Abstract
A ventricular assist device (VAD) is a form of mechanical circulatory support that uses a mechanical pump to partially or fully take over the function of a failed heart. In recent decades, the VAD has become a crucial option in the treatment of end-stage heart failure in adult patients. However, due to the lack of suitable devices and more complicated patient profiles, this therapeutic approach is still not widely used for pediatric populations. This article reviews the clinically available devices, adverse events, and future directions of design and implementation in pediatric VADs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pediatric heart failure (HF) is an important cause of mortality in childhood. The incidence of HF in children and adolescents has been reported to range from 0.87/100,000 to 7.4/100,000 [1], with a 5-year mortality or heart transplant (HT) rate of 40% [2]. The approaches to HF treatment are similar in both adult and pediatric patients and include medication, device therapy, surgical treatment, mechanical circulatory support (MCS) and HT. For those patients who retain severe and persistent symptoms of HF despite optimal guideline-directed medical treatment, due to the inefficiency of other approaches, HT was viewed as the last resort and the only long-term solution. However, the overall shortage of suitable donors is an important obstacle to their treatment.
Therefore, as one of the alternative treatments for patients suffering from HF, ventricular assist devices (VADs) have drawn increasing attention and have revolutionized this field. The concept of using a mechanical pump to assist or take over heart function was initially put into practice in the early 1960s. After the first successful clinical use of a VAD as postsurgical support in a 37-year-old female [3], the first use of VADs in pediatric patients was performed by Dr. Debakey in 1967. A 16-year-old girl received mitral valve replacement and was supported by a paracorporeal VAD postoperatively until medically stable. The technique and products have improved after decades of development since that time. However, it was not until the 2000s that pediatric VAD applications underwent a burst of continual growth. Accordingly, a significant increase in waiting-list survival was observed in the era where pediatric VADs initially started to be employed [4]. Indeed, the VAD has changed the management strategy of pediatric HF. However, we are still facing difficulties such as a lack of suitable devices, more severe and complicated patient profiles, less practice experience and higher complication rates, which all limit the application of pediatric VADs.
This article reviews the clinically available devices, adverse events, and future directions of design and implementation in pediatric VADs. Additionally, in this article, by introducing differences in the current status of VAD application in pediatric and adult patients, we try to clarify the large “gap” in devices and patient management and to provide our analysis and advice.
Classification and properties of clinically available pediatric VADs
Pediatric VADs are classified in a number of ways for better description. They can be separated by the anticipated duration of therapy: short term (temporary) and long term (durable). Short-term pediatric VADs are designed for a limited duration of support, usually ranging from several hours to up to 30 days. Temporary VADs are usually applied in acute processes for bridge to transplant (BTT), bridge to recovery (BTR) or bridge to durable VAD support; or applied for prolonged support in cases of small children and patients with complicated circulation. Long-term VADs are mostly applied for BTT and destination therapy (DT). It should be noted that in clinical practice, limitations regarding the duration of support are unclear [5].
The mode of blood flow created by the device could better reflect the mechanism of VADs, as it is strongly related to the outcome of patients, and is briefly introduced in this section. Generally, there is pulsatile flow (PF, by first generation devices) and continuous flow (CF, by second- and third-generation devices). In addition, VADs are also classified based on the location of the pump relative to the patient: percutaneous (intravascular), implantable and paracorporeal.
Based on the major registries and studies, we enrolled the devices frequently used in pediatric patients or in studies focusing on pediatric populations, regardless of whether they were specifically designed for children. Sorted by the recommended duration of support, clinically available devices are shown in (Table 1) along with their key information.
First-generation VADs
The first-generation VADs consist of a volume displacement pump that is actuated pneumatically or electrically to generate PF. Berlin Heart EXCOR VAD (Fig. 1a) is currently the most widely used pediatric PF device. First-generation VADs have shown clinical advantages over optimal medical therapy since their application [6]. Meanwhile, first-generation VADs have also shown flaws. Other than limited patient mobility due to large driving consoles and noise generated by mechanical heart valves [7], most importantly, PF devices consist of multiple moving mechanical parts and prosthetic valves, which leads to limited reliability and durability and a higher risk of thrombus formation. A study revealed that the probability of PF device failure was as high as 35% at 24 months [8], making it one of the major concerns. Moreover, another study reported that patients with PF devices had a far worse survival rate at 2 years with more frequent adverse events and device replacements than patients with CF devices [9]. This study is seen as a milestone and has revolutionized the choice of device.
Diagrams of representative VADs applied in children. Red arrows represent the direction of blood flow. a First-generation pediatric LVAD (Berlin Heart EXCOR). b Second-generation axial CF LVAD (HeartMate XVE LVAD). c Third-generation centrifugal CF LVAD (HeartMate III). d Percutaneous LVAD (Abiomed Impella)
Second- and third-generation VADs
Second- and third-generation VADs both pertain to CF pumps. All rotary blood pumps, except if full levitation of the impeller within the pump housing is achieved under normal operating conditions (referring to third-generation VADs), are defined as second-generation devices [7]. The CF pumps are widely used, and they could serve as short-term paracorporeal VADs (Maquet RotaFlow, Abbott Centrimag, etc.), percutaneous VADs (CardiacAssist TandemHeart, Abiomed Impella, etc.) (Fig. 1d) or durable intracorporeal VADs (Medtronic HeartWare HVAD, Abbott HeartMate III, etc.) (Fig. 1b, c). Theoretically, the durability and hemocompatibility could be further improved with a wear-free operation mode, which is the primary motivation for the development of third-generation VADs. The MOMENTUM 3 trial reported that, at 2 years, the composite endpoint of survival free of disabling stroke and reoperation to remove or replace a malfunctioning device was significantly better with HeartMate III (third-generation VADs) than with HeartMate II (second-generation VADs) in both the BTT and DT groups [10]. However, other third-generation devices, such as Berlin Heart INCOR and Abbott PediMag, still need constant observation to clarify their superiority in patient outcomes.
Adverse events of pediatric VADs
Pedimacs (the Pediatric Interagency Registry for Mechanical Circulatory Support), Paedi-EUROMACS (The European Registry for Patients with Mechanical Circulatory Support) and ACTION (Advanced Cardiac Therapies Improving Outcomes Network) are the three major pediatric VAD registries [11,12,13] reflecting the current situation of this field. However, their reports of adverse events are not quite comparable, as there are differences in the type of adverse events concluded between the studies, and the categorization of “early” or “late” adverse events varies. To avoid confusion, the data referred to in this section are mainly based on the Pedimacs registry, who might be more representative as it so far has included more cases than other registries along with more dispersed distribution of patients’ ages. We also reviewed studies including ≥ 25 patients published in the last 5 years (2019–2023) referring to adverse events of pediatric VADs in (Table 2), including Paedi-EUROMACS and ACTION. Numerous variables could have an impact on the VAD risk profile, including patient age, body size, anatomy, developmental hemostasis, device type, illness severity and comorbidities prior to implantation [14], and these interdependent factors greatly amplify the complexity of patient outcome.
Bleeding
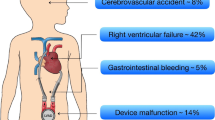
Bleeding, especially gastrointestinal (GI) bleeding, is a significantly common complication that leads to recurrent hospitalizations, along with increased lengths of stay, costs, blood transfusions, and time off anticoagulation or antiplatelet therapy [15]. The risk of bleeding in the setting of VAD support is multifactorial and is related to anticoagulation/antiplatelet therapy, dysregulated angiogenesis, arteriovenous malformations, VAD-associated von Willebrand syndrome, mucosal hypoxia induced by lack of pulsatility in CF VADs, etc. [16]. Adding up both GI and non-GI bleeding, the rate of bleeding events in the Pedimacs registry is up to 5.1 per patient-year within the first 2 weeks and 0.6 per patient-year afterward [12]. Since pediatric patients have distinct differences in blood rheology and behavior [17], the patient management should be actively and repeatedly evaluated.
