Abstract
Despite its potential use for population control, the winter ecology of nonnative fishes is still poorly understood due to the difficulty of conducting field surveys. In this study, we investigated the winter habitat use of invasive rainbow trout Oncorhynchus mykiss at the cannel unit scale (i.e., pool, riffle). Twenty-four reaches were surveyed in late December 2013 along the Obicha River, a tributary of the Otofuke River, Tokachi River basin, Hokkaido, Japan. A total of 532 fish were captured, of which 96% were rainbow trout, whereas native salmonid was only a single southern Asian Dolly Varden Salvelinus curilus. Smaller rainbow trout (< 250 mm) used reaches with low velocity, whereas larger trout (250–520 mm) aggregated in specific reaches with deep pools with abundant cover and coarse substrate. A previous tributary-scale study in the same river system showed the importance of velocity and temperature, but not depth and substrate. Therefore, habitat selection would be scale-, as well as size-, dependent. This study provides useful information on capturing large mature adults in winter for effective control of nonnative salmonids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With increasing levels of human activity, many fishes have been introduced intentionally or unintentionally, to nonnative habitats. Nonnative fishes often become invasive and seriously influence native ecosystems (Kitano 2004; Crawford and Muir 2007; Garcia de Leaniz et al. 2010; Korsu et al. 2010). Some nonnative fishes, however, play important roles in local economies, such as through aquaculture and recreational fishing (Gozlan 2008) or biological control of other invasive species (e.g., Tsurui-Sato et al. 2019). Nonnative fishes frequently pose dilemmas between economic benefits and the costs of damage to the native ecological system. The balance is dynamic and the costs can easily exceed the benefits. Thus, the preparation of effective removal methods is required to hedge risk.
Knowledge on winter ecology can provide novel insights into the management or control of nonnative fishes (Bajer et al. 2011; Shepard et al. 2014; Koizumi et al. 2017a). Winter is a severe season, and some fishes aggregate extremely to specific refugia or alternate winter habitats to avoid adverse physiochemical conditions (e.g., ice, low oxygen, winter freshet) (Cunjak 1996; Koizumi et al. 2017b), where mass removal is possible. Bajer et al. (2011) located winter aggregations of the invasive common carp Cyprinus carpio by using radio and acoustic telemetry in three Midwestern lakes, which resulted in the capture of up to 94% of the populations using seine nets. In streams, Shepard et al. (2014) suggested the efficacy of removal by electrofishing during early winter in the eradication of the invasive brook trout Salvelinus fontinalis. Thus, approaches using winter ecology may become new management tools, though ecological knowledge in winter is still limited due to the difficulty of field surveys.
Rainbow trout, freshwater salmonid fish native to North America and the Kamchatka Peninsula, have been introduced to Japan since 1887 (Taniguchi 2002). They are often stocked throughout Japan for sportfishing, and the establishment of local populations has been recognized (e.g., Kato and Yanagawa 2000). Notably, rainbow trout have been introduced in 72 river systems in Hokkaido (Takami and Aoyama 1999), and their presence has negatively influenced native salmonids through competition for foraging habitat (Hasegawa et al. 2004; Hasegawa and Maekawa 2006) and reproductive interference (Nomoto et al. 2010). Nevertheless, the trout are popular for sportfishing and play important roles in the regional economy and in environmental education (Garcia de Leaniz et al. 2010; Shimoda 2012). Thus, adaptive management or population control that depends on ecological uniqueness and local needs would be required, even for nonnative rainbow trout (Garcia de Leaniz et al. 2010; Shimoda 2012). Regarding management, although methods for effective removal should be equipped in the case of emergency, these have not been sufficiently considered. A recent tributary-scale study suggested that winter ecology offers potential applications for effective removal (Koizumi et al. 2017a). Hundreds of rainbow trout migrated from the mainstem to relatively small tributaries of the Otofuke River system, in central Hokkaido, Japan, in early winter (Koizumi et al. 2017a, b): capturing many fish is unrealistic in the large mainstem but is possible in such small tributaries.
