al., 2010; Cuoto et al., 2013; Redondo-Gómez, 2013). Translocation factors of S. alterniflora and S. perennis in the BBE are shown in Table 3. Values higher than 1 would indicate a higher allocation in aboveground tissues, and vice versa. In S. alterniflora marshes, some metals showed an opposite pattern than the observed in literature, with higher concentration in aboveground tissues. Nevertheless, we should consider that some of the translocation factors were only slightly higher than 1 and, in the case of Cd and Pb, concentrations in both types of tissues were very low and the small differences magnified in the corresponding ratios. Translocation factors for Zn and Cu, on the other hand, were lower than 1, suggesting a higher retention within the belowground component. Moreover, Zn and Cu were more concentrated in belowground tissues than in surrounding sediments. In spite of the described patterns, it should be taken into account that there is a lack of repeated measurements for S. alterniflora. Since the reported values were estimated from plant material obtained in a single sampling date, we could not assess the temporal variability of the observed trend. All the metals considered had translocation factors higher than 1 in S. perennis (Table 3), suggesting a more important role of this species in the exportation of metals bound to plant detritus. Through detritus production, salt marsh plants may facilitate the movement of toxic elements through the food webs (Luque et al., 1999).
Integrative comparison of nutrient and metal dynamics
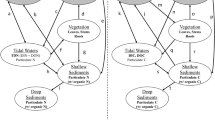
Considering NAPP values, elemental concentrations in plant tissues and their corresponding decomposition rates, we synthesized the mass balances for C, N, P, and metals for the aboveground component, through simple box schemes. According to our results, the various estimations of NAPP performed on S. alterniflora were a major source of variation for evaluating budgets, so we considered the lowest and highest NAPP values obtained to report ranges. Large variations would be also expected for S. perennis, but a more limited set of estimations is available. Additionally, decomposition estimates may be largely biased due to the methodological limitations described, and this may have a large impact on the interpretation of the mass balances. Figure 5 shows the annual rates of elemental sequestration due to NAPP (g m−2 year−1) of S. alterniflora (LM and HM) and S. perennis marshes, the annual loss of elements through decomposition (g m−2 year−1), and the in situ accumulation of elements bound to undercomposed detritus (g m−2 year−1). For the purpose of this analysis, we considered the plant material lost from litterbags as a combination of fine particulate organic matter plus dissolved organic and inorganic compounds. Due to their similar NAPP values, S. perennis and HM S. alterniflora would play a similar role in the sequestration of C and N. However, HM S. alterniflora was more efficient a P sequestration due to its higher concentration in aboveground tissues (Table 2). Metal sequestration was higher in S. perennis than in HM S. alterniflora, mainly due to their different metal concentrations in aboveground tissues, which is in turn associated with translocation factors higher than 1 in S. perennis (Table 3). It should be also considered that the aboveground tissues of S. perennis seem to decompose faster than S. alterniflora biomass, and it might have an impact on elemental cycling. The nature of the exported material (particulate or dissolved, organic or inorganic) defines the ultimate fate of nutrients and metals and their ability to re-enter grazing and detrital food webs. In the case of LM S. alterniflora, there is a large variability in NAPP estimations, and the role of these marshes in the sequestration of elements is difficult to evaluate.
Major limitations of the original data have to be carefully considered when analyzing Fig. 5. Box schemes provide an integrated understanding of the aboveground component, but decomposition rates estimates may be largely biased due to the methodological limitations. Moreover, the variability in NAPP estimations should be not dismissed. In addition, based on our data, we could not accurately estimate net belowground primary productivity, which is a key process to evaluate the role of marshes in elemental cycling and especially their impact in the soil organic storage. In the particular case of S. perennis, these marshes are being lost to erosion at a fast rate. Considering the minimum and maximum values of belowground biomass registered during the evaluated study period and the average elemental concentrations in S. perennis tissues, we estimated the available amount of each element (g m−2) (Table 4). Taking into account these values as well as a rate of erosion of 267 ha year−1 (Pratolongo et al., 2013), we also calculated the mass loss (tons year−1) for each one of the studied elements (Table 4). These estimations only consider the elemental contents in belowground biomass but, in addition to biomass, an estimated amount of 2360 tons of sediments might be daily removed (Zapperi et al., 2015), so the actual losses would be much higher if sediment contents are also considered. While S. perennis marshes are eroding, S. alterniflora marshes are expanding their distribution, and part of the eroded material may be redeposited in the expanding marshes. The observed land cover changes may have a large impact on the biogeochemical cycles within the BBE, and the actual rates of change may accelerate in response to global warming and sea level rise (e.g., Kirwan & Murray, 2007; Idazkin & Bortolus, 2011; Valentim et al., 2013). Direct human modifications derived from dredging of navigation channels, urban expansion, and industrial development in the coastal zone might also modify salt marsh cover. Moreover, net primary productivity and decomposition rates are also sensitive to climate change (e.g., Kirwan et al., 2009; Kirwan & Blum, 2011). Under this changing scenario, further research is needed to completely understand the complex dynamics of biogeochemical cycling in salt marshes.
