Abstract
Gastric cancer (GC) is one of the most common malignancies, ranking the third highest mortality rate worldwide. Due to the insidious symptoms and difficulty in early detection, patients with GS were mostly in the middle and late stages when they were diagnosed. Although ontogenetic or tumor-suppressive effects of miRNA-200a-3p have been demonstrated, the exact mechanism underlying GC is not clear. Therefore, the expression, effect, and mechanism of miRNA-200a-3p in GC progression were systematically investigated in this study. qRT-PCR, Western blotting, and immunohistochemical staining were applied to investigate the miRNA-200a-3p and deleted in liver cancer 1 (DLC-1) expression. Cell viability, proliferation, apoptosis, migration, and invasion capabilities of GC cells were assessed using cell counting kit-8 (CCK-8) colorimetry, EdU integration, flow cytometry, wound healing, and the transwell assay. The relationship between miRNA-200a-3p and tumor growth was investigated by tumor xenograft assay in vivo. A dual-luciferase reporter assay was estimated to verify the connection between miR-200-3p and DLC-1. The results showed that miRNA-200a-3p expression was significantly increased in both GC tissues and cells. Furthermore, via DLC-1, miRNA-200a-3p promotes tumor growth and development. miRNA-200a-3p, by targeting DLC-1, can function as an oncogene in GC cells. Collectively, our findings indicated that the miRNA-200a-3p/DLC axis might provide a theological basis for potential improvements in GC treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is as yet the world's third driving reason for disease-related demise (Charalampakis et al. 2018). Due to a high rate of tumor metastasis and recurrence, GC has a low 5-year survival rate (Szasz et al. 2016; Link et al. 2018). To improve the therapeutic efficacy, it is critical to understand the mechanism.
MiRNA is a form of single-abandoned endogenous particle made up of 18–24 nucleotides. MiRNAs tie to the 3′-UTR of target RNAs and are associated with various processes, including cell division, apoptosis, and cell cycle progression (Hao et al. 2017). It is accounted for abnormal expression of miRNAs act fundamental parts in the tumorigenesis in numerous diseases. For example, the forced expression of miR-675, which targets paired-like homeodomain transcription factor 1 (PITX1), promotes the malignant phenotype of GC cells (Liu et al. 2019). Furthermore, miRNA-378 inhibits GC cell migration, invasion and epithelial-mesenchymal transition (EMT) by suppressing bone morphogenetic protein-2 expression (BMP2) (Yang et al. 2019). The miRNA-200 family, which includes miRNA-200a-3p, a central regulator of EMT, acts as an oncogene or a tumor suppressor (Liu et al. 2018; Peng et al. 2018). MiRNA-200a-3p functions as a tumor suppressor in renal cell carcinoma by post-transcriptionally controlling CBL, according to Ding et al. (Ding et al. 2018). By directly inhibiting collapsin response mediator protein 1(CRMP1) in human esophageal cancer cells, miRNA-200a-3p promotes cancer cell proliferation (Zang et al. 2016). Chen et al. recently reported that miRNA-200a-3p was abnormally up-regulated in GC tissues (Chen et al. 2015). The molecular mechanism of miRNA-200a-3p in GC tumorigenesis has not yet to be reported, to our knowledge.
DLC-1, a tumor silencer gene cloned from human hepatocellular carcinoma (HCC), is believed to be a tumor silencer gene (Yuan et al. 1998). DLC-1 is reported in various cancers, including colorectal cancer (Wu et al. 2015), GC (Su et al. 2015) and HCC (Wu et al. 2018). According to bioinformatics tools (TargetScan: https://www.targetscan.org), DLC-1 is thought to be a crucial miRNA-200a-3p downstream target gene. Thus, miRNA-200a-3p might regulate DLC-1 directly. The relationship between miRNA-200a-3p and GC tissues and cells was therefore examined in this research. Overexpression of miRNA-200a-3p facilitates GC tumorigenesis by specifically targeting DLC-1, according to the findings.