In this study, to provide a more effective capture method, we examined local-scale (i.e., pool–riffle) winter habitat use by introduced rainbow trout within a tributary of the same river system (i.e., Otofuke River). We also focused on size dependency, because fishes with different body sizes use different habitats (Heggenes 1988; Höjesjö et al. 2015) and removing large fishes, especially fecund females, is more effective in population reduction (e.g., Thresher 2007). For native stream salmonids, several aspects of physical environments, such as water temperature, depth, velocity, substrate, and cover, are known to affect winter habitat selection (Cunjak 1996; Huusko et al. 2007), whereas the winter habitat use of nonnative salmonid fish is largely unknown. Because ecological characteristics may differ between native and nonnative habitats (e.g., Sax et al. 2007), the maneuvers of the species to overwinter in nonnative habitats should be studied to understand their establishment and invasibility, which also helps to predict biological invasion.
Materials and methods
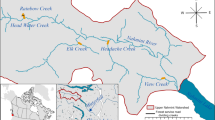
We conducted a field survey in the Obicha River, which drains to the Otofuke River in the Tokachi River basin, on 21 and 22 December 2013 (Fig. 1). The Obicha River has a length of approximately 11 km and is surrounded by agricultural land. In the Obicha River, the introduced rainbow trout is dominant, and natural reproduction should occur (spawning redds and many young-of-the-year were observed; Koizumi I., unpublished data), although some artificial releases might have occurred for recreational fishing purposes. Other native fishes captured were southern Asian Dolly Varden [Salvelinus curilus (syn. Salvelinus malma krascheninnikova)], stone loach (Barbatula oreas), freshwater sculpin (Cottus nozawae) and brook lamprey (Lethenteron sp.).
Locations of the study sections in the Obicha River, a tributary of the Otofuke River system, central Hokkaido, Japan. Detailed information on each section is provided in Table 1
We developed 24 study sections (mean: length, 22.4 m; width, 3.8 m; Fig. 1 and Table 1) where riffle–pool structures were apparent. We attempted to exclude confounding factors of environmental variables (e.g., velocity vs. depth) by selecting diverse habitats. Fish abundance was estimated by capturing all the fish in each section using an electrofisher (Model 12B, Smith-Root, Vancouver, WA, USA) with the setting of block nets (5 mm mesh size) at both ends of sections to prevent fish from emigrating from or immigrating to the sections. We continued electrofishing until every fish was considered to be captured and believe that potential biases resulting from fish remaining uncaptured are minimal. The captured fish were anesthetized with clove oil; their folk length was measured (mm), and their sex and mature/immature status determined based on external characteristics and/or sperm release by gently pressing the abandon. About 10 individuals were taken laboratory to check sex and maturation, and others were released to the captured sections.
Environmental variables (i.e., length and width, area, depth, velocity, substrate, cover) in each section were measured during the same period as the fish capture (Table 1). Water temperature was not measured because variations of temperature were considered to be small due to the short length of the stream studied (ca. 2.3 km). Depth, velocity, substrate, and cover were measured at 35 points (5 evenly spaced transects and 7 divided cross-section of each transect) at each section. Velocity was measured at the position of 60% depth from bottom to surface by using an electromagnetic velocity meter (model VE20, KENEK, Japan). Dominant substrate within a 10 cm × 10 cm quadrat at each sampling point was defined on a categorical scale (slightly modified from Bain et al. 1985): silt or sand (< 2 mm in a diameter), rank = 1; gravel (2–4 mm), rank = 2; pebble (5–64 mm), rank = 3; cobble (65–256 mm), rank = 4; boulder (> 257 mm), rank = 5. We also defined revetment by concrete block as rank = 0, because the concrete block provides no interstitial space to hide. No bedrock was present in the Obicha River. When there were 2 equally dominated substrates within sections, we recorded both and calculated the average rank score (e.g., 1.5 for a sand and gravel mixture). The presence of cover (0 or 1) was recorded at each sampling point when water plants, undercut banks, debris, or sediment of dead leaves was observed. The cover ratio of each section was then calculated as a percentage.