Conclusions, shortcomings and future perspectives
Despite limitations about methodology, statistics and interpretations, some remarkable conclusions can be drawn. NAPP of S. alterniflora and S. perennis are low, but both species play a substantial and differential role in elemental cycling in the BBE. While S. perennis was more efficient at metal sequestration, S. alterniflora was more important in the immobilization of P. Some elements were also in high concentrations in belowground tissues, which highlight the need of estimations of net belowground primary productivity. Slower decomposition rates in S. alterniflora would imply a larger contribution of this species to the accumulation of elements bound to undecomposed detritus. Nevertheless, for S. alterniflora, plant position within the tidal frame has to be considered to evaluate its role at elemental cycling. Regardless of limitations and uncertainties, results presented here synthesize most of the available information on elemental cycling in salt marshes of the BBE, Argentina. Integrative revisions on salt marsh processes are difficult to find for the region, and our findings could be a valuable tool for comparisons with different salt marshes worldwide.
Given the several shortcomings of our study, they are summarized here in order to provide a better understanding of the conclusions. The lack of a convenient procedure to estimate net belowground primary productivity is a problem that has to be addressed. In addition, for a more complete understanding of the elemental cycling in the belowground compartment, we need to perform more evaluations on sediments. Present research is being carried out to obtain estimations of C, N and P dynamics in sediments, which will add a significant piece of information to the existent knowledge. Decomposition of plant tissues is a key process in the elemental cycling within salt marshes but the methodological limitations and assumptions presented for the measurement of decomposition rates by litterbags might largely have biased our results. We should also consider that the measurements for each species were performed in different sites and in different sampling periods, which might account, at least partially, for the variability of our results. The lack of inferential statistics in some cases is another important shortcoming of this work, which is mainly related with the available information and the particular goals in each of the original papers. An additional weakness comes from the limited geographical representation of our work regarding the extent of the BBE. The studied marshes are both located in the northern section of Canal Principal, but extensive marshes exist in secondary channels south from Canal Principal. Although these marshes cover a wide area, they are virtually inaccessible for us, and still largely understudied. Our challenge for the near future is to broad our vision of the biogeochemical dynamics of the BBE studying those southern marshes.
References
Barbier, E. B., S. D. Hacker, C. Kennedy, E. W. Koch, A. C. Stier & B. R. Silliman, 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81: 169–193.
Bedford, B. L. & E. M. Preston, 1988. Developing the scientific basis for assessing cumulative effects of wetland loss and degradation of landscape functions: status, perspectives and prospects. Environmental Management 12: 751–771.
Blum, L. K., 1993. Spartina alterniflora root dynamics in a Virginia marsh. Marine Ecology Progress Series 102: 169–178.
Bocock, K. & O. Gilbert, 1957. The disappearance of leaf litter under different woodland conditions. Plant Soil 9: 179–185.
Bortolus, A., E. Schwindt, P. Bouza & Y. Idaszkin, 2009. A characterization of patagonian salt marshes. Wetlands 29: 772–780.
Botté, S.E., 2005. El rol de la vegetación en el ciclo biogeoquímico de metales pesados en humedales del estuario de Bahía Blanca. Thesis dissertation, Universidad Nacional del Sur, Bahía Blanca, Argentina.
Botté, S. E., R. H. Freije & J. E. Marcovecchio, 2007. Dissolved heavy metal (Cd, Pb, Cr, Ni) concentrations in surface water and porewater from Bahía Blanca Estuary tidal flats. Bulletin of Environmental Contamination and Toxicology 79: 415–421.