Materials and methods
Patients and tissue samples
GC patients' tumors and adjoining tissues were gathered (30 sets) after surgical section from Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University between December 2018 and April 2020. All patients were pathologically diagnosed without receiving any chemotherapy, radiotherapy or any adjuvant therapy. All experimental processes were followed by the Ethics Committee of Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University. Each participant signed informed consent before the study.
Cell culture
Human gastric mucosa fibroblasts cell line GES-1, gastric cancer cell lines SGC-7901 and HGC-27 were bought from American Type Culture Collection (ATCC, Manassas, VA, USA), and cultured in a modified RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS fetal bovine serum, 100 units/mL penicillin, and 100 µg/mL streptomycin. Cells were then cultured with 5% CO2 and 37 °C in a humidified environment.
Cell transfection
In the cell transfection assay, miRNA-200a-3p mimics, inhibitors, and negative controls (miR-200c-3p mimics NC, miR-200c-3p inhibitors NC) were used (Shanghai GenePharma Co., Ltd., Shanghai, China). The DNA of DLC-1 by PCR amplification was inserted into the pcDNA 3.1 vector to achieve pc-DLC-1 plasmid while pc-NC functioned as the negative control. Before transfection, cells were incubated to 70% confluence. Then, transfection was conducted by Lipofectamine 2000 (Invitrogen) with miRNA mimics/inhibitors/negative controls (50 mM) or vectors (2 μg) following the manual.
In vivo experiment
The Center for Animal Experiment at Nanjing Medical University provided BALB/c nude mice (female, 4-week). Mice were randomly divided into mimics, inhibitors and negative controls groups. The formula V = (length × width2)/2 was used to calculate the tumors' volume. Mice were sacrificed twenty-eight days after the injection, and tumors were collected for immunohistochemical examination.
Luciferase reporter assay
Cells were co-transfected with miRNA-200a-3p mimics or miR-200c-3p mimics NC. The DLC-1 3′UTR was also added to the Luciferase miRNA expression reporter (wild or mutant type) (Promega, Madison, WI, USA). The Dual-Luciferase Reporter Assay System provided the results after a 48 h incubation period (Promega).
Quantitative real-time PCR (qRT-PCR)
The Trizol reagent from Invitrogen was used to extract total RNA. Full RNAs are then reverse-transcribed into cDNA using a PrimeScript TMRT reagent package (TaKaRa Biotechnology, Shiga, Japan). Denaturation at 94 °C for 10 s, annealing at 56 °C for 20 s, extension at 67 °C for 20 s, and amplification for 40 cycles were changed from the standard protocol for PCR amplification of cDNA. SYBR Green PCR Kits (TaKaRa Biotechnology) were applied to measure the miRNA-200a-3p and DLC-1 mRNA level using the 2−△△CT method. Internal references were added in the form of U6 or GAPDH. The following primers were used: miRNA-200a-3p-F: 5′-CCTACGCCACAATTAACAAGCC-3′ and R: 5′-GCCGTCTAACACTGTCTGGTA-3′; U6-F: 5′-CGCAAGGATGACACGCAAAT-3′ and R: 5′-GTGCAGGGTCCGAGGTATTC-3′; DLC-1-F: 5′-GGACACCATGATCCTAACAC-3′ and R: 5′-CTCATCCTCGTCTGAATCGT-3′; GAPHD-F: 5′-ACAACTTTGGTATCGTGGAAGG-3′ and R: 5′-GCCATCACGCCACAGTTTC-3′.
Western blotting
The total proteins in cultured cells were extracted and electroblotted onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Primary antibodies were incubated overnight at 4 °C on membranes after adding 5% skim milk for 2 h. The secondary antibody was then inserted and left to incubate for two hours. The primary antibodies are listed below: DLC-1 (ab126257; Abcam, Cambridge, UK), Bax (#14796), Bcl-2 (#4223), Caspase-3 (#14220), MMP-2(#40994), Caspase-9 (#9502), MMP-9 (#13667) and GAPDH (#5174) (Cell Signaling Technology, Boston, MA, USA) were diluted 1000 times. An enhanced chemiluminescence reagent was selected to analyze the protein profile.
