Abstract
The pathophysiological mechanism of carotid atherosclerosis (CAS) involves endothelial cell dysfunction, vascular smooth muscle cells (VSMCs), and macrophage activation, which ultimately leads to fibrosis of the vessel wall. lncRNA works weightily in the formation of CAS, but the function and mechanism of lncRNA LINC01123 in stable plaque formation are still equivocal. We collected blood samples from 35 CAS patients as well as 33 healthy volunteers. VSMCs treated with oxidized low-density lipoprotein (ox-LDL) were utilized as the CAS cell models. We applied qRT-PCR for detecting LINC01123, miR-1277-5p and KLF5 mRNA expression, CCK-8 method and BrdU test for determining cell proliferation, Transwell test for measuring cell migration, as well as Western blot for assaying KLF5 protein expression. Dual-luciferase reporter experiment was adopted for assessing the interaction between LINC01123 and miR-1277-5p, as well as KLF5 and miR-1277-5p. LINC01123 and KLF5 expression were dramatically up-regulated, while miR-1277-5p expression was down-regulated in CAS patients and ox-LDL-induced CAS cell models. Overexpressed LINC01123 notedly promoted VSMCs migration and proliferation. LINC01123 knockdown repressed cell proliferation and migration. Also, LINC01123 targeted miR-1277-5p and down-regulated its expression, while miR-1277-5p could negatively regulate KLF5 expression. LINC01123 is highly expressed in CAS patients, and promotes cell proliferation and migration via regulating miR-1277-5p/KLF5 axis in ox-LDL-induced VSMCs. It might be involved in the fibrous plaque formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis (AS), the momentous causation of death in patients, is the common pathological basis of cardiovascular and cerebrovascular diseases (Hansson and Hermansson 2011). Carotid atherosclerosis (CAS) is a part of systemic atherosclerosis. According to research investigations, the annual incidence of cerebrovascular accidents is 250/100,000, which is 5 times that of coronary heart disease (Hankey and Stroke 2017). Considerable studies have evidenced that CAS is the staple incentive of ischemic cerebral infarction (Gepner et al. 2017). The degree of carotid artery stiffness, blood flow, and cerebral infarction have a very close link (Yahagi et al. 2017). Currently, the specific regulatory mechanism of the emergence and progress of CAS at the gene and molecular level is still not fully explicit.
Long non-coding RNA (lncRNA) is a class of non-coding RNA transcripts with over 200 nucleotides and no protein-coding capability, which modulates gene expression in the form of RNA at the levels of transcription, post-transcription, and epigenetics (Zhang et al. 2018a, b). Research has authenticated that lncRNA is a crucial modulatory factor engaged in the emergence and progress of cardiovascular and cerebrovascular diseases, and is tightly connected to the formation of CAS (Wu et al. 2020). Research has recently uncovered that lncRNA acts vitally in the modulation of AS, and is probably a biomarker for diagnosing cerebrovascular diseases. According to a previous report, lncRNA p21 can curb VSMCs proliferation of ApoE-/- mice and induce their apoptosis through fortifying the transcriptional activity of p53 (Wu et al. 2014). lncRNA H19 is highly expressed in AS patients, fosters VSMCs proliferation, and represses their apoptosis via modulating the MAPK and NF-κB signal pathways (Pan 2017). LINC01123 exerts significant functions in cancer progression (Hua et al. 2019; Shang et al. 2020; Yang et al. 2020). Nevertheless, the function and mechanism of LINC01123 in CAS vascular smooth muscle cells (VSMCs) are still nebulous.