Botté, S. E., R. H. Freije & J. E. Marcovecchio, 2010. Distribution of several heavy metals in tidal flats sediments within Bahía Blanca Estuary (Argentina). Water, Air, Soil Pollution 210: 371–388.
Bouchard, V. & J. C. Lefeuvre, 2000. Primary production and macrodetritus dynamics in a European salt marsh: carbon and nitrogen budgets. Aquatic Botany 67: 23–42.
Brinson, M., 1988. Strategies for assessing the cumulative effects of wetland alteration on water quality. Environmental Management 12: 655–662.
Caçador, I. M., B. Duarte Caetano & C. Vale, 2009. Stock and losses of trace metals from salt marsh plants. Marine Environmental Research 67: 75–82.
Caetano, M., C. Vale, R. Cesário & N. Fonseca, 2008. Evidence for preferential depths of metal retention in roots of salt marsh plants. Science of the Total Environment 390: 466–474.
Chmura, G. L., S. Anisfeld, D. Cahoon & J. Lynch, 2003. Global carbon sequestration in tidal, saline wetland soils. Global Biogeochemical Cycles 17: 1–12.
Couto, T., B. Duarte, D. Barroso, I. Caçador & J. C. Marques, 2013. Halophytes as sources of metals in estuarine systems with low levels of contamination. Functional Plant Biology 40(9): 931–939.
Davy, A. J., G. F. Bishop, H. Mossman, S. Redondo-Gómez, J. M. Castillo, E. M. Castellanos, T. Luque & M. E. Figueroa, 2006. Biological flora of the British isles: Sarcocornia perennis (Miller) A.J. Scott. Journal of Ecology 94: 1035–1048.
Day, J. W., B. C. Crumpt, M. W. Kemp & A. Yáñez-Arancibia, 1989. Estuarine Ecology. Wiley-Interscience, New York.
Duarte, B., M. Caetano, P. R. Almeida, C. Vale & I. Caçador, 2010. Accumulation and biological cycling of heavy metal in four salt marsh species, from Tagus estuary (Portugal). Environmental Pollution 158: 1661–1668.
Enriquez, S., C. M. Duarte & K. Sand-Jensen, 1993. Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N: P content. Oecologia 94: 457–471.
Ferrer, L., E. Contardi, S. J. Andrade, R. Asteasuain, A. E. Pucci & J. E. Marcovecchio, 2000. Environmental cadmium and lead concentrations in the Bahía Blanca Estuary (Argentina). Potential toxic effects of Cd and Pb on crab larvae. Oceanologia 42: 493–504.
Freije, R. H., C. V. Spetter, J. E. Marcovecchio, C. A. Popovich, S. E. Botté, V. L. Negrin, A. H. Arias, F. Delucchi & R. O. Astesuain, 2008. Water chemistry and nutrients of the Bahía Blanca Estuary. In Neves, R., J. Baretta & M. Mateus (eds), Perspectives on Integrated Coastal Zone Management in South America. IST Press, Lisboa: 241–254.
Gedan, K. B., B. R. Silliman & M. D. Bertness, 2009. Centuries of human-driven change in salt marsh ecosystems. Annual Review of Marine Science 1: 117–141.
González, M. A., 1989. Holocene levels in the Bahía Blanca estuary, Argentina Republic. Journal of Coastal Research 5: 65–77.
González Trilla, G., P. Kandus, V. L. Negrin, R. Vicari & J. E. Marcovecchio, 2009. Tiller dynamic and production on a SW Atlantic Spartina alterniflora marsh. Estuarine, Coastal and Shelf Science 85: 126–133.
González Trilla, G., M. M. Borro, N. S. Morandeira, F. Schivo, P. Kandus & J. E. Marcovecchio, 2013. Allometric scaling of dry weight and leaf area for Spartina densiflora and Spartina alterniflora in two Southwest Atlantic saltmarshes. Journal of Coastal Research 29: 1373–1381.
Gross, M., M. Hardisky, P. Wolf & V. Klemas, 1991. Relationship between aboveground and belowground biomass of Spartina alterniflora (smooth cordgrass). Estuaries 14: 180–191.
Hempel, M., S. E. Botté, V. L. Negrin, M. N. Chiarello & J. E. Marcovecchio, 2008. The role of the smooth cordgrass Spartina alterniflora and associated sediments in the heavy metal biogeochemical cycle within Bahía Blanca estuary salt marshes. Journal of Soils and Sediments 8: 289–297.