Cell viability (CCK-8) assay
Cells were transfected and cultured in 96-well plates with CCK-8 reagent (Beyotime, Shanghai, China). The absorbance was measured at 450 nm after a 2 h incubation period.
EdU assay
Cells were cultured in 6-well plates with 500L EdU solution (RiboBio, Guangzhou, China). The mixture was incubated for 2 h at 37 °C. The labeled cells were digested using 0.25%t Tyrisin and then fixed with 4 percent paraformaldehyde and incubated with 0.5% Triton X-100. Hoechst staining solution was used to stain the cell nuclei. The staining process lasted for 30 min, and cells were measured by a fluorescence microplate reader (Millipore, Bedford, MA, USA).
Cell apoptosis assay
Cells were transfected and washed in PBS after 48 h. The cells were then centrifuged and fixed in ethanol for two hours at 4 °C. The cells were then resuspended in PBS and stained with Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit for 30 min at 4 °C (BD Pharmingen, Heidelberg, Germany). The procedures described above were carried out in complete darkness. Flow cytometry was used to investigate the findings (Beckman Coulter, Fullerton, CA, USA).
Migration assay
The migration assay was adapted from a previously published procedure. A 10 µl-pipette tip was used to scrape transfected cells. The cells were then washed in PBS and resuspended in a culture medium before being cultured for 24 h at 37 °C. A light microscope was used to examine the results (Leica Microsystems, Wetzlar, Germany).
Transwell invasion assay
The upper chamber (coated with Matrigel) was filled with cells, while the lower chamber was filled with a culture medium containing FBS. Then, Paraformaldehyde was used to fix the cells moved through the membrane. Finally, crystal violet hydrate solution (JRDUN Biotechnology Co., Ltd., Shanghai, China) was used to dye them and counted under a light microscope after a 24 h incubation (Leica Microsystems).
Histopathological examination
Tissue samples were collected and immersed in paraffin (with 10% formalin) overnight at 4 °C. Afterward, xylene and graded ethanol were used to deparaffinized and rehydrate the samples. Hematoxylin and eosin (H&E) were used to stain the sample for further histologic assessment.
In situ cell death detection
Tissues were immersed in paraformaldehyde for fixation for 15 min. After that, 0.25% Triton-X 100 was used to permeabilize the fixed tissues. In Situ Cell Death Detection Kits were used to conduct the TUNEL assay. All protocols followed the manuals from the manufacturer. Cell counts were carried out under a fluorescence microscope (Olympus, Hamburg, Germany) at × 400 magnification.
Immunohistochemical staining
Paraffin-embedded slices were mixed with diluted anti-Ki-67 antibody (400 times; Cell Signaling Technology, Boston, MA, USA) at 4 °C overnight. After that, the samples were washed in PBS and incubated with a 2000Xdilution biotinylated secondary antibody for 30 min at 37 °C (Abcam, Cambridge, UK). The samples were then counterstained with hematoxylin for 2 min after reacting with diaminobenzidine (DAB). Results were overserved in duplicate by different researchers.
Statistical analysis
The data was provided in the form of a mean and standard deviation (SD). The disparity was investigated using student's t-test and one-way ANOVA in SPSS 19.0. (IBM, Armonk, NY, USA). A P value of less than 0.05 was used to define statistical significance.
Results
MiRNA-200a-3p expression was significantly increased in GC tissues and cells
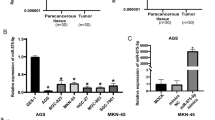
GC tissues had higher levels of miRNA-200a-3p expression than normal tissues, as shown in Fig. 1A. We also looked at the expression of miRNA-200a-3p in HGC-27, SGC-7901 and GES-1 cells. As shown in Fig. 1B, the miRNA-200a-3p level was significantly higher in GC.