Infection
Infection is another common adverse event occurring in VAD-supported children. The Pedimacs registry found that infections occurred in 12% of pediatric patients within 2 weeks of implantation and 21% after 2 weeks or more [12]. Among adult patients, the most common VAD-specific infections are driveline infections, with an overall event rate of 14 to 48% between various studies [18]. The ACTION registry further reveals that, in children, the three most common types of infection are sepsis, localized non-device infection, and percutaneous site and/or pocket infection [19]. Paracorporeal devices are strongly related to a higher risk of infection than intracorporeal devices [19].
Device malfunction/pump thrombus formation
Device malfunction/pump thrombus formation is the most frequently reported major adverse event in pediatric population [5, 11]. Pump thrombus is the most common reason for device exchange and could be a risk factor for stroke leading to morbidity [20]. Although pump thrombus is a significant adverse event, a study noted that only 13% to 15% of device malfunctions are attributable to pump failure [21]. The true incidence of malfunction of the broader system components might have been neglected. CF-VADs greatly ameliorated the outcome, with 90.4% of adult patients at 1 year and 82.6% at 2 years free from device malfunction/pump thrombi [22]. Similarly, in a recent study of pediatric patients implanted with the HeartMate 3 device, there were no episodes of pump thrombosis or pump dysfunction requiring operative exchange with a median 78 days of follow-up [23]. However, due to the difference in device type and the lack of experience, it seems much less optimistic for the overall pediatric patients, especially those supported by paracorporeal devices [24].
Neurological dysfunction
Neurological adverse events are defined and recorded differently among studies, most of which mainly focus on cerebrovascular accidents (CVAs). Ischemic strokes usually result from embolic sources on the aortic valve, the inflow cannula, or intracardiac chamber, and hemorrhagic strokes may occur mainly secondarily to hypertension or coagulopathy [25]. The Pedimacs report reveals that stroke occurs in 11% of all pediatric patients [12]. CVAs are one of the leading causes of death in Paedi-EUROMACS (24.17% of deaths), as in the Pedimacs cohort [11, 12]. It is important to recognize that adverse neurological events comprise a broad category of complications. Other than CVAs, there are seizures, encephalopathy, asymptomatic neuroradiological findings, confusion, extra-axial bleeding, etc. [26].
Beyond these complications, right ventricular failure [27], aortic regurgitation [28, 29], peripheral infarction [30], arrhythmia [31, 32], renal dysfunction, respiratory failure, wound dehiscence, allosensitization, psychiatric episodes, and hemolysis are also observed adverse events post-VAD implantation [22, 33] and yet are less discussed in pediatric cohorts. Despite improvements in VAD technology and increasing familiarity with pediatric VAD patients, the outcome and survival of pediatric patients are still not optimal and not comparable with those of adults. For better determination of the incidence of adverse events and for improved reporting of how to effectively manage them, sharing experiences across centers is of great value.
Future directions of pediatric VADs design
Ideally, VADs should be able to provide suitable cardiac output both at rest and during exertion, high durability, a less invasive implantation approach, a nonblood contact design, and a fully implantable system to avoid skin barrier penetration [34]. Whereas each of these ideals may have been achieved and implemented in different designs, we are far from fulfilling all of these criteria. Here, we put forward a few possible trends of pediatric VADs design and research.
Suitable devices for different age groups
Lack of suitable devices designed to address the unique anatomic and physiologic needs of children in different age groups remains the biggest obstacle. The development of pediatric VADs lags behind that of adults due to both technological limitations and economic unsustainability. As a result, many larger children are implanted with adult devices. Using adult-sized devices in children (“off-label” or “off-design” application) results in patient-device size mismatches, creative implantation strategies, and blood flow beyond the range recommended [35]. Adapting adult VADs for children has the risk of unexpected complications. Indeed, worse patient outcomes compared to adult patients have been observed. A multicenter registry analysis showed promising outcomes in a cohort of pediatric and younger adult patients undergoing implantation of HeartMate III [23], but only 57% of these patients were discharged. While in adult patients supported by HeartMate III, the discharge rate could reach 94.2% [36], showing an obvious gap between the two populations. Similarly, in another study, the rate of discharge for HVAD was 80% in young adults and only 48% in children [31]. Numeric simulations and in vitro measurements indicate that in the pediatric condition, HVAD washout of old blood is 2 times slower and the residence time of blood within the pump is twofold prolonged compared with a typical adult case [37], which are potentially unfavorable mechanisms in terms of blood trauma and thrombogenicity. Thus, adapting adult VADs for larger children could be a temporal solution in this era, but devices specially designed for them are expected.
As to smaller children supported by VADs, the outcome is even worse and with more limited options. When we look at survival by age group, the youngest patients have the lowest overall survival [5]. Factors associated with the lowest survival are frequently observed together (infants, Pedimacs patient profile level 1, paracorporeal continuous VADs, and congenital heart disease) [12]. In these cases, implantable devices are barely possible to be applied due to the body size. Challenges such as a complex anatomy, a more invasive procedure for implantation and risks related to mechanical valves make the durable PF VAD support less ideal. On the one hand, innovations of PF VADs, such as a valveless design (Fig. 2), could be beneficial to them in the future; on the other hand, short term paracorporeal CF VADs has currently become a very important option. Although the devices were designed for short-term support, a prolonged duration is proven to be feasible. The median duration varies between 6 to 20 days [38,39,40,41,42] with a longest duration of 227 days [41] reported in different studies. A study shows that 71% of patients had positive primary end point [41], which is acceptable considering the patient profile, but still far from optimal. The safety duration of temporary devices should be reevaluated with more clinical evidence collected, and the development of extracorporeal PF and CF devices with suitable range of output is also crucial for small children.
Mechanism of TORVAD pulsatile pump. “A” and “B” are two pistons that cyclically move around a toroidal pumping chamber via magnetic coupling to a motor. Black arrows indicate the movement of pistons, and red arrows represent blood flow generated. By cyclically actuating one piston around a toroidal pumping chamber via magnetic coupling to a motor, TORVAD could generate a pulsatile blood flow without an area of stasis
In conclusion, there are challenges and an urgent need for suitable devices for each age groups. Devices specifically designed for children should be further developed, and the full potential of VAD therapy for children has yet to be realized.
Non-blood-contacting devices
The development of non-blood-contacting devices is a very attractive research direction. Reduction or even elimination of direct contact between the blood flow and the device could theoretically avoid the use of anticoagulation agents and could reduce complications such as gastrointestinal bleeding, neurologic injury and device-related thrombosis. Non-blood-contacting devices directly compress the heart using artificial muscle or pressurized cups that either cover the entire epicardial surface or target just one of the diseased ventricles. Several non-blood-contacting devices have been tested in animals [43]. For example, a “soft robotic sleeve” could use compressed air to power artificial silicone muscles [44]. By being selectively activated to compress and twist, the silicon muscles mimic the movements of the normal human heart (Fig. 3). However, complications related to local mechanical lesions, such as bleeding, ecchymosis and adhesions, should not be ignored, and the outcome and stability require further research.
The structure of a bioinspired nonblood-contacting VAD with “soft robotic sleeves”. a The design is inspired by the muscle fiber orientations of the outer two layers of the myocardium. b Individual active layers composed of fluidic actuator contractile elements could perform compression and decompression or twisting and untwisting, or could simultaneously perform both actions to provide proper support
Wireless power system for implantable VAD
A wireless power system could greatly reduce percutaneous site infections (PSIs), especially in pediatric patients supported by implantable devices. All current VADs are either paracorporeal/intravenous devices that have catheters inserted across the skin barrier to transfuse blood or implantable devices with a percutaneous drive line to power the VAD, which are associated with PSIs. It has been reported that at 1 year after implantation, nearly 19% of adult CF left ventricular assist device (LVAD) recipients develop a PSI [45]. Younger age may be a predictor of a higher incidence of PSIs, and being more physically active is suspected to be an important reason [45]. With less control of their actions, pediatric patients could more likely disrupt the integrity of the driveline–integument barrier, and the self-nursing of percutaneous sites may not be well performed unless there is intense assistance and supervision from adults. As a result, the elimination of the driveline of implantable devices is feasible and very advantageous for pediatric patients.