Idaszkin, Y. L. & A. Bortolus, 2011. Does low temperature prevent Spartina alterniflora from expanding toward the austral-most salt marshes? Plant ecology 212: 553–561.
Idaszkin, Y. L., P. J. Bouza, C. H. Marinho & M. N. Gil, 2014. Trace metal concentrations in Spartina densiflora and associated soil from a Patagonian salt marsh. Marine Pollution Bulletin 89: 444–450.
Isacch, J., C. Costa, L. Rodriguez-Gallego, D. Conde, M. Escapa, D. Gagliardini & O. Iribarne, 2006. Distribution of salt marsh plant communities associated with environmental factors along a latitudinal gradient on the south-west Atlantic coast. Journal of Biogeography 33: 888–900.
Kirwan, M. L. & A. B. Murray, 2007. A coupled geomorphic and ecological model of tidal marsh evolution. Proceedings of the National Academy of Sciences 104: 6118–6122.
Kirwan, M. L., G. R. Guntenspergen & J. T. Morris, 2009. Latitudinal trends in Spartina alterniflora productivity and the response of coastal marshes to global change. Global Change Biology 15: 1982–1989.
Kirwan, M. L. & L. K. Blum, 2011. Enhanced decomposition offsets enhanced productivity and soil carbon accumulation in coastal wetlands responding to climate change. Biogeosciences 8: 987–993.
Koretsky, C. M., M. Haveman, A. Cuellar, L. Beuving, T. Shattuck & M. Wagner, 2008. Influence of Spartina and Juncus on saltmarsh sediments. I. Porewater geochemistry. Chemical Geology 255: 87–99.
La Colla, N. S., V. L. Negrin, J. E. Marcovecchio & S. E. Botté, 2015. Dissolved and particulate metals dynamics in a human impacted estuary from the SW Atlantic. Estuarine, Coastal and Shelf Science. doi:10.1016/j.ecss.2015.05.009.
Lanfredi, N. W., E. E. D’Onofrio & C. A. Mazio, 1988. Variations of the mean sea level in the southwest Atlantic Ocean. Continental Shelf Research 8: 1211–1220.
Limbozzi, F. & T. E. Leitào, 2008. Characterization of Bahía Blanca main existing pressures and their effects on the state indicators for surface and groundwater quality. In Neves, R., J. Baretta & M. Mateus (eds), Perspectives on Integrated Coastal Zone Management in South America. IST Press, Lisboa: 315–331.
Linthurst, R. A. & R. J. Reimold, 1978. An evaluation of methods for estimating the net aerial primary productivity of estuarine angiosperms. Journal of Applied Ecology 15: 919–931.
Luque, C. J., E. M. Castellanos, J. M. Castillo, M. González, M. C. González-Vilches & M. E. Figueroa, 1999. Metals in halophytes of a contaminated estuary (Odiel Saltmarshes, SW Spain). Marine Pollution Bulletin 38: 49–51.
Menéndez, M. & N. Sanmartí, 2007. Geratology and decomposition of Spartina versicolor in a brackish Mediterranean marsh. Estuarine, Coastal and Shelf Science 74: 320–330.
National Biological Information Infrastructure (NBII) & IUCN/SSC Invasive Species Specialist Group (ISSG) (Comp.), 2005. Global Invasive Species Database. http://www.issg.org/database/species/.
Negrin, V.L. 2011. El rol de las marismas del estuario de Bahía Blanca en el ciclo bio-geoquímico de nutrientes inorgánicos y de materia orgánica. Thesis dissertation, Universidad Nacional del Sur, Bahía Blanca, Argentina.
Negrin, V. L., C. V. Spetter, R. O. Asteasuain, G. M. E. Perillo & J. E. Marcovecchio, 2011. Influence of flooding and vegetation on carbon, nitrogen, and phosphorus dynamics in the pore water of a Spartina alterniflora salt marsh. Journal of Environmental Sciences 23: 212–221.
Negrin, V. L., A. E. de Villalobos, G. González Trilla, S. E. Botté & J. E. Marcovecchio, 2012a. Above and belowground biomass and nutrient pools of Spartina alterniflora (smooth cordgrass) in a South American salt marsh. Chemistry and Ecology 28: 391–404.