MiRNA-200a-3p inhibited apoptosis while increased GC cell proliferation, migration, and invasion
We used gain-of- and loss-of-function analyses to learn more about the biological functions of miRNA-200a-3p in GC cells. As depicted in Fig. 2A, miR-200c-3p was prominently increased after transfected with miR-200c-3p mimics while decreased after transfection with miR-200c-3p inhibitors in SGC-7901 and HGC-27 cells, implying the transfection was successful. CCK-8 assays showed that the viabilities of GC cells increased in miRNA-200a-3p treatment but decreased in miRNA-200a-3p treatment (Fig. 2B). Referring to Fig. 2C, miRNA-200a-3p expression was positively related to the number of EdU incorporated cells, implying that miR-200c-3p enhanced the SGC-7901 and HGC-27 cell proliferation. Figure 2D showed the result of the apoptosis assay. The result indicated that GC apoptotic rate was negatively related to miRNA-200a-3p expression. After the miRNA-200a-3p overexpression in GC cells, Bcl-2 expression led to reduction of Bax, Caspase-3 and Caspase-9, versa vice. Figure 3B showed the result of the migration assay, which indicated that miRNA-200a-3p enhanced migration. Conversely, a reduced migration rate was observed in miRNA-200a-3p inhibitors treatments. Thus, miRNA-200a-3p was positively related to cell invasion (Fig. 3C). We further examined the expression of the metastasis-related protein. As represented in Fig. 3D, MMP-2 and MMP-9 in GC cells were due to upregulation of miRNA-200a-3p, versa vice.
MiRNA-200a-3p inhibited apoptosis while increased GC cell proliferation. MiRNA-200a-3p mRNA expression was measured in SGC-7901 and HGC-27 cells transfected with mimics NC, miRNA-200a-3p mimics, inhibitors NC and miRNA-200a-3p inhibitors, or blank control cells. (A), CCK-8 cell viability test (B), EdU cell proliferation test (C), Annexin V-FITC/PI staining test for apoptosis discovery (D), *P < 0.05, **P < 0.01 vs. miR-200c-3p mimics NC or miR-200c-3p inhibitors NC
MiRNA-200a-3p enhanced GC cell migration and invasion. The Bcl-2, Bax, Caspase-3 and Caspase-9 protein levels were measured in SGC-7901 and HGC-27 cells transfected with mimics NC, miRNA-200a-3p mimics, inhibitors NC, and miRNA-200a-3p inhibitors, or in blank control cells. (A), wound healing migration test (B), Transwell invasion test (C), and MMP-2 and MMP-9 protein levels (D). **P < 0.01 vs. miR-200c-3p mimics NC or miR-200c-3p inhibitors NC
MiRNA-200a-3p added GC cell proliferation and tumor development in vivo
In order to confirm the tumorigenic activity of miRNA-200a-3p in vivo, naked mice were subcutaneously inoculated with SGC-7901 cells stably transfected with miRNA-200a-3p mimics or inhibitors. The miRNA-200a-3p-overexpressing group had a greater xenografted tumor size than the mimic NC group; the tumor size was smaller in the miRNA-200a-3p inhibitor group than the inhibitor NC group (Fig. 4A). Furthermore, in comparison to the miRNA-200a-3p-overexpressing population, H&E staining of the xenograft tissues revealed substantial pathologic mitosis in tumors, whereas miRNA-200a-3p knockdown in vivo resulted in a reduction of histopathological damages (Fig. 4B). Besides, miRNA-200a-3p overexpression ameliorated, and suppression of miRNA-200a-3p expression exacerbated tumor cell death which was revealed by the TUNEL assay (Fig. 4C). Accordingly, immunohistochemical staining for Ki-67 revealed that the tumor cell proliferation rate was higher in the miRNA-200a-3p mimics group than the NC group, as calculated by the percentage of Ki-67-positive cells. In contrast, the miRNA-200a-3p knockdown group exhibited declined cell proliferation rate (Fig. 4D).