MicroRNA (miRNA) is a type of small single-stranded RNA with a length of about 22 nucleotides, which is highly conservative, sequential, and tissue-specific. miRNA is engaged in diverse biological processes, including cell differentiation, proliferation, development, tumorigenesis, and metastasis, etc. (Bartel 2004). Evidence has displayed that miRNAs function substantially in the physiological and pathological processes of multiple diseases, especially closely concerned with the emergence and progress of cardiovascular and cerebrovascular diseases (Small et al. 2010). Recent research has exhibited that various miRNAs take part in the emergence and progress of AS lesions. For instance, miR-181b contributes to the emergence of AS via modulating TIMP-3 and elastin expression (Gregoli et al. 2017). miR-181a-5p and miR-181a-3p block NF-κB activation and vascular inflammation through targeting Table 1 and NEMO separately, thus deferring AS progression (Su et al. 2019). Nonetheless, the function of miR-1277-5p in CAS pathogenesis and the causative role remain cryptic.
Kruppel like factor 5 (KLF5) is a staple member of the KLFs family and a transcription factor intimately associated with cell differentiation, proliferation, development, and apoptosis (Jono et al. 2000). Studies have manifested that KLF5 down-regulation in VSMCs is correlated with rupture of abdominal aortic aneurysm, an age-related vascular disease (Ma et al. 2020). Scholars have corroborated that KLF5 exerts a momentous part in vascular remodeling diseases, particularly in AS, vascular restenosis, and cardiac hypertrophy (Nagai et al. 2005). Howbeit the causative role of KLF5 in the emergence and formation of fibrous plaque has not yet been fully illustrated.
This work aimed to dig into the expression and function of LINC01123 in CAS, and to inquire into its potential mechanism. We disclosed that LINC01123 was highly expressed in the serum of CAS patients and the ox-LDL-induced AS cell models. LINC01123 governed KLF5 expression through sponging miR-1277-5p to foster VSMCs migration and proliferation, thus facilitating the formation of fibrous plaque.
Materials and methods
Clinical samples
Serum samples were harvested from 35 CAS patients treated in the Department of Neurology of our institution from September 2018 to December 2019 and 33 healthy volunteers. Atherosclerotic plaques were collected from patients who had undergone carotid endarterectomy and were diagnosed with atherosclerosis. Arteries without macroscopic evidence of atherosclerosis were collected from cerebral edema. The exclusion criteria were patients with cancer, congestive heart failure, valvular heart disease, hematological system diseases, autoimmune disease, and/or infection. The collected samples were immediately preserved at − 80 °C until analysis. The medical ethics committee had endorsed this research protocol. The clinical features of the patients were presented in Table 2.
Cell culture
Human VSMC cell line was purchased from Yichen Biotechnology (Shanghai, China). These VSMCs were originated from an 11-month-old white female. VSMCs were cultivated using Ham’s F-12 K (Kaighn’s) Medium (Gibco/BRL life Technology, Eggenstein, Germany) supplemented with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA), 100 U/mL penicillin, 100 mg/mL streptomycin and 1% antibiotic mixture (Sangon Biotech, Shanghai, China) at 37℃ with 5% CO2. When cells reached 90% confluence, 20% of the cells were re-plated and cultured until they reached 90% confluence again for the next passage and were then passaged every 3–4 days. The utilization of VSMCs has gotten the permission of the Ethic Committee of Hainan Provincial Hospital of Chinese Medicine.
Cell transfection and treatment
LINC01123-overexpression plasmid and KLF5-overexpression plasmid were constructed by cloning full length cDNA of LINC01123 of KLF5 into pcDNA3.1 empty vector (Invitrogen). Small interferernce RNAs (siRNAs) specifically targeting LINC01123 (si-LINC01123) or KLF5 (si-KLF5) were obtained from GenePharma (Shanghai, China) to down-regulate the expression of LINC01123 and KLF5, with corresponding scrambled oligonucleotides (si-NC) as control. miR-1277-5p mimic was used to simulate miR-1277-5p and miR-1277-5p inhibitor (miR-1277-5p-in) was employed to deplete miR-1277-5p, with miR-NC and inhibitor-NC as respective control. Transient transfection with plasmids or nucleotides was carried out at 80% confluency using Lipofectamine® 2000 (Invitrogen). Cells were harvested 48 h after transfection following overnight serum starvation. To detect the effect of ox-LDL on the expressions of LINC01123 and miR-1277-5p, VSMCs were incubated with the culture medium in the presence of different concentrations of ox-LDL (0, 25, 50, 100 and 150 mg/L) for 24 h, or grown in the culture medium containing ox-LDL at a final concentration of 100 mg/L for different times (0, 6, 12, 24 and 48 h).
