Abstract
Previous studies of implantable cardiac resynchronization therapy plus defibrillator (CRT-D) therapy used for primary prevention of sudden cardiac death have suggested that CRT-D therapy is less effective in patients with mild heart failure and a wide QRS complex. However, the long-term benefits are variable. We performed a meta-analysis of randomized trials identified in systematic searches of MEDLINE, EMBASE, and the Cochrane Database. Three studies (3858 patients) with a mean follow-up of 66 months were included. Overall, CRT-D therapy was associated with significantly lower all-cause mortality than was implantable cardioverter defibrillator (ICD) therapy (OR, 0.78; 95 % CI, 0.63–0.96; P = 0.02; I 2 = 19 %). However, the risk of cardiac mortality was comparable between two groups (OR, 0.74; 95 % CI, 0.53–1.01; P = 0.06). CRT-D treatment was associated with a significantly lower risk of hospitalization for heart failure (OR, 0.67; 95 % CI, 0.50–0.89; P = 0.005; I 2 = 55 %). The composite outcome of all-cause mortality and hospitalization for heart failure was also markedly lower with CRT-D therapy than with ICD treatment alone (OR, 0.67; 95 % CI, 0.57–0.77; P < 0.0001; I 2 = 0 %). CRT-D therapy decreased the long-term risk of mortality and heart failure events in patients with mild heart failure with a wide QRS complex. However, long-term risk of cardiac mortality was similar between two groups. More randomized studies are needed to confirm these findings, especially in patients with NYHA class I heart failure or patients without LBBB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with heart failure (HF) are subject to arrhythmia-related sudden cardiac death (SCD) and HF events [1]. Previous studies have shown that implantable cardioverter defibrillator (ICD) treatment represents an effective treatment strategy for lowering mortality and improving survival in selected patients [2]. However, several smaller randomized trials and meta-analyses have reported that ICD treatment is associated with an increased risk of HF events. Meanwhile, recent studies have demonstrated that cardiac resynchronization therapy plus defibrillator (CRT-D) therapy can improve the cardiac structure and function through reverse left ventricular remodeling for patients with moderate to severe HF (New York Heart Association [NYHA] class III–IV) and a prolonged QRS interval [3]. For patients with mildly symptomatic HF and a wide QRS complex, the CONTAK CD trial [4] showed a reduction in left ventricular diameter but failed to demonstrate improvement in the composite outcome end point with CRT-D therapy. Another two large randomized controlled trials, the REVERSE and MADIT-CRT, showed that CRT-D therapy reduces the risk of HF events [5, 6]. However, the long-term effectiveness of CRT-D treatment is still variable.
Because of this recent increase in evidence, we performed a meta-analysis to evaluate the long-term effectiveness of CRT-D therapy on mortality and HF events for patients with mild, symptomatic HF (NYHA functional class I–II) and a prolonged QRS duration.
Methods
Data sources and search strategies
We searched MEDLINE (source, PubMed from January 2005 to June 2015), EMBASE (January 2005–June 2015), the Cochrane Library (to June 2015), and the ClinicalTrials.gov Web site (to June 2015) using the terms “heart failure,” “cardiac resynchronization therapy,” “implantable cardioverter defibrillator,” “mortality,” and “sudden cardiac death.” We manually checked the references of all relevant articles. No restrictions were applied.
Study selection
We initially screened all titles and abstracts and then performed a full-text review. Trials were considered eligible if they met the following criteria: (1) The study was a prospective randomized controlled trial conducted in patients with mildly symptomatic HF (NYHA functional class I–II), (2) the intervention was CRT-D therapy, (3) the follow-up was >12 months, and (4) the outcomes of interest were mortality and HF events. The primary outcome was the risk of cardiac mortality.
Data extraction
Two reviewers independently extracted data on patient characteristics, the CRT-D therapy used, study quality, and clinical outcomes using a standard data collection form. Disagreements were resolved by discussion.
Quality assessment
Two reviewers assessed the quality of the selected trials. Components used for quality assessment were means of random sequence generation, allocation concealment, blinding of outcome assessment, and selective outcome reporting. The Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) statement [7] was followed.
Data synthesis and analysis
Results were analyzed quantitatively with Review Manager 5.1 (Nordic Cochrane Center, Copenhagen, Denmark). We calculated the pooled odds ratio (OR) for dichotomous outcomes with 95 % confidence intervals (CIs) [8].