Previous transcutaneous energy transfer systems have been limited by restrictions on the separation distance and alignment between the transmit and receive coils [46], and there are some breakthroughs. For example, a coplanar energy transfer system characterized by coil-within-the-coil topology could ensure high and robust resonance energy powering. It has been tested in animal trials for up to 6 months, and the first two applications in human use were reported in 2019 (both supported for more than 30 days) [47]. The free-range resonant electrical energy delivery (FREE-D) system allows power delivery at larger distances without compromising safety and efficiency [48]. The wireless powering system may offer a new perspective on quality of life (QoL), a decrease in the caregiver burden, and the elimination of driveline infection for patients supported by implanted devices [49].
In addition, better anticoagulation surface materials, better anticoagulation management protocols [50] (to consist with developmental hemostasis [14]), a larger range of support, miniaturization and better mobility are all directions worth working on. Here we reviewed published materials on pediatric VADs under development (Table 3) mostly focusing on blood pump innovation. Other than basic information, their unique innovations of design are also provided. We look forward to encouraging outcomes in future investigations to provide better choices for children.
Future directions of pediatric VADs implementation exploration
Exploration of PF generated by CF pumps for children
PF devices are currently less preferred when CF pumps are optional, but the pulsatility of blood flow does have positive effects on patients. It has been proved in both pediatric and adult population that patients with PF devices had a far worse outcome compared to CF devices [9, 12], there has been a clear trend of transition in device type in the adult population over time, and PF devices are almost abandoned for long-term support. Of all durable VADs implanted in adult patients in the last decade, only 0.4% have been driven by PF pumps [51]. While as to pediatric patients, PF VADs are implanted in more cases, accounting for approximately 27.6% of all pediatric VADs implanted in North America and for 52.9% in Europe [11, 12], and this could be explained by the difficulty of device implantation into children, different preferences for treatment strategies and the lack of suitable CF devices for smaller and younger children. PF pumps seem to be applied only in unavoidable circumstances. However, it is mainly because of how the pumps work, instead of the pulsatility of blood flow. It is undeniable that PF has many advantages over CF, including reduced incidence of aortic valve complications [52], gastrointestinal bleeding [53, 54], ventricular suction and pulmonary congestion [6]; and improved volume unloading [55] and bridge to recovery outcomes [56].
Thus, combining the safety of the CF pump and the hemocompatibility of PF could be beneficial, but it should be prudently applied. Other than the pulsatility generated by the intrinsic cardiac cycle, a PF can also be actively generated with CF pumps by periodically adjusting the pump speed. A few attempts have been made in adult population. The Lavare cycle [57] is a periodic speed modulation designed for better washout of the pump. It significantly reduced the rates of stroke, sepsis and right heart failure, with no difference in the transplant or recovery rates [57]. Similarly, by periodically changing the pump speed, the HeartMate III system could generate an artificial pulse every 2 s [58]. By evaluating middle cerebral artery flow dynamics, it was found that the pulsatility and improved hemocompatibility of HeartMate III may improve cerebrovascular metabolic reactivity compared with HeartMate II, which matches the decreased rate of stroke and better clinical outcomes [59]. A computational fluid dynamic simulation showed that the artificial pulse contributes to the removal of blood components from pump surfaces [58]. These evidences show potential in pediatric patients’ outcomes, especially for those supported by CF pumps. Despite all the advantages, similar “artificial pulse” applications in pediatric patients should be highly cautiously reviewed. It is currently not well understood how pediatric patients tolerate mechanical support in either pulsatile or continuous-flow scenarios [60]. It has been observed that artificial pulses increase turbulence and total stresses, whose biological effects are not known in detail but might contribute to clinically observed issues related to hemocompatibility [58]. In addition, if introduced into pediatric application, the range of pump speed change for generating PF should be carefully titrated and dynamically adjusted for each patient, as a very high speed may lead to suction events and arrhythmias [25]. In conclusion, attempts to adjust the currently available CF devices to mimic PF could be beneficial and are worth exploring for children; and to avoid collateral harm, both the biological and clinical implications of the technique remain to be resolved.
From salvage therapy to standard routine
Due to lack of experience and fear of device-related complications, pediatric VADs were mainly used as an approach to rescue patients with critical conditions. In the Pedimacs cohort, 87.1% of patients had INTERMACS profile 1 (critical cardiogenic shock) or 2 (progressive decline) at VAD implantation, which is more advanced than the 50.9% in the adult population [12, 51]. As improvements in technology and medical care continue to reduce the risk of morbidity associated with VAD support, strategies regarding candidacy and timing for device implantation should also evolve. Outcomes are worse when patients have developed cardiogenic shock with significant end-organ dysfunction prior to implantation [5, 61]. A more proactive device placement strategy could stop and even reverse the worsening general condition, and mechanical unloading has many positive effects on preservation and recovery of cardiac function [62]. Thus, VAD should gradually shift to a component of standard pediatric HF therapy, rather than primarily as a means of hemodynamic support [63], and proactive implantation may promote the process of recovery.
Better choice of duration VAD “bridge”
A recent analysis showed that more than 1200 children have been bridged to heart transplant in the last 15 years with MCS, including VADs and total artificial hearts [64]. For pediatric patients, there is yet no consensus reached regarding the duration of “bridge”, and how to make full use of both VAD support and heart transplantation to optimize patient survival is worth discussing.
Heart transplantation seems to be carried out more radically in pediatric patients. At 6 months after VAD implantation, more than half of pediatric patients in North America were reported to receive a heart transplant [65], as did 33% of patients in Europe [66]. Meanwhile, for adult patients, only 7.3% of them received a heart transplant, and 76.6% survived on support at 1 year post-implantation [51]. Possible reasons are that the proportion of qualified receivers in children is higher, and the mindset of pediatric physicians overemphasizes the benefit of minimizing the support duration [67], which results in anticipative transplantation. Once such support commences, medical teams seem to be in a race against the “complication clock,” and they try to shorten the bridge to transplantation.
However, some experts propose that being in a rush to eliminate VAD support may not be beneficial. This “bridge” of the VAD here plays an important role, more than just helping the patients to live long enough for a suitable donor graft. Prolonged BTT support duration leads to stabilization and rehabilitation of the patient prior to transplantation, with improved end-organ function, decreased inotrope and ventilator dependence, and improved nutritional and functional status [4], which can all improve patients’ pretransplant conditions and candidacy for heart transplantation. Moreover, this can also provide an opportunity for myocardial recovery [65] and, in certain cases, free them from having a heart transplant [68, 69]. Additionally, transplantation that is performed too soon has potential risks, such as graft failure and a missed opportunity for recovery [70]. Keeping both VAD-related complications and risks of early heart transplantation in mind, the optimal balance between these two competing risks determines the optimal timing of explantation. A study concerning 1064 children who underwent VAD implantation prior to a heart transplant indicated that a longer duration (within 30 days versus ≥ 30 days) of VAD support prior to heart transplantation is associated with a one-year survival benefit in children [71]. A multicenter review of pediatric VAD support analyzing the association between the duration of support and posttransplant survival identified a potentially optimal duration of VAD support: 2–4 months in patients supported with a paracorporeal pulsatile VAD and any time after 3 weeks in patients supported with an intracorporeal continuous VAD [72]. Texas Children’s Hospital has adopted a 3-month waiting period after CF VAD implantation [65]. These patients are inactivated on the transplant waitlist after implantation to allow sufficient time for systemic recovery, which has shown promising survival, cardiac recovery and QoL improvements. In conclusion, a prolonged “bridging period” is likely to be beneficial, but the optimal timing is yet to be determined.