Quantitative real-time PCR (qRT-PCR)
We applied TRIzol reagent (Invitrogen, Unite State) for extracting total RNA from clinical specimens and cells. Our member used NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States) for checking RNA concentration, PrimeScrip-RT kit (Takara, Dalian, China), and ABI7900 system (Applied Biosystems) for generating cDNA. Next, we performed reverse transcription reaction and used GAPDH or U6 as an internal control. The primer sequence were: LINC01123, F: 5’-FACAGTGGCCGCACGCATAGCTG-3’, R: 5’-RCTGACGACCGAGGTGACAACGATGA-3’; miR-1277-5p, F: 5’-GCCGAGTATATATATATGTACGTAT-3’, R: 5’-CTCAACTGGTGTCGTGGA-3 ’; KLF5, F: 5’-ACCTCAGCTTCCTCCAGTTC-3’, 5’-CGCATGGTCTCTGGGATTTG-3’; U6, F: 5’-CTCGCTTCGCRCAGCACA-3’, R: 5’-AACGCTTCACGAATTTGCGT-3’; GAPDH, F: 5’ -CCTGACCTGCGTGTGGACT-3’, R: 5’-GCTGTGGATGGGGAGGTGTC-3’. Using the 2−ΔΔCt method, the relative expression of genes was computed. We completed each experiment in triplicate and done each measurement in triplicate too.
MTT assay
After serum starvation, cells were induced with 10% serum-containing media for 24 h. Our crew seeded the transfected VSMCs into a 96-well plate comprising ox-LDL (100 mg/L) at a density of 5 × 103 cells per well and incubated them at 37 °C. After incubating for 12, 24, 48, 72, and 96 h, MTT (Sigma-Aldrich) was added to each well. We incubated the mixture under 37 °C and 5% CO2 condition lasting 4 h, supplemented it with 150 mL DMSO and dissolved it under room temperature lasting 20 min. The absorbance value of each well was assayed at 490 nm via a microplate reader.
BrdU analysis
After serum starvation for 48 h, cells were stimulated with 10% fetal bovine serum for 24 h. After seeding the transfected cells into a 96-well culture plate at a density of 2 × 103 cells/well, we fostered them lasting 24 or 48 h, and incubated them with a final concentration of 10 µM BrdU (BD Pharmingen) 2 to 24 h. In the next step, we withdrew the medium, fixed the cells at room temperature lasting 30 min, incubated them with the peroxidase-conjugated anti-BrdU antibody (Sigma-Aldrich) at room temperature lasting 60 min, washed them 3 times applying PBS, and incubated them with the oxidase substrate lasting 30 min. The absorbance value was assayed at 450 nm. Background BrdU immunofluorescence was assayed in cells not exposed to BrdU but dyed with BrdU antibody.
Transwell assay
For synchronizing cells were stimulated by 10% serum after serum starvation for 24 h. The cell migration detection was accomplished by making use of a 24-well Transwell chamber (8 μm pore size; BD Biosciences). About 1 × 105 cells were resuspended in 100 µL serum-free medium, and DMEM comprising 10% FBS was filled into the lower chamber. After incubating lasting 24 h under 37 °C condition, the upper chamber was fixed in anhydrous methanol lasting 20 min, dyed applying 0.1% crystal violet lasting 20 min, then washed and dried. An inverted microscope (Nikon, Japan) was applied for measuring the number of migrating cells.