In vivo, MiRNA-200a-3p aided GC cell proliferation and tumor development. Tumor size (A), H&E (B), TUNEL (C) or immunohistochemical staining for Ki-67 (D) in the xenograft tissues of nude mice subcutaneously inoculated with the blank control cells or SGC-7901 cells transfected with mimics or inhibitors NC, miRNA-200a-3p mimics or inhibitors. **P < 0.01 vs. miR-200c-3p mimics NC or miR-200c-3p inhibitors NC
MiRNA-200a-3p directly targeted DLC-1 in GC cells
DLC-1 expression at the sub-atomic level in GC cells was lower than in GES-1 cells, as shown in Fig. 5A and B. DLC-1 was anticipated to be an up-and-comer target gene of miRNA-200a-3p by bioinformatics research (TargetScan) (Fig. 5C). To check whether DLC-1 was an immediate objective of miRNA-200a-3p, we utilized a luciferase activity assay. The outcomes uncovered that when SGC-7901 and HGC-27 cells were co-transfected with miRNA-200a-3p copy within sight of the wild-type DLC-1 3′-UTR, the relative luciferase activity was decreased. In contrast, luciferase activity of Mut-3′UTR-DLC-1 in miRNA-200a-3p overexpression samples experienced no significant change (Fig. 5D). Subsequently, DLC-1 expression was noticeably diminished in GC cells transfected with miRNA-200a-3p mimics, versa vice (Fig. 5E, F).
MiRNA-200a-3p directly targeted DLC-1 in GC cells. (A and B) DLC-1 mRNA and protein levels in cells as determined by qRT-PCR and western blotting; (C) miRNA-200a-3p and DLC-1 targeted binding sites. (D) Luciferase activity of cells co-transfected with DLC-1-3′UTR-WT or DLC-1-3′UTR-MUT and miRNA-200a-3p mimics. (E, F) DLC-1 mRNA and protein levels in cells transfected with NC mimics or inhibitors, miRNA-200a-3p mimics or inhibitors, or blank control cells. *P < 0.01 vs. miR-200c-3p mimics NC or miR-200c-3p inhibitors NC
MiRNA-200a-3p functioned as an oncogene in GC through downregulating DLC-1
To see if the function of miRNA-200a-3p in GC cells was mediated by downregulating DLC-1 expression, researchers looked into it further. First, we restored DLC-1 in miRNA-200a-3p-overexpressed GC cells by transfection of the DLC-1 expression vector (Fig. 6A). Then, we found that upregulation of DLC-1 reduced cell viability (Fig. 6B), enhanced cell apoptosis (Fig. 6C), up-regulated Bax, and Caspase-3/9 and downregulated Bcl-2 expression level (Fig. 6D), inhibited cancer cell invasion and migration (Fig. 7A, B), and decreased MMP-2 and MMP-9 protein levels (Fig. 7C). Overexpression of miRNA-200a-3p, on the other hand, could counteract the effects of DLC-1 upregulation in GC cells.
MiRNA-200a-3p functioned as an oncogene in GC through downregulating DLC-1. DLC-1 mRNA and protein expression (A and B), CCK-8 cell viability assay (C), Annexin V-FITC/PI staining for apoptosis detection (D), and Bcl-2, Bax, Caspase-3, and Caspase-9 protein levels (E) in blank control cells, SGC-7901 or HGC-27 cells transfected with pc-NC or pc-DLC-1 or together with miRNA-200a-3p mimics. **P < 0.01 vs. pc-NC group; #P < 0.05, ##P < 0.01 vs. pc-DLC group
MiRNA-200a-3p functioned as an oncogene in GC through downregulating DLC-1. In the blank control cells, SGC-7901 or HGC-27 cells transfected with pc-NC or pc-DLC-1 or together with miRNA-200a-3p mimics, wound healing migration assay (A), Transwell invasion test (B), and the MMP-2 and MMP-9 protein levels (C). **P < 0.01 vs. pc-NC group; ##P < 0.01 vs. pc-DLC group
Discussion
Since GC is still prevailing in China, it remains an urgent challenge to elucidate the molecular mechanism underlying GC carcinogenesis. Recently, miRNAs have been shown to cause specific mRNA degradation or translational inhibition by engaging in posttranscriptional gene regulation by matching the 3′UTR of mRNA targets (Liu et al. 2017). This finding suggests that miRNAs are proposed to be a possible way of improving disease therapy.