Negrin, V. L., G. González Trilla, P. Kandus & J. E. Marcovecchio, 2012b. Decomposition and nutrient dynamics in a Spartina alterniflora marsh of the Bahía Blanca estuary, Argentina. Brazilian Journal of Oceanography 60: 259–263.
Negrin, V. L., C. V. Spetter, V. A. Guinder, G. M. E. Perillo & J. E. Marcovecchio, 2013. The role of Sarcocornia perennis and tidal flooding on sediment biogeochemistry in a South American wetland. Marine Biology Research 9: 703–715.
Negrin, V. L., P. D. Pratolongo, A. E. de Villalobos, S. E. Botté & J. E. Marcovecchio, 2015. Biomass, decomposition and nutrient cycling in a SW Atlantic Sarcocornia perennis marsh. Journal of Sea Research 97: 50–55.
Newell, S. Y., R. D. Fallon & J. D. Miller, 1989. Decomposition and microbial dynamics for standing, naturally positioned leaves of the salt-marsh grass Spartina alterniflora. Marine Biology 101: 471–481.
Odum, E. P., 2000. Tidal marshes as outwelling/pulsing systems. In Weinstein, M. & D. A. Kreeger (eds), Concepts and Controversies in Tidal Marsh Ecology. Kluwer Academic Publishing, Dordrecht: 3–7.
Palomo, L. & F. X. Niell, 2009. Primary production and nutrient budgets of Sarcocornia perennis ssp. alpini (Lag.) Castroviejo in the salt marsh of the Palmones River estuary (Southern Spain). Aquatic Botany 91: 130–136.
Perillo, G. M. E. & M. C. Piccolo, 1991. Tidal response in the Bahia Blanca estuary, Argentina. Journal of Coastal Research 7: 437–449.
Piccolo, M. C., 2008. Climatological features of the Bahía Blanca Estuary. In Neves, R., J. Baretta & M. Mateus (eds), Perspectives on Integrated Coastal Zone Management in South America. IST Press, Lisboa: 231–239.
Piccolo, M. C., G. M. E. Perillo & W. D. Melo, 2008. The Bahía Blanca Estuary: an integrated overview of its geomorphology and dynamics. In Neves, R., J. Baretta & M. Mateus (eds), Perspectives on Integrated Coastal Zone Management in South America. IST Press, Lisboa: 219–229.
Piovan, M. J, P. Pratolongo, G. Zapperi & G. M. E. Perillo, 2014. Aplicación de herramientas de teledetección satelital y SIG para caracterizar a los humedales costeros de Bahía Blanca. Proceedings of II Jornadas de Tecnologías de la Información Geográfica del Sur Argentino. Bahía Blanca, Argentina, 78–82.
Pozo, J. & R. Colino, 1992. Decomposition processes of Spartina maritima in a salt marsh of the Basque Country. Hydrobiologia 231: 165–175.
Pratolongo, P. D., G. M. E. Perillo & M. C. Piccolo, 2010. Combined effects of waves and plants on a mud deposition event at a mudflat-saltmarsh edge in the Bahía Blanca Estuary. Estuarine, Coastal and Shelf Science 87: 207–212.
Pratolongo, P., C. Mazzon, G. Zapperi, M. J. Piovan & M. M. Brinson, 2013. Land cover changes in tidal salt marshes of the Bahía Blanca estuary (Argentina) during the past 40 years. Estuarine, Coastal and Shelf Science 133: 23–31.
Rascio, N. & F. Navari-Izzo, 2011. Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Science 180: 169–181.
Reboreda, R. & I. Caçador, 2007. Halophyte vegetation influences in salt marsh retention capacity for heavy metals. Environmental Pollution 146: 147–154.
Reboreda, R., I. Caçador, S. Pedro & P. Raposo Almeida, 2008. Mobility of metals in salt marsh sediments colonized by Spartina maritima (Tagus estuary, Portugal). Hydrobiologia 606: 129–137.
Redondo-Gómez, S., 2013. Bioaccumulation of heavy metals in Spartina. Functional Plant Biology 40: 913–921.
Rejmánková, E. & K. Houdková, 2006. Wetland plant decomposition under different nutrient conditions: what is more important, litter quality or site quality? Biogeochemistry 80: 245–262.
Scarton, F., J. Day & A. Rismondo, 2002. Primary production and decomposition of Sarcocornia fruticosa (L.) Scott and Phragmites australis Trin. Ex Steudel in the Po Delta, Italy, Estuaries 25: 325–336.