Dual-Luciferase reporter gene assay
Dual-luciferase reporter gene assay was carried out, as described below. The LINC01123 or KLF5 fragment encompassing the forecasted miR-1277-5p binding site, as well as the wild-type or mutant hypothetical sequence of the binding site, was subcloned into the pMiRGLO dual-luciferase vector (Promega) to construct report vectors, pMiRGLO-LINC01123-wild type (LINC01123-WT), pMiRGLO-KLF5-wild type (KLF5-WT) or pMiRGLO-LINC01123-mutant (LINC01123-MUT), pMiRGLO-KLF5-mutant (KLF5-MUT). Subsequently, we applied Lipofectamine® 2000 for co-transfecting SNHG16-WT/KLF5-WT or LINC01123-MUT/KLF5-MUT with miR-1277-5p mimic or miR-con into 293T. After transfecting for 48 h, we measured the luciferase activity making use of the dual-luciferase reporter assay system (Promega).
Western blot analysis
The total cell protein was extracted by applying RIPA buffer (Pierce, Thermo Sciencific). The protein concentration was determined utilizing the Bradford method (Pierce, Thermo Sciencific). The same amount of protein (40 µg) was disassociated on 10% SDS-PAGE and then transferred to PVDF membranes (Millipore). We blocked the PVDF membrane by applying 5% bovine serum albumin in TBST for 2 h at 37 ℃. Subsequently, the membrane was incubated with anti-KLF5 antibody (Abcam, ab137676, 1:500) or anti-GAPDH antibody (Abcam, ab8245, 1:3000) at 4 °C nightlong. We washed the membrane 3 times adopting TBST and incubated it with goat anti-rabbit HRP (IgG) (Abcam, ab6721, 1:2000) at 37 °C lasting 1 h. An enhanced chemiluminescence detection system (ECL) was adopted to visualize proteins.
Statistical analysis
All the data were expressed as the mean ± standard deviation (SD). Each assay was applied at least three independent experiments or replicates. The Student’s t-tests was utilized for analyzing the significance between groups. When a P value was less than 0.05, the result was considered to be of statistical significance. All statistical analyses were performed by Mann-Whitney U tests employing SPSS 18.0 (SPSS Inc, Chicago, IL) software and GraphPad Prism 8.0 (GraphPad Software Inc.) software.
Results
LINC01123 was up-regulated in the serum of CAS patients and ox-LDL-induced cells
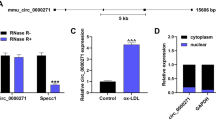
To delve into LINC01123 expression level in CAS, first, we used qRT-PCR for assaying LINC01123 expression in the serum of CAS patients. The results exhibited that compared with the control group, LINC01123 was noticeably higher in the serum of CAS patients (Fig. 1A). Subsequently, we performed LINC01123 expression analysis on aortic atherosclerotic plaques (from 3 patients) and normal aortic tissues (from 3 individuals). The analysis showed that the level of LINC01123 was significantly higher in the atherosclerotic plaques than in the normal arterial intimae (Fig. 1B). Besides, human VSMCs were treated with ox-LDL and detected via qRT-PCR. The results displayed that LINC01123 expression in ox-LDL-treated VSMCs was notably up-regulated compared with the 0 mg/L groups (24 h of induction) or the 0 h group (100 mg/L ox-LDL induction) (Fig. 1C, D). The above data implicated that LINC01123 was up-regulated in the serum of CAS patients and ox-LDL-induced CAS cell lines.