MiRNA-200a-3p were found to be is correlated with the occurrence and development of various tumors according to previous studies. For instance, according to Shi et al., ectopic expression of miRNA-200a-3p decreased HCC cell migration, viability and invasion by altering cell cycle progression (Shi et al. 2017). Wei et al. discovered that miRNA-200a-3p increased tumor cell apoptosis and prevented metastasis by directly targeting programmed death-ligand 1 (Wei et al. 2019). Notwithstanding this, few examinations have shown that miRNA-200a-3p is overexpressed in an assortment of tumors. MiNA-200a-3p, for instance, was discovered to be altogether up-regulated in epithelial ovarian malignancy (EOC) tissues, proposing its likely qualities as a novel biomarker for the finding of EOC (Teng et al. 2016). MiRNA-200a-3p attacked CRMP1 and aggravated the progression of esophageal cancer, according to Zang et al. (Zang et al. 2016). Wang et al. recently discovered a high miRNA-200a-3p level in the serum and tumor tissues of patients with gastric cardia adenocarcinoma (GCA) (Wang et al. 2018).
In this study, we found that MiRNA-200a-3p was highly expressed in GC. The overexpression of miRNA-200a-3p worked with the expansion, relocation, and intrusion of GC cells. Furthermore, miRNA-200a-3p upregulation may exert inhibitory activities against cell apoptosis. To more readily comprehend the sub-atomic capacity of miRNA-200a-3p in GC carcinogenesis, potential target genes of miRNA-200a-3p were screened and DLC-1 was discovered to be a novel target of miRNA-200a, according to the findings.
DLC-1 belongs to the RhoGTPase activating protein (GAP) family and is often silenced in multiple common tumors, including GC (Ullmannova and Popescu 2006; Verma et al. 2019). In this examination, an altogether lower level of DLC-1 was seen in GC tissues and cells. According to bioinformatics and luciferase reporter assays, miRNA-200a-3p is involved in the mechanism by directly targeting DLC-1. Rescue assay verified that co-transfection of miRNA-200a-3p abrogated the tumor suppressive role of DLC-1 overexpression in GC cells, suggesting that miRNA-200a-3p exerted carcinogenic role in GC by directly targeting DLC-1.
In conclusion, this research revealed that miRNA-200a-3p was a possible carcinogen in GC by directly targeting DLC-1 and controlling tumor proliferation, migration, and invasion. Thus, the MiRNA-200a-3p/DLC axis provides a theological basis for potential improvements in GC treatment strategies.
References
Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA, Psyrri A (2018) Medical management of gastric cancer: a 2017 update. Cancer Med 7(1):123–133
Chen Z, Liu X, Hu Z, Wang Y, Liu M, Liu X, Li H, Ji R, Guo Q, Zhou Y (2015) Identification and characterization of tumor suppressor and oncogenic miRNAs in gastric cancer. Oncol Lett 10(1):329–336
Ding M, Sun X, Zhong J, Zhang C, Tian Y, Ge J, Zhang CY, Zen K, Wang JJ, Zhang C et al (2018) Decreased miR-200a-3p is a key regulator of renal carcinoma growth and migration by directly targeting CBL. J Cell Biochem 119(12):9974–9985
Hao NB, He YF, Li XQ, Wang K, Wang RL (2017) The role of miRNA and lncRNA in gastric cancer. Oncotarget 8(46):81572–81582
Link H, Angele M, Schuller M, Ganschow P, Machetanz L, Guba M, Werner J, Kirchner T, Neumann J (2018) Extra-capsular growth of lymph node metastasis correlates with poor prognosis and high SOX9 expression in gastric cancer. BMC Cancer 18(1):483
0 C, Hu W, Li LL, Wang YX, Zhou Q, Zhang F, Song-Yang YY, Zhu W, Sun CC, Li DJ (2018) Roles of miR-200 family members in lung cancer: more than tumor suppressors. Future Oncol 14(27):2875–2886
Liu L, Ren W, Chen K (2017) MiR-34a promotes apoptosis and inhibits autophagy by targeting HMGB1 in acute myeloid leukemia cells. Cell Physiol Biochem 41(5):1981–1992