Further improvement in physical activity performance after VAD implantation
Patients on LVAD support demonstrate improved physical activity and QoL, but well below that of healthy people [73]. Low physical activity levels are associated with increased risk factors for cardiometabolic diseases, impairments in cognitive function and lower academic achievement for children [74], and such activity deficits also affect muscular strength, patient-reported health outcomes [75], functional capacity, social interactions and mental health [76]. Thus, generalized treatment, rehabilitation and exercise prescriptions should timely step in. To coordinate this process, finely adjusted VAD output is needed for proper support.
Upmodulation of pump speed within a limited range during exercise is worth exploring but yet debatable. In the healthy population, cardiac output increases threefold to fivefold to meet the demands of exercising [77]. Under VAD support, the pump speed, delta P (pressure difference between systemic arterial blood pressure and left ventricular end diastolic pressure) and native heart contractility determine the actual total cardiac output (CO) [78]. Since mechanical pumps have approximately half the sensitivity of the natural heart to preload and three times greater sensitivity to afterload [78], when exercise is completed at the baseline pump speed of CF LVAD, an increase in total output can be observed but is not sufficient to maintain low filling pressures. Thus, increasing total CO during body exercise by increasing the pump speed within a limited range may provide better support. Several studies have shown positive effects of upmodulation of pump speed on CO and on tolerance of body exercise [77, 79, 80], including both adult and pediatric cohorts. In contrast, some studies indicate that the high-speed setting does not improve exercise tolerance [81, 82]. Given the limited patient volume, the variety of patients and pumps involved, and the heterogeneity of modulation and exercise protocols of these studies, the conclusion is still controversial and needs to be discussed separately. Additionally, the exercise physiology needs to be further elucidated in VAD patients, and smarter and automatically adjusting device algorithms are expected.
Balancing interagency and international development
The accessibility of proper medical care, convenience and cost of follow-up largely depend on the distribution of qualified centers. It is clearly seen that pediatric VADs are not yet widely applied even in developed countries and regions, and there is an obvious interagency imbalance. For example, only 13 large-volume hospitals (defined by > 30 patients reported) out of 47 hospitals carry out 63% of pediatric VAD implantations in North America [12], and the situation is quite similar in Europe [11]. From an international perspective, fewer centers and cases are reported in other countries and regions, and the gap is much wider. Taking China as an example, a nation-wide survey indicates that by June 2017, the total case number of pediatric VAD implantation in mainland China was 39 [83]. The first application of implantable LVADs in pediatric patients was carried out in 2022 at Fuwai Hospital using Corheart 6 (full magnetic levitated LVAD designed and fabricated by a local company) [84]. Thus, pediatric VAD support is far from widely applied. Although the superiority of VAD support over extracorporeal membrane oxygenation (ECMO) is widely observed [85, 86], ECMO is still the most attainable option of MCS in most cases. The imbalanced interagency and international development of pediatric VAD largely restricts the accessibility of medical care and overall survival of patients, and the road ahead will be long.
Conclusion
VAD support plays an important role in the management of end-stage HF. Great accomplishments have been made in recent decades, but VAD application to pediatric patients very much lags behind that in adult patients in many aspects, and there are still many unsettled questions to be answered. To overcome these challenges, more registries enrolling a larger number of pediatric patients should be established to provide comparative data and to guide clinical decisions. Regarding the technological development of pediatric VADs, forward-thinking design solutions are needed. We believe that VAD will better serve pediatric HF patients in the future.
References
Shaddy RE, George AT, Jaecklin T, Lochlainn EN, Thakur L et al (2018) Systematic literature review on the incidence and prevalence of heart failure in children and adolescents. Pediatr Cardiol 39:415–436. https://doi.org/10.1007/s00246-017-1787-2
Watanabe K, Shih R (2020) Update of pediatric heart failure. Pediatr Clin North Am 67:889–901. https://doi.org/10.1016/j.pcl.2020.06.004
DeBakey ME (1971) Left ventricular bypass pump for cardiac assistance. Clinical experience. Am J Cardiol 27
Zafar F, Castleberry C, Khan MS, Mehta V, Bryant R 3rd et al (2015) Pediatric heart transplant waiting list mortality in the era of ventricular assist devices. J Heart Lung Transplant 34:82–88. https://doi.org/10.1016/j.healun.2014.09.018
Rossano JW, VanderPluym CJ, Peng DM, Hollander SA, Maeda K et al (2021) Fifth Annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) report. Ann Thorac Surg. https://doi.org/10.1016/j.athoracsur.2021.10.001
Wu EL, Stevens MC, Pauls JP, Steinseifer U (2018) Chapter 3 - First-generation ventricular assist devices. In:Gregory SD, Stevens MC, Fraser JF(ed) Mechanical circulatory and respiratory support. Academic Press, pp 93–115
Graefe R, Groß-Hardt S (2018) Chapter 4 - Second-generation ventricular assist devices. In:Gregory SD, Stevens MC, Fraser JF(ed) Mechanical circulatory and respiratory support. Academic Press, pp 117–150
Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW et al (2001) Long-term use of a left ventricular assist device for end-stage heart failure. N Eng J Med 345:1435–1443
Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV et al (2009) Advanced heart failure treated with continuous-flow left ventricular assist device. N Eng J Med 361:2241–2251. https://doi.org/10.1056/NEJMoa0909938
Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J et al (2020) Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the multicenter study of MagLev technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) randomized clinical trial. JAMA Cardiol 5:411–419. https://doi.org/10.1001/jamacardio.2019.5323
de By TMMH, Schweiger M, Hussain H, Amodeo A, Martens T et al (2022) The European Registry for Patients with Mechanical Circulatory Support (EUROMACS): third Paediatric (Paedi-EUROMACS) report. Eur J Cardiothorac Surg : Official Journal of the European Association For Cardio-thoracic Surgery 62. https://doi.org/10.1093/ejcts/ezac355
Adachi I, Peng DM, Hollander SA, Simpson KE, Davies RR et al (2022) Sixth Annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) Report. Ann Thorac Surg. https://doi.org/10.1016/j.athoracsur.2022.10.042
Lorts A, Smyth L, Gajarski RJ, VanderPluym CJ, Mehegan M et al (2020) The creation of a pediatric health care learning network: the ACTION quality improvement collaborative. ASAIO Journal (American Society For Artificial Internal Organs : 1992) 66:441–446. https://doi.org/10.1097/MAT.0000000000001133