Expression characteristics of LINC01123 in CAS. A qRT-PCR to assay LINC01123 expression in the serum of CAS patients and healthy volunteers. B LINC01123 expression levels in human normal arterial tissues and atherosclerotic plaque tissues, quantified by qRT-PCR. Column chart shows the fold differences in the mean ± SD of LINC01123 levels; n = 8 subjects in each group; each assayed in triplicate. C-D. qRT-PCR to assay LINC01123 expression in VSMCs after ox-LDL treatment in a concentration and time dependent manners. LINC01123 level in three independent experiments repeated in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001
LINC01123 affected VSMCs migration and proliferation
For probing into the biological functions of LINC01123 in the formation of fibrous plaque, we successfully transfected pcDNA-LINC01123 and LINC01123 siRNAs into VSMCs and established a cell model of LINC01123 overexpression or knockdown (Fig. 2 A). Then we adopted the MTT method, BrdU experiment, and Transwell method for determining VSMC migration and proliferation. It was unveiled that compared with the control group (Vector or si-NC), overexpressed LINC01123 noteworthily promoted VSMCs migration and proliferation, whereas LINC01123 knockdown suppressed VSMCs migration and proliferation (Fig. 2B-F). These outcomes revealed that LINC01123 fostered VSMCs migration and proliferation.
LINC01123 facilitated VSMCs proliferation and migration. A Cultured VSMCs were transfeted with either Vector or LINC01123, qRT-PCR to detect LINC01123 expression in VSMCs that overexpress or knockdown LINC01123. B–D Cultured VSMCs were transfeted with either Vector or LINC01123, MTT and BrdU methods to detect VSMCs proliferation, scale bar = 50 μm. E–F Cultured VSMCs were transfeted with either Vector or LINC01123, Transwell experiments to assay the migration ability of VSMCs, scale bar = 100 μm. Data presented are the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
LINC01123 acted as a molecular sponge for miR-1277-5p in VSMCs
We previously corroborated that LINC01123 functioned in promoting CAS cell migration and proliferation. Therefore, it was pretty momentous to inquire into the causative role of LINC01123 in modulating the progress of CAS. We used the StarBase (http://starbase.sysu.edu.cn) database and observed that miR-1277-5p was likely one of the functional target miRNAs of LINC01123 (Fig. 3A). For further verifying the targeting association between LINC01123 and miR-1277-5p, we conducted a dual-luciferase reporter experiment. The results manifested that miR-1277-5p mimics markedly repressed the wild-type LINC01123 luciferase activity, but did not remarkably change the mutant LINC01123 (Fig. 3B). Furthermore, we revealed that miR-1277-5p expression was visibly down-regulated in VSMCs overexpressing LINC01123, whereas LINC01123 knockdown up-regulated miR-1277-5p expression in VSMCs (Fig. 3C). qRT-PCR evinced that miR-1277-5p was observably lower expressed in the serum of CAS patients and ox-LDL-induced CAS cell lines (Fig. 3D-F). In a word, this study validated that LINC01123 could adsorb miR-1277-5p and negatively modulate its expression.
miR-1277-5p was the target of LINC01123. A Bioinformatics analysis to forecast the binding sequence of miR-1277-5p and LINC01123. B Dual-luciferase reporter experiments to test the luciferase activity of overexpressed miR-1277-5p combined with clinical 123 wild-type or mutant type. C qRT-PCR to monitor miR-1277-5p expression in VSMCs overexpressing or knocking down LINC01123. D qRT-PCR to determine miR-1277-5p expression in the serum of CAS patients and healthy volunteers. E-F qRT-PCR to assess miR-1277-5p expression in VSMCs after ox-LDL treatment. Data presented are the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
miR-1277-5p reversed the effect of LINC01123 in CAS
The aforementioned research suggested that LINC01123 could bind to miR-1277-5p. In the next experiment, we aimed to inquire into whether LINC01123 can target miR-1277-5p to govern CAS progress. MTT methods, BrdU experiments, and Transwell experiments evinced that overexpressed miR-1277-5p restrained VSMCs migration and proliferation, and knocking down miR-1277-5p fostered VSMCs migration and proliferation. On the other hand, the effect of overexpressed LINC01123 on VSMCs migration and proliferation was partially impaired by overexpressed miR-1277-5p. miR-1277-5p knockdown greatly reversed the inhibitory influence of LINC01123 knockdown on VSMCs migration and proliferation (Fig. 4A-F). These outcomes indicated that LINC01123 governed VSMCs migration and proliferation via adsorbing miR-1277-5p.