Liu L, Tian YC, Mao G, Zhang YG, Han L (2019) MiR-675 is frequently overexpressed in gastric cancer and enhances cell proliferation and invasion via targeting a potent anti-tumor gene PITX1. Cell Signal 62:109352
Peng L, Fu J, Ming Y (2018) The miR-200 family: multiple effects on gliomas. Cancer Manag Res 10:1987–1992
Shi T, Hua Q, Ma Z, Lv Q (2017) Downregulation of miR-200a-3p induced by hepatitis B Virus X (HBx) Protein promotes cell proliferation and invasion in HBV-infection-associated hepatocarcinoma. Pathol Res Pract 213(12):1464–1469
Su Y, Lin L, Zhang J, Jiang Y, Pan C, Sun L, Duan J, Liao W (2015) Low expression of DLC1 is predictive of poor therapeutic efficiency of fluoropyrimidine and oxaliplatin as adjuvant chemotherapy in gastric cancer. Mol Med Rep 12(4):5771–5779
Szasz AM, Lanczky A, Nagy A, Forster S, Hark K, Green JE, Boussioutas A, Busuttil R, Szabo A, Gyorffy B (2016) Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget 7(31):49322–49333
Teng Y, Su X, Zhang X, Zhang Y, Li C, Niu W, Liu C, Qu K (2016) miRNA-200a/c as potential biomarker in epithelial ovarian cancer (EOC): evidence based on miRNA meta-signature and clinical investigations. Oncotarget 7(49):81621–81633
Ullmannova V, Popescu NC (2006) Expression profile of the tumor suppressor genes DLC-1 and DLC-2 in solid tumors. Int J Oncol 29(5):1127–1132
Verma R, Agarwal AK, Sakhuja P, Sharma PC (2019) Microsatellite instability in mismatch repair and tumor suppressor genes and their expression profiling provide important targets for the development of biomarkers in gastric cancer. Gene 710:48–58
Wang J, Zhang H, Zhou X, Wang T, Zhang J, Zhu W, Zhu H, Cheng W (2018) Five serum-based miRNAs were identified as potential diagnostic biomarkers in gastric cardia adenocarcinoma. Cancer Biomark 23(2):193–203
Wei S, Wang K, Huang X, Zhao Z, Zhao Z (2019) LncRNA MALAT1 contributes to non-small cell lung cancer progression via modulating miR-200a-3p/programmed death-ligand 1 axis. Int J Immunopathol Pharmacol 33:2058738419859699
Wu HT, Xie CR, Lv J, Qi HQ, Wang F, Zhang S, Fang QL, Wang FQ, Lu YY, Yin ZY (2018) The tumor suppressor DLC1 inhibits cancer progression and oncogenic autophagy in hepatocellular carcinoma. Lab Invest 98(8):1014–1024
Wu PP, Zhu HY, Sun XF, Chen LX, Zhou Q, Chen J (2015) MicroRNA-141 regulates the tumour suppressor DLC1 in colorectal cancer. Neoplasma 62(5):705–712
Yang YJ, Luo S, Wang LS (2019) Effects of microRNA-378 on epithelial-mesenchymal transition, migration, invasion and prognosis in gastric carcinoma by targeting BMP2. Eur Rev Med Pharmacol Sci 23(12):5176–5186
Yuan BZ, Miller MJ, Keck CL, Zimonjic DB, Thorgeirsson SS, Popescu NC (1998) Cloning, characterization, and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res 58(10):2196–2199
Zang Y, Tai Y, Wan B, Jia X (2016) miR-200a-3p promotes the proliferation of human esophageal cancer cells by post-transcriptionally regulating cytoplasmic collapsin response mediator protein-1. Int J Mol Med 38(5):1558–1564
Funding
This study was supported by the Hospital-level topic of Jiangsu Cancer Hospital (ZM202011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest among the writers.
Ethical approval
All experimental processes followed the Ethics Committee of Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University. In addition, each participant signed informed consent before the study. Both methods used in studies involving human subjects have complied with the institutional study committee's ethical guidelines and the Helsinki Declaration (WMA Declaration of Helsinki, 2013).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Wang, Y., Liu, S. et al. MiR-200a-3p promotes gastric cancer progression by targeting DLC-1. J Mol Histol 53, 39–49 (2022). https://doi.org/10.1007/s10735-021-10037-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-021-10037-7