Lorts A, Conway J, Schweiger M, Adachi I, Amdani S et al (2021) ISHLT consensus statement for the selection and management of pediatric and congenital heart disease patients on ventricular assist devices Endorsed by the American Heart Association. J Heart Lung Transplant 40:709–732. https://doi.org/10.1016/j.healun.2021.04.015
Shah R, Qayed E (2018) Outcomes and predictors of readmissions with GI bleeding in patients with left ventricular assist devices. South Med J 111:666–673. https://doi.org/10.14423/SMJ.0000000000000883
Kataria R, Jorde UP (2019) Gastrointestinal bleeding during continuous-flow left ventricular assist device support: state of the field. Cardiol Rev 27. https://doi.org/10.1097/CRD.0000000000000212
Sharp MK, Gregg M, Brock G, Nair N, Sahetya S et al (2017) Comparison of blood viscoelasticity in pediatric and adult cardiac patients. Cardiovasc Eng Technol 8:182–192. https://doi.org/10.1007/s13239-017-0300-7
Aslam S (2018) Ventricular assist device infections. Cardiol Clin 36:507–517. https://doi.org/10.1016/j.ccl.2018.06.005
Bansal N, Auerbach SR, Shezad MF, Patel AB (2021) The initial analysis of infectious adverse events in pediatric ventricular assist devices reported to the Action Registry. actionlearningnetwork.org. https://www.actionlearningnetwork.org/wp-content/uploads/ISHLT-Abstract_Infection-Neha-Bansal.pdf. Accessed 13 Oct 2022
Lichtenstein KM, Tunuguntla HP, Peng DM, Buchholz H, Conway J (2021) Pediatric ventricular assist device registries: update and perspectives in the era of miniaturized continuous-flow pumps. Ann Cardiothorac Surgery 10:329–338. https://doi.org/10.21037/acs-2020-cfmcs-18
Kormos RL, McCall M, Althouse A, Lagazzi L, Schaub R et al (2017) Left ventricular assist device malfunctions: it is more than just the pump. Circulation 136:1714–1725. https://doi.org/10.1161/CIRCULATIONAHA.117.027360
Shah P, Yuzefpolskaya M, Hickey GW, Breathett K, Wever-Pinzon O et al (2022) Twelfth Interagency Registry for Mechanically Assisted Circulatory Support Report: readmissions after left ventricular assist device. Ann Thorac Surg 113:722–737. https://doi.org/10.1016/j.athoracsur.2021.12.011
O’Connor MJ, Lorts A, Davies RR, Fynn-Thompson F, Joong A et al (2020) Early experience with the HeartMate 3 continuous-flow ventricular assist device in pediatric patients and patients with congenital heart disease: a multicenter registry analysis. J Heart Lung Transplant 39:573–579. https://doi.org/10.1016/j.healun.2020.02.007
Morales DLS, Rossano JW, VanderPluym C, Lorts A, Cantor R et al (2019) Third Annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) report: preimplant characteristics and outcomes. Ann Thorac Surg 107:993–1004. https://doi.org/10.1016/j.athoracsur.2019.01.038
Castrodeza J, Ortiz-Bautista C, Fernández-Avilés F (2022) Continuous-flow left ventricular assist device: current knowledge, complications, and future directions. Cardiol J 29:293–304. https://doi.org/10.5603/CJ.a2021.0172
Morales DLS, Adachi I, Peng DM, Sinha P, Lorts A et al (2020) Fourth Annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) Report. Ann Thorac Surg 110:1819–1831. https://doi.org/10.1016/j.athoracsur.2020.09.003
Simpson KE, Kirklin JK, Cantor RS, Mehegan M, Lamour JM et al (2020) Right heart failure with left ventricular assist device implantation in children: an analysis of the Pedimacs registry database. J Heart Lung Transplant 39:231–240. https://doi.org/10.1016/j.healun.2019.11.012
Imamura T, Narang N, Kim G, Nitta D, Fujino T et al (2020) Aortic Insufficiency during HeartMate 3 left ventricular assist device support. J Card Fail 26:863–869. https://doi.org/10.1016/j.cardfail.2020.05.013
Zhang Q, Gao B, Yu C (2018) The effects of left ventricular assist device support level on the biomechanical states of aortic valve. Med Sci Monit 24:2003–2017. https://doi.org/10.12659/msm.906903
Lammers AE, Sprenger KS, Diller G-P, Miera O, Lebherz C et al (2021) Ventricular assist devices in paediatric cardiomyopathy and congenital heart disease: an analysis of the German National Register for Congenital Heart Defects. Int J Cardiol 343:37–44. https://doi.org/10.1016/j.ijcard.2021.08.047
VanderPluym CJ, Adachi I, Niebler R, Griffiths E, Fynn-Thompson F et al (2019) Outcomes of children supported with an intracorporeal continuous-flow left ventricular assist system. J Heart Lung Transplant 38:385–393. https://doi.org/10.1016/j.healun.2018.09.015
Auerbach SR, Simpson KE (2021) HVAD usage and outcomes in the current pediatric ventricular assist device field: an Advanced Cardiac Therapies Improving Outcomes Network (ACTION) Analysis. Asaio j 67:675–680. https://doi.org/10.1097/mat.0000000000001373
Nandi D, Auerbach SR, Bansal N, Buchholz H, Conway J et al (2023) Initial multicenter experience with ventricular assist devices in children and young adults with muscular dystrophy: an ACTION registry analysis. J Heart Lung Transplant 42:246–254. https://doi.org/10.1016/j.healun.2022.09.003
Criscione JC (2017) Cardiovascular devices: soft hugs for healing hearts. Nat Biomed Eng 1:0046. https://doi.org/10.1038/s41551-017-0046
Conway J, Tunuguntla H (2020) Big devices in small patients: adapting adult ventricular assist devices for children. J Heart Lung Transplant 39:580–581. https://doi.org/10.1016/j.healun.2020.04.001
Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M et al (2019) A fully magnetically levitated left ventricular assist device - final report. N Engl J Med 380:1618–1627. https://doi.org/10.1056/NEJMoa1900486
Granegger M, Thamsen B, Schlöglhofer T, Lach S, Escher A et al (2020) Blood trauma potential of the HeartWare Ventricular Assist Device in pediatric patients. J Thorac Cardiovasc Surg 159:1519-1527.e1511. https://doi.org/10.1016/j.jtcvs.2019.06.084
Yarlagadda VV, Maeda K, Zhang Y, Chen S, Dykes JC et al (2017) Temporary circulatory support in U.S. children awaiting heart transplantation. J Am Coll Cardiol 70:2250–2260. https://doi.org/10.1016/j.jacc.2017.08.072
Conway J, Al-Aklabi M, Granoski D, Islam S, Ryerson L et al (2016) Supporting pediatric patients with short-term continuous-flow devices. J Heart Lung Transplant 35:603–609. https://doi.org/10.1016/j.healun.2016.01.1224
Cho J, Fuentes-Baldemar AA, Tunuguntla HP, Spinner JA, Tume SC et al (2023) Outcomes of temporary ventricular assist device: a pediatric institutional experience over 25 years. J Thorac Cardiovasc Surg 166. https://doi.org/10.1016/j.jtcvs.2022.10.041
Lorts A, Eghtesady P, Mehegan M, Adachi I, Villa C et al (2018) Outcomes of children supported with devices labeled as “temporary” or short term: a report from the Pediatric Interagency Registry for Mechanical Circulatory Support. J Heart Lung Transplant 37:54–60. https://doi.org/10.1016/j.healun.2017.10.023
Lim JH, Kwak JG, Min J, Kwon HW, Song MK et al (2020) Experience with temporary centrifugal pump bi-ventricular assist device for pediatric acute heart failure: comparison with ECMO. Pediatr Cardiol 41:1559–1568. https://doi.org/10.1007/s00246-020-02412-0
Weymann A, Foroughi J, Vardanyan R, Punjabi PP, Schmack B et al (2023) Artificial muscles and soft robotic devices for treatment of end-stage heart failure. Adv Mater (Deerfield Beach, Fla) 35:e2207390. https://doi.org/10.1002/adma.202207390
Roche ET, Horvath MA, Wamala I, Alazmani A, Song S-E et al (2017) Soft robotic sleeve supports heart function. Sci Transl Med 9. https://doi.org/10.1126/scitranslmed.aaf3925