We constructed a generalized linear model (GLM) to examine the relationship between abundance (the number of captured rainbow trout) and environmental factors (depth, velocity, substrate, cover) with Poisson or negative binomial error distribution depending on overdispersion. Section area was set as an offset term. Median depth, velocity, and substrate score, as well as cover ratio, at the 35 points of each section were used for statistical analysis (we used the medians because some of the averages were highly influenced by outlier values within sections). Model selection was performed using Akaike’s information criterion (AIC). As models with ΔAIC values less than 2 are considered as equivalently supported models (Richards 2005), such models were averaged using the “model.avg” function in the R package “MuMIn” (Burnham and Anderson 2002; Richards 2005). Because fish with different size classes often use different habitat (Heggenes 1988; Höjesjö et al. 2015), we separated the size class based on sexual maturity and analyzed the data separately (see Results). All statistical analysis was conducted using R version 4.0.2. (R core team 2020) and significance level was set at 0.05. For GLM, we used the “MASS” R package (Venables and Ripley 2002). For model averaging, we used the “MuMIn” R package (Barton 2020).
Results
We caught 512 nonnative rainbow trout (96.2%) in a total capture of 532 (Fig. 2; Table 1). Other fishes were as follows: 1 southern Asian Dolly Varden, 10 freshwater sculpin, 8 stone loach, and 1 brook lamprey. Lengths of rainbow trout ranged from 62 to 520 mm, and most fish (63.0%) were between 76 and 125 mm (Fig. 2). Mature individuals captured were 17 females and 26 males, and sexual maturity was usually attained over 280 mm and 230 mm in females and males, respectively, whereas a few males matured within 100 to 150 mm. Because fish larger than 250 mm were largely mature, we defined individuals over and under 250 mm as adult or young and analyzed them separately. The number of adults was 44 (8.6%), and the number of young was 465 (91.4%). The abundance of rainbow trout was heterogeneous among the 24 sections: notably, only a few adults (0 to 3 individuals) were caught at 20 sections, whereas much adults (9 to 13 individuals) were caught at the other 4 sections (Table 1). The correlation of abundances (/m2) between young and adult fish was significantly positive but not strong (Spearman’s rank correlation coefficient: r = 0.51, P < 0.05, n = 24 sections).
Size distribution of nonnative rainbow trout in the Obicha River. Black, gray, and white bars represent mature female, mature male, and immature individuals, respectively. a All 512 individuals captured (3 individuals of the total catch were not measured due to escape); b the same figure but with the y-axis cut at 30 for clarity for large fish
A GLM with Poisson distribution was conducted for adult abundance. Results of model averaging selected the depth, cover ratio and substrate as meaningful variables (Table 2). Each of the selected environmental factors correlated positively with abundance (Fig. 3). A GLM with a negative binomial distribution was constructed for young abundance to address overdispersion. Results of model averaging selected only the median velocity as a meaningful variable (Table 2), which negatively correlated with young abundance (Fig. 3).
Relationship between trout abundance and each environmental factor that significantly affects the abundance. The curved lines were estimated from the conditional averaged model (ΔAIC < 2) in Table 2. a < 250 mm (young) vs. velocity (b = -0.04 ± 0.02, z = 2.2, P = 0.03); b ≥ 250 mm (adults) vs. depth (b = 0.22 ± 0.04, z = 4.61, P < 0.01); c > 250 mm (adults) vs. cover (b = 9.56 ± 2.11, z = 4.21, P < 0.01); d ≥ 250 mm (adults) vs. substrate (b = 0.42 ± 0.15, z = 2.62, P = 0.01)
Discussion
Winter is a harsh season for stream fishes and, therefore, wintering habitat is highly restricted, which often results in large aggregations of individuals (Cunjak 1996; Huusko et al. 2007). If invasive nonnative fishes also aggregate to specific habitat during winter, we could effectively control or manage such nonnative species (e.g., Bajer et al. 2011; Koizumi et al. 2017a). While ecological differences between native and nonnative ranges should be considered for the control of nonnative species (e.g., Sax et al. 2007), no study has compared the winter habitat use between native and nonnative rainbow trout. In their native range, the preferable winter habitat has been characterized as deep areas, slow water current, abundant substrate, and cover such as aquatic macrophytes (Baltz et al. 1991; Riehle and Griffith 1993; Simpkins et al. 2000). Importantly, we found that all the variables were selected as the expected directions. Deep pools, large substrate and abundant cover, such as that of woody debris, aquatic macrophytes, or undercut banks, provide refuges from avian and mammal predators (Peterson 1982; Baltz et al. 1991; Penaluna et al. 2016). In addition, low velocity minimizes energy expenditure (Cunjak 1988). Lack of energy may be the main factor in winter mortality (Huusko et al. 2007). These four variables are also known to affect winter habitat selection among other stream salmonids (Cunjak 1996; Huusko et al. 2007). Because winter is a severe period that induces highly constrained physiological processes, knowledge regarding the winter habitat selection or aggregation of native salmonids can be readily applied to nonnative salmonids.