Heterogeneity was examined by the I 2 statistic and the Chi-squared test. An I 2 value of >50 % was considered a substantial level of heterogeneity [9]. A fixed-effect model was applied to evaluate the pooled effect when the heterogeneity was not significantly different; otherwise, a random-effect model was used. Sensitivity analyses and meta-regression were performed only when significant heterogeneity appeared. All analyses were performed according to the intention-to-treat principle. Statistical significance was set at 0.05 for the I 2 test for heterogeneity.
Results
Search results
We initially identified 348 potentially relevant articles. Of these, 331 articles were excluded based on their titles and abstracts, and 17 articles were considered to be of interest and were retrieved for full-text review. Thirteen articles were excluded because they were reviews (n = 4), presented incorrect comparisons (n = 3), had a short follow-up (n = 4), or described no clinical outcomes (n = 3). Ultimately, three studies were included in the analysis. Figure 1 is a flowchart showing the process of study selection.
Study characteristics
Three published randomized controlled trials [10–12] included a total of 3858 patients. The total number of patients in each study ranged from 600 to 1820. The participants’ ages ranged from 18 to 85 years (mean age, 61 years). Most patients (78 %) were men, 72 % had a left bundle branch block (LBBB), and the mean QRS duration was 157 ms. The baseline systolic and diastolic blood pressures were comparable between two groups. Two studies reported the long-term (>5 years) outcomes of CRT-D treatment. The mean duration of CRT-D therapy was 66 months (Table 1).
Methodological quality assessment
All three trials [10–12] randomized the participants and used satisfactory methods of concealed treatment allocation. Blinding of participants and personnel was reported in three studies. There was low risk of attrition bias and reporting bias in most of the studies (Fig. 2).
Primary outcome
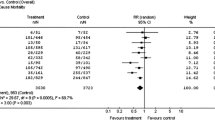
The long-term risk of all-cause mortality was reported in four studies. There were 2216 patients in the CRT-D group, of whom 447 died (20.1 %). There were 1652 patients in the ICD alone group, of whom 479 died (28.9 %). CRT-D therapy was associated with a significantly lower all-cause mortality rate than was the control group (OR, 0.78; 95 % CI, 0.63–0.96; P = 0.02; I 2 = 19 %) (Fig. 3). However, because of the limited data, the cardiac mortality rate was comparable between the CRT-D and ICD groups (OR, 0.74; 95 % CI, 0.53–1.01; P = 0.06).
HF events
The risk of hospitalization for HF was reported in three studies with a mean follow-up of 50 months. There were 2216 patients in the CRT-D group, of whom 306 were diagnosed with HF events (13.8 %). There were 1652 patients in the ICD group, of whom 344 had HF events (20.8 %). The risk of hospitalization for HF events was significantly lower in the CRT-D group (OR, 0.67; 95 % CI, 0.50–0.89; P = 0.005; I 2 = 55 %) (Fig. 4). Furthermore, compared with ICD treatment alone, the composite outcome of mortality and hospitalization for HF was also markedly lower in the CRT-D therapy group (OR, 0.67; 95 % CI, 0.57–0.77; P < 0.0001; I 2 = 0 %) (Fig. 5).
Discussion
In this study, we have shown that CRT-D therapy can significantly decrease the risk of mortality. Furthermore, compared with ICD alone, the incidence of adverse HF events was markedly lower with CRT-D therapy. Overall, given the clinical benefit, CRT-D therapy appears to be a safe and effective treatment for patients with mildly symptomatic HF with a wide QRS complex.
HF probably leads to arrhythmia-related SCD, and ICD can detect and terminate potentially life-threatening tachyarrhythmias via defibrillation. Many randomized trials and meta-analyses have demonstrated the efficacy of ICDs for primary prevention of SCD. However, in most patients with chronic HF, dyssynchrony manifests with a QRS duration of >120 ms, commonly presenting as an LBBB. This leads to a shortened filling time and abnormal septal motion with an increase in left ventricular end-systolic/end-diastolic diameter and decreased left ventricular function [13]. Over time, this dyssynchrony has further deleterious effects on heart structure and function. CRT-D therapy not only improves survival, but also improves the functional status and symptoms of HF [14]. Among patients with advanced HF (NYHA functional class III–IV), a left ventricular ejection fraction (LVEF) of ≤35 %, and a QRS duration of >130 ms, the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) study showed that CRT-D treatment resulted in a significant decrease in the 6-min walk, NYHA functional class, peak VO2, and LVEF [15]. Furthermore, there was also a decrease in hospitalization for HF. Meanwhile, the Cardiac Resynchronization-Heart Failure (CARE-HF) study demonstrated that patients who underwent CRT with pacemaker (CRT-P) therapy showed a reduction in all-cause mortality and unplanned hospitalizations for cardiovascular events [16]. Additionally, patients had improvements in LVEF and reverse remodeling. The COMPANION trial [17] and the CARE-HF study established CRT as a treatment for HF (NYHA functional class III–IV). Thus, CRT is recommended for patients with an LVEF of ≤35 %; sinus rhythm; LBBB with a QRS duration of ≥150 ms or 120–149 ms; and NYHA class II, III, or ambulatory IV symptoms on medical therapy in the 2013 ESC guidelines (class I) [18].