Goldstein DJ, Naftel D, Holman W, Bellumkonda L, Pamboukian SV et al (2012) Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant 31:1151–1157. https://doi.org/10.1016/j.healun.2012.05.004
Fu Y, Hu L, Ruan X, Fu X (2015) A transcutaneous energy transmission system for artificial heart adapting to changing impedance. Artif Organs 39:378–387. https://doi.org/10.1111/aor.12384
Amodeo A, Filippelli S, Perri G, Iacobelli R, Adorisio R et al (2020) First human implantation of a miniaturized axial flow ventricular assist device in a child with end-stage heart failure. J Heart Lung Transplant 39:83–87. https://doi.org/10.1016/j.healun.2019.09.003
Waters BH, Park J, Bouwmeester JC, Valdovinos J, Geirsson A et al (2018) Electrical power to run ventricular assist devices using the Free-range Resonant Electrical Energy Delivery system. J Heart Lung Transplant 37:1467–1474. https://doi.org/10.1016/j.healun.2018.08.007
Wang JX, Smith JR, Bonde P (2014) Energy transmission and power sources for mechanical circulatory support devices to achieve total implantability. Ann Thorac Surg 97:1467–1474. https://doi.org/10.1016/j.athoracsur.2013.10.107
VanderPluym CJ, Cantor RS, Machado D, Boyle G, May L et al (2020) Utilization and outcomes of children treated with direct thrombin inhibitors on paracorporeal ventricular assist device support. ASAIO J (American Society For Artificial Internal Organs : 1992) 66:939–945. https://doi.org/10.1097/MAT.0000000000001093
Yuzefpolskaya M, Schroeder SE, Houston BA, Robinson MR, Gosev I et al (2023) The Society of Thoracic Surgeons Intermacs 2022 annual report: focus on the 2018 Heart Transplant Allocation System. Ann Thorac Surg 115:311–327. https://doi.org/10.1016/j.athoracsur.2022.11.023
Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD et al (2010) The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail 3:668–674. https://doi.org/10.1161/CIRCHEARTFAILURE.109.917765
Wang Y, Nguyen KT, Ismail E, Donoghue L, Giridharan GA et al (2022) Effect of pulsatility on shear-induced extensional behavior of Von Willebrand factor. Artif Organs 46:887–898. https://doi.org/10.1111/aor.14133
Crow S, John R, Boyle A, Shumway S, Liao K et al (2009) Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg 137:208–215. https://doi.org/10.1016/j.jtcvs.2008.07.032
Kato TS, Chokshi A, Singh P, Khawaja T, Cheema F et al (2011) Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circ Heart Fail 4:546–553. https://doi.org/10.1161/CIRCHEARTFAILURE.111.962142
Krabatsch T, Schweiger M, Dandel M, Stepanenko A, Drews T et al (2011) Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? Ann Thorac Surg 91:1335–1340. https://doi.org/10.1016/j.athoracsur.2011.01.027
Zimpfer D, Strueber M, Aigner P, Schmitto JD, Fiane AE et al (2016) Evaluation of the HeartWare ventricular assist device Lavare cycle in a particle image velocimetry model and in clinical practice. Eur J Cardiothorac Surg : Official Journal of the European Association For Cardio-thoracic Surgery 50:839–848
Wiegmann L, Thamsen B, de Zélicourt D, Granegger M, Boës S et al (2019) Fluid dynamics in the HeartMate 3: influence of the artificial pulse feature and residual cardiac pulsation. Artif Organs 43:363–376. https://doi.org/10.1111/aor.13346
Stöhr EJ, Ji R, Akiyama K, Mondellini G, Braghieri L et al (2021) Cerebral vasoreactivity in HeartMate 3 patients. J Heart Lung Transplant 40:786–793. https://doi.org/10.1016/j.healun.2021.05.005
Palazzolo T, Hirschhorn M, Garven E, Day S, Stevens RM et al (2022) Technology landscape of pediatric mechanical circulatory support devices: a systematic review 2010–2021. Artif Organs. https://doi.org/10.1111/aor.14242
Butto A, Teele SA, Sleeper LA, Thrush PT, Philip J et al (2020) The impact of pre-implant illness severity on the outcomes of pediatric patients undergoing durable ventricular assist device. J Heart Lung Transplant 39:666–674. https://doi.org/10.1016/j.healun.2020.02.011
Burkhoff D, Topkara VK, Sayer G, Uriel N (2021) Reverse remodeling with left ventricular assist devices. Circul Res 128:1594–1612. https://doi.org/10.1161/CIRCRESAHA.121.318160
Friedland-Little JM, Joong A, Shugh SB, O’Connor MJ, Bansal N et al (2022) Patient and device selection in pediatric MCS: a review of current consensus and unsettled questions. Pediatr Cardiol. https://doi.org/10.1007/s00246-022-02880-6
Thangappan K, Zafar F, Lorts A, Adachi I, Rosenthal D et al (2022) MILESTONE: more than 1,200 children bridged to heart transplantation with mechanical circulatory support. ASAIO J (American Society For Artificial Internal Organs : 1992) 68:577–583. https://doi.org/10.1097/MAT.0000000000001635
Adachi I, Zea-Vera R, Tunuguntla H, Denfield SW, Elias B et al (2019) Centrifugal-flow ventricular assist device support in children: a single-center experience. J Thorac Cardiovasc Surg 157. https://doi.org/10.1016/j.jtcvs.2018.12.045
D’Addese L, Joong A, Burch M, Pahl E (2019) Pediatric heart transplantation in the current era. Curr Opin Pediatr 31:583–591. https://doi.org/10.1097/MOP.0000000000000805
Adachi I (2019) Pediatric ventricular assist device support as a permanent therapy: clinical reality. J Thorac Cardiovasc Surg 158:1438–1441. https://doi.org/10.1016/j.jtcvs.2019.02.145
CHUV (2023) Une première au CHUV : un enfant placé sous assistance cardiaque bi-ventriculaire a pu être sevré de la machine sans greffe cardiaque. Centre Hospitalier Universitaire Vaudois. https://www.chuv.ch/fr/chuv-home/espace-pro/journalistes/communiques-de-presse/detail/une-premiere-au-chuv-un-enfant-place-sous-assistance-cardiaque-bi-ventriculaire-a-pu-etre-sevre-de-la-machine-sans-greffe-cardiaque. Accessed 26 Jan 2024
Hospital FW (2022) After the "artificial heart" treatment for 100 days, little boy got back to his "wholeheart". WeChat Official Account of Chinese Academy of Medical Sciences Fuwai Hospital. https://mp.weixin.qq.com/s/bDGHRXDIoCZFFDoJv85VQw. Accessed 26 Jan 2024
Spigel ZA, Cho J, Adachi I (2020) Current status of pediatric mechanical circulatory support. Curr Opin Organ Transplant 25:231–236. https://doi.org/10.1097/mot.0000000000000761
Butto A, Mao CY, Wright L, Wetzel M, Kelleman MS et al (2022) Relationship of ventricular assist device support duration with pediatric heart transplant outcomes. J Heart Lung Transplant 41:61–69. https://doi.org/10.1016/j.healun.2021.09.011
Riggs KW, Zafar F, Lorts A, Villa CR, Bryant R 3rd et al (2020) Optimizing postcardiac transplantation outcomes in children with ventricular assist devices: how long should the bridge be? Asaio j 66:787–795. https://doi.org/10.1097/mat.0000000000001075
Jakovljevic DG, McDiarmid A, Hallsworth K, Seferovic PM, Ninkovic VM et al (2014) Effect of left ventricular assist device implantation and heart transplantation on habitual physical activity and quality of life. Am J Cardiol 114:88–93. https://doi.org/10.1016/j.amjcard.2014.04.008
Dring KJ, Hatch LA, Williams RA, Morris JG, Sunderland C et al (2022) Effect of 5-weeks participation in The Daily Mile on cognitive function, physical fitness, and body composition in children. Sci Rep 12:14309. https://doi.org/10.1038/s41598-022-18371-w
Kerrigan DJ, Cowger JA, Keteyian SJ (2022) Exercise in patients with left ventricular devices: the interaction between the device and the patient. Prog Cardiovasc Dis 70:33–39. https://doi.org/10.1016/j.pcad.2021.12.002