Interestingly, however, each size class showed different habitat usage. Larger adults used deep pools with abundant cover and large substrate, whereas smaller young used low velocity areas. This suggests that adults and young may avoid predation and starvation, respectively. Small individuals can preserve less energy (Huusko et al. 2007) and the habitat that save energy has a priority. Large fishes, by contrast, store more energy and hence have a lower probability of starvation; thus, habitats with lower predation risks may be more important.
Alternatively, there are two other possibilities explaining the size dependent habitat use, although these are not mutually exclusive. The first explanation is intra-specific competition. Salmonids generally form a social hierarchy depending on body size (Nakano 1995; Fausch et al. 2020) and compete for resources (e.g., habitat) even during winter (Harwood et al. 2002). Thus, large individuals may select the best wintering habitat first and, then, smaller fish may choose the second best due to interference competition from larger adult. The other explanation is maturity dependent, instead of size dependent, habitat selection. Since rainbow trout spawn in spring (Taniguchi et al. 2000; Nomoto et al. 2010), mature fish might preserve energy by selecting specific habitats during winter, although the field evidence is scarce. Because most of the large (i.e., ≥ 250 mm) and small fish (< 250 mm) were mature and immature, respectively (except a few small precocial males), we could not distinguish the effects of size and maturation on habitat usage. To solve the problem, further research is needed to compare the winter habitat use among the populations with different maturation sizes.
Limiting factors for fish abundance and distributions are often scale-dependent (e.g., Fausch et al. 1994; Rieman and Dunham 2000). Among the nonnative rainbow trout in the Otofuke River system, water temperature and velocity negatively influenced winter abundance at the tributary level (Koizumi et al. 2017a), whereas depth, velocity, cover, and substrate were important at the pool-riffle level (the current study). If winter habitat use is similar among species and locations, due to highly restricted winter habitat (e.g., Cunjak 1996; Huusko et al. 2007), our results might lead to an effective management strategy for an invasive nonnative salmonid in a stream network. First, we should target tributaries with low water temperature and low water velocity where fish may immigrate from the mainstem during winter (Koizumi et al. 2017a). Next, as intensive capture of mature adults, especially females, minimizes population size (e.g., Thresher 2007), it is recommended that larger adults be targeted at deep pools with abundant cover and large substrate within the tributaries. Additional capture of smaller individuals in low velocity areas may be effective in further reducing population size. Although it is usually difficult to control nonnative salmonids in a large stream network, this strategy can partially resolve the problem. On the other hand, caution should be needed for the populations where the size at maturation is smaller because smaller mature adults may use different habitats as discussed above.
Even when nonnative salmonids are economically or educationally important (e.g., Garcia de Leaniz et al. 2010; Shimoda 2012), effective removal methods should be prepared for emergencies: for example, nonnative species may spread their distributions and/or become highly invasive because of climate change (Roberts et al. 2017). Threatened native southern Asian Dolly Varden inhabit some tributaries in the Otofuke River system and critically endangered Sakhalin taimen Parahucho perryi live in the Tokachi River basin. The negative effects of rainbow trout on these native Japanese salmonids have been widely reported (Taniguchi et al. 2000; Hasegawa et al. 2004; Hasegawa and Maekawa 2006; Baxter et al. 2007; Nomoto et al. 2010; Sahashi and Morita 2016). Because ecological damage could result in irreversible consequences (e.g., Takami et al. 2002), careful management or control for nonnative trout would be necessary to minimize the potential damages.