Many studies have confirmed the short-term effectiveness of decreasing HF events associated with CRT-D therapy in patients with mild HF and a prolonged QRS interval. The efficacy of CRT-D therapy in patients with mild HF was suggested by the CONTAK CD study, which demonstrated left ventricular reverse remodeling across NYHA functional classes II–IV [4]. However, the benefits of CRT-D therapy in these populations were closely examined in the MIRACLE ICD II, which demonstrated left ventricular reverse remodeling in patients with HF of NYHA class II characterized by a prolonged QRS duration (≥130 ms) and LVEF of ≤35 % [19]. The REVERSE trial and MADIT-CRT study validated the benefits of CRT in this group [5, 6]. The MADIT-CRT is the largest trial of patients with mild HF. Enrolled patients had NYHA class I/II, QRS duration of ≥130 ms, and LVEF of ≤30 %. This study showed the benefits of CRT-D therapy over ICD therapy with a significant reduction in HF events in the CRT-D group [11]. However, the short-term results of both the MADIT-CRT and REVERSE study failed to demonstrate an independent effect on the risk of all-cause mortality. Combing these studies, AI-Majed et al. [20] confirmed the short-term benefit of CRT-D therapy in reduced mortality and hospitalizations in patients with mildly symptomatic HF and a wide QRS complex.
The long-term results of the REVERSE and MADIT-CRT showed remarkably low mortality in patients who underwent CRT-D treatment. At 5 years of follow-up, CRT-D showed a significant improvement in survival over CRT-P (P = 0.02) [12]. Together with the RAFT study, these results provide compelling evidence in support of CRT in patients with mild HF. Combining 3858 patients with mean follow-up of 66 months, we found that CRT-D therapy was associated with a significantly lower risk of mortality than was ICD therapy. However, the majority of patients in MIRACLE ICD II, REVERSE, MADIT-CRT, and RAFT studies had NYHA class II heart disease (>90 %) [5, 6, 10, 19]. Whether CRT-D therapy improved the outcomes of patients with NYHA functional class I heart disease is still variable. Future studies designed specifically to answer this question may change current recommendations. Additionally, at 7 years of follow-up, the MADIT-CRT study found that CRT-D therapy was not associated with any clinical benefit and possibly with harm in patients without LBBB [11]. Future studies are needed to identify the long-term effect of CRT-D treatment for patients without LBBB.
Compared with previous meta-analyses [21–24], our study had several advantages (Table 2). First, our meta-analysis included 3858 patients with mild heart failure (NYHA I-II). However, the other four studies enrolled participants with mild to advanced heart failure (NYHA II-IV). Second, the short-term efficacy of CRT therapy in patients with advanced heart failure was confirmed by many studies. Therefore, we aim to evaluate the long-term efficacy of CRT-D therapy. However, most of the other four meta-analyses did not report the long-term follow-up result [21–23]. Finally, most previous meta-analyses were compared between CRT and medical therapy. Only the study by Wells was in consistent with our meta-analysis, which showed the result between CRT-D therapy and implantable defibrillator alone.
Study limitations
Several limitations deserve consideration. First, although the inclusion criteria were broad across the study population, all three studies exhibited slight differences in the characteristics of the patients enrolled. Second, although the present study comprised a large-scale comparison of CRT-D vs. ICD, the sample size of several studies was relatively small and the overall sample size was inadequate to exclude small differences in outcomes between two groups. Therefore, more studies with larger numbers of patients, carefully matched key clinical and technical variables, and longer follow-up periods are needed to definitively quantify the potential clinical benefits of CRT-D therapy, especially for patients with NYHA class I heart disease or without LBBB.
Conclusions
Overall, CRT-D therapy significantly decreases the long-term risk of mortality. However, larger studies are needed to evaluate whether patients with NYHA class I heart disease or without LBBB can benefit from CRT-D treatment.