Karapolat H, Engin C, Eroglu M, Yagdi T, Zoghi M et al (2013) Efficacy of the cardiac rehabilitation program in patients with end-stage heart failure, heart transplant patients, and left ventricular assist device recipients. Transplant Proc 45:3381–3385. https://doi.org/10.1016/j.transproceed.2013.06.009
Burstein DS, McBride MG, Rossano JW, O’Connor MJ, Lin KY et al (2021) Increasing pump speed during exercise training improves exercise capacity in children with ventricular assist devices. Asaio j 67:449–456. https://doi.org/10.1097/mat.0000000000001231
Severin R, Sabbahi A, Ozemek C, Phillips S, Arena R (2019) Approaches to improving exercise capacity in patients with left ventricular assist devices: an area requiring further investigation. Expert Rev Med Devices 16:787–798. https://doi.org/10.1080/17434440.2019.1660643
Vignati C, Apostolo A, Cattadori G, Farina S, Del Torto A et al (2017) Lvad pump speed increase is associated with increased peak exercise cardiac output and vo, postponed anaerobic threshold and improved ventilatory efficiency. Int J Cardiol 230:28–32. https://doi.org/10.1016/j.ijcard.2016.12.112
Jung MH, Houston B, Russell SD, Gustafsson F (2017) Pump speed modulations and sub-maximal exercise tolerance in left ventricular assist device recipients: a double-blind, randomized trial. J Heart Lung Transplant 36:36–41. https://doi.org/10.1016/j.healun.2016.06.020
Muthiah K, Robson D, Prichard R, Walker R, Gupta S et al (2015) Effect of exercise and pump speed modulation on invasive hemodynamics in patients with centrifugal continuous-flow left ventricular assist devices. J Heart Lung Transplant 34:522–529. https://doi.org/10.1016/j.healun.2014.11.004
Hayward CS, Salamonsen R, Keogh AM, Woodard J, Ayre P et al (2011) Effect of alteration in pump speed on pump output and left ventricular filling with continuous-flow left ventricular assist device. ASAIO J 57:495–500. https://doi.org/10.1097/MAT.0b013e318233b112
Ai X, Gong Y, Hong X, Wang W, Liu J et al (2018) Extracorporeal life support in perioperative care of pediatric cardiac surgical patients in China. Chin J ECC 16:3–6. https://doi.org/10.13498/j.cnki.chin.j.ecc.2018.01.02
Hospital FW (2022) The first pediatric LVAD implantation in China! Academician Hu Shengshou's team from Fuwai Hospital of the Chinese Academy of Medical Sciences successfully implanted Corheart 6 LVAD in a 14-year-old child. WeChat Official Account of Chinese Academy of Medical Sciences Fuwai Hospital. https://mp.weixin.qq.com/s/vlqLAY22YU5kPbV1hzBCfw. Accessed 26 Jan 2024
Ricci Z, Amodeo A (2012) Prospective trial of a pediatric ventricular assist device. N Engl J Med 367. https://doi.org/10.1056/NEJMc1212304
Jeewa A, Manlhiot C, McCrindle BW, Van Arsdell G, Humpl T et al (2010) Outcomes with ventricular assist device versus extracorporeal membrane oxygenation as a bridge to pediatric heart transplantation. Artif Organs 34:1087–1091. https://doi.org/10.1111/j.1525-1594.2009.00969.x
Geller BJ, Sinha SS, Kapur NK, Bakitas M, Balsam LB et al (2022) Escalating and de-escalating temporary mechanical circulatory support in cardiogenic shock: a scientific statement from the American Heart Association. Circulation 146:e50–e68. https://doi.org/10.1161/CIR.0000000000001076
MAQUET (2002) ROTAFLOW with ICU Kit(User’s Manual, 1.0). the Alfred. https://ecmo.icu/wp-content/uploads/pdfs/ROTAFLOW_UserManual.pdf. Accessed 18 Jan 2024
Abbott (2019) PediMag® blood pump instructions for use. Abbott manuals. https://manuals.eifu.abbott/en/hcp/home.html. Accessed 19 Jan 2024
Abbott (2019) CentriMagTM circulatory support systemoperation manual. Abbott manuals. https://manuals.eifu.abbott/en/hcp/home.html. Accessed 19 Jan 2024
Zein R, Patel C, Mercado-Alamo A, Schreiber T, Kaki A (2022) A review of the impella devices. Interv Cardiol (London, England) 17:e05. https://doi.org/10.15420/icr.2021.11
Heart B (2016) INCOR® Implantable ventricular assist device instructions for clinical use Berlin Heart. https://www.berlinheart.de/fileadmin/user_upload/Berlin_Heart/Dokumente/Downloads/Downloads_IFU/INCOR/clinic/5000013x13_A08_INC_GA_K_en.pdf. Accessed 26 Jan 2024
Heart B (2018) Product Catalog-EXCOR® Pediatric, the ventricular assist device for children. Berlin Heart Inc. https://www.berlinheart.com/fileadmin/user_upload/Berlin_Heart/Bilder/US_Website/Berlin_Heart_Inc_Product_Catalog_MPC21_6_zusammengefuehrte_Seiten.pdf. Accessed 26 Jan 2024
Abbott (2020) HeartMate II left ventricular assist system instructions for use Abbott manuals. https://manuals.eifu.abbott/en/hcp/home.html. Accessed 19 Jan 2024
Abbott (2020) HeartMate 3 Left ventricular assist system instructions for use Abbott manuals. https://manuals.eifu.abbott/en/hcp/home.html. Accessed 27 Jan 2024
HeartWare (2023) HeartWare™ HVAD™ system instructions for use. Medtronic manual library. https://www.medtronic.com/content/dam/emanuals/crdm/M034727C001_2_IFU_COLOR_view.pdf. Accessed 27 Jan 2024
Bleiweis MS, Philip J, Peek GJ, Stukov Y, Janelle GM et al (2023) World J Pediatr Congenit Heart Surg 14:117–124. https://doi.org/10.1177/21501351221146150
Rohde S, Antonides CFJ, Muslem R, de Woestijne PCv, der Meulen MHv et al (2020) Pediatric ventricular assist device support in the Netherlands. World J Pediatr Congenit Heart Surg 11:275–283. https://doi.org/10.1177/2150135120902114
Mantell B, Addonizio L, Jain N, LaPar D, Chai P et al (2020) Evolution of pediatric ventricular assist devices and their neurologic and renal complications-A 24-year single-center experience. Artif Organs 44:987–994. https://doi.org/10.1111/aor.13696
Menéndez JJ, Sánchez-Galindo AC, Balcells J, Tejero-Hernández MÁ, Ferrer-Barba Á et al (2023) Short- and long-term survival of children treated with ventricular assist devices in Spain, based on 15 years' experience. Eur J Cardiothorac Surg : Official Journal of the European Association For Cardio-thoracic Surgery 63. https://doi.org/10.1093/ejcts/ezad050
Joong A, Maeda K, Peng DM (2022) Ventricular assist device outcomes in infants and children with stage 1 single ventricle palliation. ASAIO J (American Society For Artificial Internal Organs : 1992) 68:e188–e195. https://doi.org/10.1097/MAT.0000000000001817
Fu H-Y, Chou H-W, Lai C-H, Tsao C-I, Lu C-W (2023) Outcomes of pediatric patients supported with ventricular assist devices single center experience. J Formos Med Assoc = Taiwan Yi Zhi 122:172–181. https://doi.org/10.1016/j.jfma.2022.09.008
Rohde S, Sandica E, Veen K, Miera O, Amodeo A et al (2022) Cerebrovascular accidents in paediatric patients supported by the Berlin Heart EXCOR. Eur J Cardiothorac Surg : Official Journal of the European Association For Cardio-thoracic Surgery 62. https://doi.org/10.1093/ejcts/ezac381
Yu J, Murray J, Ramamoorthy C, Chen S, Lee S et al (2021) Neurosurgical intervention in children with ventricular assist devices: a single-center case series review. Paediatr Anaesth 31:1208–1215. https://doi.org/10.1111/pan.14287
Adachi I (2018) Current status and future perspectives of the PumpKIN trial. Transl Pediatr 7:162–168. https://doi.org/10.21037/tp.2018.02.04
Olia SE, Wearden PD, Maul TM, Shankarraman V, Kocyildirim E et al (2018) Preclinical performance of a pediatric mechanical circulatory support device: the PediaFlow ventricular assist device. J Thorac Cardiovasc Surg 156:1643-1651.e1647. https://doi.org/10.1016/j.jtcvs.2018.04.062