References
Bain MB, Finn JT, Booke HE (1985) Quantifying stream substrate for habitat analysis studies. N Am J Fish Manag 5:499–500
Bajer PG, Chizinski CJ, Sorensen PW (2011) Using the Judas technique to locate and remove wintertime aggregations of invasive common carp. Fish Manag Ecol 18:497–505
Baltz DM, Vondracek B, Brown LR, Moyle PB (1991) Seasonal changes in microhabitat selection by rainbow trout in a small stream. Trans Am Fish Soc 120:166–176
Barton K (2020) MuMIn: multi-model inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn. Accessed 13 November 2020
Baxter CV, Fausch KD, Murakami M, Chapman PL (2007) Invading rainbow trout usurp a terrestrial prey subsidy from native charr and reduce their growth and abundance. Oecologia 153:461–470
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Second edition. Springer-Verlag, New York
Crawford SS, Muir AM (2007) Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Rev Fish Biol Fish 18:313–344
Cunjak RA (1988) Behavior and microhabitat of young Atlantic salmon (Salmo salar) during winter. Can J Fish Aquat Sci 45:2156–2160
Cunjak RA (1996) Winter habitat of selected stream fishes and potential impacts from land-use activity. Can J Fish Aquat Sci 53:267–282
Fausch KD, Nakano S, Ishigaki K (1994) Distribution of two congeneric charrs in stream of Hokkaido Island, Japan: considering multiple factors across scales. Oecologia 100:1–12
Fausch KD, Nakano S, Kitano S, Kanno Y, Kim S (2020) Interspecific social dominance networks reveal mechanisms promoting coexistence in sympatric charr in Hokkaido, Japan. J Anim Ecol. https://doi.org/10.1111/1365-2656.13384
Garcia de Leaniz C, Gajardo G, Consuegra S (2010) From best to pest: changing perspectives on the impact of exotic salmonids in the Southern Hemisphere. Syst Biodivers 8:447–459
Gozlan RE (2008) Introduction of non-native freshwater fish: is it all bad? Fish Fish 9:106–115
Harwood AJ, Metcalfe NB, Griffiths SW, Armstrong JC (2002) Intra- and inter-specific competition for winter concealment habitat in juvenile salmonids. Can J Fish Aquat Sci 59:1515–1523
Hasegawa K, Maekawa K (2006) Effect of introduced salmonids on two native stream-dwelling salmonids through interspecific competition. J Fish Biol 68:1123–1132
Hasegawa K, Yamamoto T, Murakami M, Maekawa K (2004) Comparison of competitive ability between native and introduced salmonids: Evidence from pairwise contests. Ichthyol Res 51:191–194
Heggenes J (1988) Physical habitat selection by brown trout (Salmo trutta) in riverine systems. Nord J Freshw Res 64:74–90
Höjesjö J, Kaspersson R, Armstrong JD (2015) Size-related habitat use in juvenile Atlantic salmon: the importance of intercohort competition. Can J Fish Aquat Sci 8:1–8
Huusko A, Greenberg L, Stickler M, Linnansaari T, Nykänen M, Vehanen T, Koljonen S, Louhi P, Alfredsen K (2007) Life in the ice lane: the winter ecology of stream salmonids. River Res Appl 23:469–491
Kato K, Yanagawa T (2000) A reproductive population of rainbow trout introduced into the Sanjo River, eestern Japan. Suisanzoshoku 48:603–608 (in Japanese)
Kitano S (2004) Ecological impacts of rainbow, brown and brook trout in Japanese inland waters. Glob Environ Res 8:41–50
Koizumi I, Kanazawa Y, Yamazaki C, Tanaka Y, Takaya K (2017a) Extreme winter aggregation of invasive rainbow trout in small tributaries: implications for effective control. Ichthyol Res 64:197–203
Koizumi I, Tanaka Y, Kanazawa Y (2017b) Mass immigration of juvenile fishes into a small, once-dried tributary demonstrates the importance of remnant tributaries as wintering habitats. Ichthyol Res 64:353–356
Korsu K, Huusko A, Muotka T (2010) Impacts of invasive stream salmonids on native fish: using meta-analysis to summarize four decades of research. Boreal Environ Res 15:491–500
Nakano S (1995) Individual differences in resource use, growth and emigration under the influence of a dominance hierarchy in fluvial red-spotted masu salmon in a natural habitat. J Anim Ecol 64:75–84