Schubauer, J. P. & C. S. Hopkinson, 1984. Above-and belowground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia. Limnology and Oceanography 29: 1052–1065.
Scian, B. & J. Pierini, 2013. Variability and trends of extreme dry and wet seasonal precipitation in Argentina. A retrospective analysis. Atmósfera 26: 3–26.
Simões, M. P., M. L. Calado, M. Madeira & L. C. Gazarini, 2011. Decomposition and nutrient release in halophytes of a Mediterranean salt marsh. Aquatic Botany 94: 119–126.
Sousa, A. I., A. I. Lillebø, I. Caçador & M. Â. Pardal, 2008. Contribution of Spartina maritima to the reduction of eutrophication in estuarine systems. Environmental Pollution 156: 628–635.
Sousa, A. I., A. I. Lillebø, M. Â. Pardal & I. Caçador, 2010. Productivity and nutrient cycling in salt marshes: contribution to ecosystem health. Estuarine, Coastal and Shelf Science 87: 640–646.
Tangahu, B.V., S. R. Sheikh Abdullah, H. Basri, M. Idris, N. Anuar & M. Mukhlisin, 2011. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. International Journal of Chemical Engineering, 2011.
Taylor, D. I. & B. R. Allanson, 1995. Organic carbon fluxes between a high marsh and estuary, and the inapplicability of the outwelling hypothesis. Marine Ecology Progress Series 120: 263–270.
Turner, R. E., E. M. Swenson & C. S. Milan, 2000. Organic and inorganic contributions to vertical accretion in salt marsh sediments. In Weinstein, M. & D. A. Kreeger (eds), Concepts and Controversies in Tidal Marsh Ecology. Kluwer Academic Publishing, Dordrecht: 583–595.
Turner, R. E., E. M. Swenson, C. S. Milan, J. M. Lee & T. A. Oswald, 2004. Belowground biomass in healthy and impaired salt marshes. Ecological Research 19: 29–35.
Valentim, J. M., N. Vaz, H. Silva, B. Duarte, I. Caçador & J. M. Dias, 2013. Tagus estuary and Ria de Aveiro salt marsh dynamics and the impact of sea level rise. Estuarine, Coastal and Shelf Science 130: 138–151.
Wang, Y., L. Zhou, X. Zheng, P. Qian & Y. Wu, 2013. Influence of Spartina alterniflora on the mobility of heavy metals in salt marsh sediments of the Yangtze River Estuary, China. Environmental Science and Pollution Research 20: 1675–1685.
Watanabe, F. S. & S. R. Olsen, 1965. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Science Society of America Journal 29: 677–678.
Weis, J. S. & P. Weis, 2004. Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environment International 30: 685–700.
Windham, L., J. S. Weis & P. Weis, 2003. Uptake and distribution of metals in two dominant salt marsh macrophytes, Spartina alterniflora (cordgrass) and Phragmites australis (common reed). Estuarine, Coastal and Shelf Science 56: 63–72.
Zapperi, G., P. Pratolongo, M. J. Piovan & J. E. Marcovecchio, 2015. Benthic-pelagic coupling in an intertidal mudflat in the Bahía Blanca Estuary (SW Atlantic). Journal of Coastal Research. doi:10.2112/JCOASTRES-D-14-00064.1.
Zilio, M. I., S. London, G. M. E. Perillo & M. C. Piccolo, 2013. The social cost of dredging: the Bahia Blanca Estuary case. Ocean and Coastal Management 71: 195–202.
Zotelo, C., 2011. Variabilidad Climática y Ciclos Naturales. Anales 2011 de la Academia Nacional de Agronomía y Veterinaria, Tomo LXV: 374–381.
Acknowledgments
We thank an anonymous reviewer, who carefully read the manuscript, and we thank Dr. Valeria Guinder for their useful comments to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Pierluigi Viaroli, Marco Bartoli & Jan Vymazal / Wetlands Biodiversity and Processes: Tools for Management and Conservation
Rights and permissions
About this article
Cite this article
Negrin, V.L., Botté, S.E., Pratolongo, P.D. et al. Ecological processes and biogeochemical cycling in salt marshes: synthesis of studies in the Bahía Blanca estuary (Argentina). Hydrobiologia 774, 217–235 (2016). https://doi.org/10.1007/s10750-015-2582-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2582-9