Cooper BT, Roszelle BN, Long TC, Deutsch S, Manning KB (2008) The 12 cc Penn State pulsatile pediatric ventricular assist device: fluid dynamics associated with valve selection. J Biomech Eng 130:041019. https://doi.org/10.1115/1.2939342
Weiss WJ, Carney EL, Clark JB, Peterson R, Cooper TK et al (2012) Chronic in vivo testing of the Penn State infant ventricular assist device. ASAIO J (American Society For Artificial Internal Organs : 1992) 58:65–72. https://doi.org/10.1097/MAT.0b013e318239feb4
Lukic B, Clark JB, Izer JM, Cooper TK, Finicle HA et al (2019) Chronic ovine studies demonstrate low thromboembolic risk in the Penn State Infant Ventricular Assist Device. ASAIO J (American Society For Artificial Internal Organs : 1992) 65:371–379. https://doi.org/10.1097/MAT.0000000000000945
Good BC, Ponnaluri SV, Weiss WJ, Manning KB (2022) Computational modeling of the Penn State Fontan Circulation Assist Device. ASAIO J (American Society For Artificial Internal Organs : 1992) 68:1513–1522. https://doi.org/10.1097/MAT.0000000000001708
Ponnaluri SV, Christensen EJ, Good BC, Kubicki CJ, Deutsch S et al (2022) Experimental hemodynamics within the Penn State Fontan Circulatory Assist Device. J Biomech Eng 144. https://doi.org/10.1115/1.4053210
Letsou GV, Pate TD, Gohean JR, Kurusz M, Longoria RG et al (2010) Improved left ventricular unloading and circulatory support with synchronized pulsatile left ventricular assistance compared with continuous-flow left ventricular assistance in an acute porcine left ventricular failure model. J Thorac Cardiovasc Surg 140:1181–1188. https://doi.org/10.1016/j.jtcvs.2010.03.043
Gohean JR, Larson ER, Hsi BH, Kurusz M, Smalling RW et al (2017) Scaling the low-shear pulsatile TORVAD for Pediatric Heart Failure. ASAIO J 63:198–206. https://doi.org/10.1097/mat.0000000000000460
Bartoli CR, Hennessy-Strahs S, Gohean J, Villeda M, Larson E et al (2019) A novel toroidal-flow left ventricular assist device minimizes blood trauma: implications of improved ventricular assist device hemocompatibility. Ann Thorac Surg 107:1761–1767. https://doi.org/10.1016/j.athoracsur.2018.11.053
Gohean JR, Larson ER, Longoria RG, Kurusz M, Smalling RW (2019) Preload sensitivity with TORVAD counterpulse support prevents suction and overpumping. Cardiovasc Eng Technol 10:520–530. https://doi.org/10.1007/s13239-019-00419-0
Sarkisyan H, Stevens R, Tchantchaleishvili V, Rossano J, Throckmorton A (2021) Integrated long-term multifunctional pediatric mechanical circulatory assist device. Artif Organs 45:E65–E78. https://doi.org/10.1111/aor.13863
Krawiec C, Wang S, Kunselman AR, Ündar A (2014) Impact of pulsatile flow on hemodynamic energy in a Medos Deltastream DP3 pediatric extracorporeal life support system. Artif Organs 38:19–27. https://doi.org/10.1111/aor.12117
Wang S, Kunselman AR, Ündar A (2014) In vitro performance analysis of a novel pulsatile diagonal pump in a simulated pediatric mechanical circulatory support system. Artif Organs 38:64–72. https://doi.org/10.1111/aor.12181
Speth M, Münch F, Purbojo A, Glöckler M, Toka O et al (2016) Pediatric extracorporeal life support using a third generation diagonal pump. ASAIO J (American Society For Artificial Internal Organs : 1992) 62:482–490. https://doi.org/10.1097/MAT.0000000000000385
Stiller B, Houmes RJ, Rüffer A, Kumpf M, Müller A et al (2018) Multicenter experience with mechanical circulatory support using a new diagonal pump in 233 children. Artif Organs 42:377–385. https://doi.org/10.1111/aor.13016
Wang S, Force M, Moroi MK, Patel S, Kunselman AR et al (2019) Effects of pulsatile control algorithms for diagonal pump on hemodynamic performance and hemolysis. Artif Organs 43:60–75. https://doi.org/10.1111/aor.13284
Patel S, Wang S, Pauliks L, Chang D, Clark JB et al (2015) Evaluation of a novel pulsatile extracorporeal life support system synchronized to the cardiac cycle: effect of rhythm changes on hemodynamic performance. Artif Organs 39:67–76. https://doi.org/10.1111/aor.12454
Force M, Moroi M, Wang S, Kunselman AR, Ündar A (2018) In vitro hemodynamic evaluation of ECG-synchronized pulsatile flow using i-Cor pump as short-term cardiac assist device for neonatal and pediatric population. Artif Organs 42:E153-e167. https://doi.org/10.1111/aor.13136
Petukhov D, Korn L, Walter M, Telyshev D (2019) A novel control method for rotary blood pumps as left ventricular assist device utilizing aortic valve state detection. Biomed Res Int 2019:1732160. https://doi.org/10.1155/2019/1732160
Pugovkin AA, Markov AG, Selishchev SV, Korn L, Walter M et al (2019) Advances in hemodynamic analysis in cardiovascular diseases investigation of energetic characteristics of adult and pediatric Sputnik left ventricular assist devices during mock circulation support. Cardiol Res Pract 2019:4593174. https://doi.org/10.1155/2019/4593174
Kimura M, Nishimura T, Kinoshita O, Kashiwa K, Kyo S et al (2012) Hemodynamic influence of tilting disc valve type on pump performance with the NIPRO-ventricular assist device. J Artif Organs : the Official Journal of the Japanese Society For Artificial Organs 15:134–139. https://doi.org/10.1007/s10047-011-0616-2
Naito N, Takewa Y, Kishimoto S, Iizuka K, Mizuno T et al (2018) Preclinical animal study of the NIPRO-ventricular assist device for use in pediatric patients. J Artif Organs 21:156–163. https://doi.org/10.1007/s10047-017-1009-y
Tompkins LH, Gellman BN, Morello GF, Prina SR, Roussel TJ et al (2021) Design and computational evaluation of a pediatric MagLev rotary blood pump. ASAIO J (American Society For Artificial Internal Organs : 1992) 67:1026–1035. https://doi.org/10.1097/MAT.0000000000001323
Monreal G, Koenig SC, Slaughter MS, Morello GF, Prina SR et al (2022) Feasibility testing of the inspired therapeutics NeoMate mechanical circulatory support system for neonates and infants. PLoS ONE 17:e0266822. https://doi.org/10.1371/journal.pone.0266822
Tompkins LH, Gellman BN, Prina SR, Morello GF, Roussel T et al (2022) Development of inspired therapeutics pediatric VAD: computational analysis and characterization of VAD V3. Cardiovasc Eng Technol 13:624–637. https://doi.org/10.1007/s13239-021-00602-2
Nissim L, Karnik S, Smith PA, Wang Y, Frazier OH et al (2023) Machine learning based on computational fluid dynamics enables geometric design optimisation of the NeoVAD blades. Sci Rep 13:7183. https://doi.org/10.1038/s41598-023-33708-9
Funding
This work was supported by the National Natural Science Foundation of China (82000389), the project of Shanghai Municipal Science and Technology Commission (20MC1920400) and Innovative research team of high-level universities in Shanghai (2021).
Author information
Authors and Affiliations
Contributions
Hao Zhang contributed to the conceptualization of this work. Literature search and visualization were performed by Xu Huang. The original draft of the manuscript was written by Xu Huang, and Yi Shen and Yiwei Liu contributed to the review and editing of the manuscript. Funding acquisition was provided by Yiwei Liu and Hao Zhang. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Shen, Y., Liu, Y. et al. Current status and future directions in pediatric ventricular assist device. Heart Fail Rev 29, 769–784 (2024). https://doi.org/10.1007/s10741-024-10396-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-024-10396-9