Nomoto K, Omiya H, Sugimoto T, Akiba K, Edo K, Higashi S (2010) Potential negative impacts of introduced rainbow trout on endangered Sakhalin taimen through redd disturbance in an agricultural stream, eastern Hokkaido. Ecol Freshw Fish 19:116–126
Penaluna BE, Jason BD, David LGN (2016) Instream cover and shade mediate avian predation on trout in semi-natural streams. Ecol Freshw Fish 25:405–411
Peterson NP (1982) Immigration of juvenile coho salmon (Oncorhynchus kisutch) into riverrine ponds. Can J Fish Aquat Sci 39:1308–1310
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 21 December 2020
Richards SA (2005) Testing ecological theory using the information-theoretic approach: examples and cautionary results. Ecology 86:2805–2814
Riehle MD, Griffith JS (1993) Changes in habitat utilization and feeding chronology of juvenile rainbow trout (Oncorhynchus mykiss) in fall and onset of winter in Silver Creek, Idaho. Can J Fish Aquat Sci 50:2119–2128
Rieman BE, Dunham JB (2000) Metapopulations and salmonids: a synthesis of life history patterns and empirical observations. Ecol Freshw Fish 9:51–64
Roberts JJ, Fausch KD, Hooten MB, Peterson DP (2017) Nonnative trout invasions combined with climate change threaten persistence of isolated cutthroat trout populations in the southern Rocky Mountains. N Am J Fish Manag 37:314–325
Sahashi G, Morita K (2016) Potential threat of introduced rainbow trout Oncorhynchus mykiss to native salmonids in the western part of Hokkaido, Japan. Ichthyol Res 63:540–544
Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, Hastings A, Holt RD, Mayfield MM, O’Connor MI, Rice WR (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evol 22:465-71
Shepard B, Nelson LM, Taper ML, Zale AV (2014) Factors influencing successful eradication of nonnative brook trout from four small Rocky Mountain streams using electrofishing. N Am J Fish Manag 34:988–997
Shimoda K (2012) Alien fish problems in Hokkaido (introduced salmonidae fishes). Nippon Suisan Gakkaishi 78:754–757 (in Japanese)
Simpkins DG, Hubert WA, Wesche TA (2000) Effects of fall-to-winter changes in habitat and frazil ice on the movements and habitat use of juvenile rainbow trout in a Wyoming tailwater. Trans Am Fish Soc 129:101–118
Takami T, Aoyama T (1999) Distribution of rainbow and brown trouts in Hokkaido, northern Japan. Wildlife Conservation Japan 4:41–48 (in Japanese)
Takami T, Yoshihara T, Miyakoshi Y, Kuwabara R (2002) Replacement of whit-spotted charr Salvelinus leucomaenis by brown trout Salmo trutta in a branch of the Chitose River, Hokkaido. Nippon Suisan Gakaishi 68:24–28 (in Japanese with English summary)
Taniguchi Y (2002) Oncorhynchus mykiss. In: The Ecological Society of Japan (ed) Handbook of Alien Species in Japan. Chijin Shokan, Tokyo, p 112 (in Japanese)
Taniguchi Y, Miyake Y, Saito T, Urabe H, Nakano S (2000) Redd super-imposition by introduced rainbow trout on native charrs in a Japanese stream. Ichthyol Res 47:149–156
Thresher RE (2007) Genetic options for the control of invasive vertebrate pests: prospects and constraints. In: Witmer GW, Pitt WC, Fagerstone KA (eds) Managing vertebrate invasive species 52: proceedings of an international symposium. USDA/APHIS Wildlife Services, National Wildlife Research Center, Fort Collins, Colorado, pp 318–331
Tsurui-Sato K, Fujimoto S, Deki O, Suzuki T, Tatsuta H, Tsuji K (2019) Reproductive interference in live‐bearing fish: the male guppy is a potential biological agent for eradicating invasive mosquitofish. Sci Rep 9:5439
Venables WN, Ripley BD (2002) Modern applied statistics with S. Fourth Edition. Springer, New York
Acknowledgements
We appreciated valuable comments of two anonymous reviewers on the earlier version of the draft. This study was partly supported by the research fund provided by Ministry of Land, Infrastructure, Transport and Tourism, Japan. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Furusawa, C., Suehiro-Kanazawa, Y., Tanaka, Y. et al. Local factors affecting winter habitat use of non-native rainbow trout in a boreal stream in northern Japan. Ichthyol Res 69, 125–131 (2022). https://doi.org/10.1007/s10228-021-00820-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-021-00820-7