References
Fan X, Hua W, Xu Y, Ding L, Niu H, Chen K, Xu B, Zhang S (2014) Incidence and predictors of sudden cardiac death in patients with reduced left ventricular ejection fraction after myocardial infarction in an era of revascularisation. Heart 100(16):1242–1249
Earley A, Persson R, Garlitski AC, Balk EM, Uhlig K (2014) Effectiveness of implantable cardioverter defibrillators for primary prevention of sudden cardiac death in subgroups a systematic review. Ann Intern Med 160(2):111–121
Leyva F, Nisam S, Auricchio A (2014) 20 years of cardiac resynchronization therapy. J Am Coll Cardiol 64(10):1047–1058
Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, Boehmer JP, Higginbotham MB, De Marco T, Foster E, Yong PG (2003) Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol 42(8):1454–1459
Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C, REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group (2008) Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 52(23):1834–1843
Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W, MADIT-CRT Trial Investigators (2009) Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 361(14):1329–1338
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Hardy RJ, Thompson SG (1998) Detecting and describing heterogeneity in meta-analysis. Stat Med 17(8):841–856
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL, Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators (2010) Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 363(25):2385–2395
Goldenberg I, Kutyifa V, Klein HU, Cannom DS, Brown MW, Dan A, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Kautzner J, Klempfner R, Kuniss M, Merkely B, Pfeffer MA, Quesada A, Viskin S, McNitt S, Polonsky B, Ghanem A, Solomon SD, Wilber D, Zareba W, Moss AJ (2014) Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med 370(18):1694–1701
Gold MR, Padhiar A, Mealing S, Sidhu MK, Tsintzos SI, Abraham WT (2015) Long-term extrapolation of clinical benefits among patients with mild heart failure receiving cardiac resynchronization therapy: analysis of the 5-Year Follow-Up From the REVERSE Study. JACC Heart Fail 3(9):691–700
Kutyifa V, Pouleur AC, Knappe D, Al-Ahmad A, Gibinski M, Wang PJ, McNitt S, Merkely B, Goldenberg I, Solomon SD, Moss AJ, Zareba W (2013) Dyssynchrony and the risk of ventricular arrhythmias. JACC Cardiovasc Imaging 6(4):432–444
Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, Dickstein K, Ford I, Gorcsan J 3rd, Gras D, Krum H, Sogaard P, Holzmeister J, EchoCRT-Study-Group (2013) Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 369(15):1395–1405
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J, MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation (2002) Cardiac resynchronization in chronic heart failure. N Engl J Med 346(24):1845–1853
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352(15):1539–1549
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators (2004) Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350(21):2140–2150
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas P, ESC Committee for Practice Guidelines (CPG), Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document Reviewers, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bänsch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM (2013) 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 34(29):2281–2329
Abraham WT, Young JB, León AR, Adler S, Bank AJ, Hall SA, Lieberman R, Liem LB, O’Connell JB, Schroeder JS, Wheelan KR, Multicenter InSync ICD II Study Group (2004) Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation 110(18):2864–2868
Al-Majed NS, McAlister FA, Bakal JA, Ezekowitz JA (2011) Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann Intern Med 154(6):401–412
Bradley DJ, Bradley EA, Baughman KL, Berger RD, Calkins H, Goodman SN, Kass DA, Powe NR (2003) Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA 289(6):730–740
Salukhe TV, Dimopoulos K, Francis D (2004) Cardiac resynchronisation may reduce all-cause mortality: meta-analysis of preliminary COMPANION data with CONTAK-CD, InSync ICD, MIRACLE and MUSTIC. Int J Cardiol 93(2–3):101–103
Rivero-Ayerza M, Theuns DA, Garcia-Garcia HM, Boersma E, Simoons M, Jordaens LJ (2006) Effects of cardiac resynchronization therapy on overall mortality and mode of death: a meta-analysis of randomized controlled trials. Eur Heart J 27(22):2682–2688
Wells G, Parkash R, Healey JS, Talajic M, Arnold JM, Sullivan S, Peterson J, Yetisir E, Theoret-Patrick P, Luce M, Tang AS (2011) Cardiac resynchronization therapy: a meta-analysis of randomized controlled trials. CMAJ 183(4):421–429
Acknowledgments
This work was financially supported by the grants from Natural Science Foundation of china (81373076) and Beijing Municipal Commission of Education (SQKM201210025010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sun, WP., Li, CL., Guo, JC. et al. Long-term efficacy of implantable cardiac resynchronization therapy plus defibrillator for primary prevention of sudden cardiac death in patients with mild heart failure: an updated meta-analysis. Heart Fail Rev 21, 447–453 (2016). https://doi.org/10.1007/s10741-016-9550-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9550-y