LINC01123/miR-1277-5p axis was engaged in regulating VSMCs proliferation and migration. A–D Cell proliferation evaluated by MTT methods and BrdU tests in VSMCs treated with ox-LDL for 24 h. E–F Transwell experiments to evaluate cell migration in VSMCs with si-LINC01123, si-NC, miR-1277-5p inhibitor, or co-transfectedd with si-LINC01123 and miR-1277-5p inhibitor (LINC01123, Vector, miR-1277-5p mimic, or co-transfected with LINC01123 and miR-1277-5p mimic), and treated with 100 mg/L ox-LDL for 24 h. Data presented are the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01
KLF5 was the immediate target of miR-1277-5p
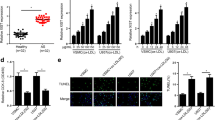
For further looking into the potential mechanism of the LINC01123/miR-1277-5p axis in the proliferation and migration of VSMCs, we investigated the downstream targets of miR-1277-5p. We screened the potential target genes of miR-1277-5p through the StarBase database (http://starbase.sysu.edu.cn). It was discovered that KLF5 was one of the potential targets of miR-1277-5p (Fig. 5A). Subsequently, we took advantage of the dual-luciferase reporter experiment for detecting the targeting relation between miR-1277-5p and KLF5. The results manifested that overexpressed miR-1277-5p strikingly sapped the luciferase activity of KLF5-WT, KLF5-MUT1, KLF5-MUT2, and KLF5-MUT3, whereas the luciferase activity of KLF5-MUT1&2&3 did not change dramatically (Fig. 5B). qRT-PCR indicated that KLF5 mRNA was down-regulated in the serum of CAS patients and ox-LDL-induced VSMCs (Fig. 5C-E). Moreover, miR-1277-5p overexpression or LINC01123 knockdown restrained KLF5 mRNA and protein expression, while miR-1277-5p knockdown or overexpressed LINC01123 enhanced KLF5 mRNA and protein expression. On the other hand, overexpressed miR-1277-5p debilitated the promotion of overexpressed LINC01123 on KLF5 expression, and the inhibition of LINC01123 knockdown on KLF5 could be greatly reversed by miR-1277-5p inhibitors (Fig. 5F-I). These data implied that KLF5 was the downstream target of miR-1277-5p in VSMCs, and its expression was modulated negatively or positively by miR-1277-5p and LINC01123.
KLF5 was the target of miR-1277-5p. A Bioinformatics analysis to forecast the binding sequence of miR-1277-5p and KLF5. B Dual-Luciferase reporter experiments to observe the luciferase activity of overexpressed miR-1277-5p combined with wild-type or mutant KLF5. C qRT-PCR to observe KLF5 mRNA expression in the serum of CAS patients and volunteers. D–E qRT-PCR to investigate KLF5 mRNA expression in VSMCs after ox-LDL treatment. F qRT-PCR was employed to explore KLF5 mRNA levels in the transfected VSMCs treated with ox-LDL for times (0, 6, 12, 24 and 48 h) and concentrations (0, 25, 50, 100,150 mg/L). G–J Western blot was used to detected the KLF5 protein levels in VSMCs trasfected with miR-NC, miR-1277-5p mimic, pc-LINC01123, or co-transfected with miR-1277-5p mimic and pc-LINC01123 (inhibitor-NC, miR-1277-5p inhibitor, si-INC01123, or co-transfected with miR-1277-5p inhibitor and si-LINC01123), and treated with ox-LDL for 24 h.Column chart shows the fold differences in bands intensities of KLF5, standardized against the band intensity of GAPDH. Data presented are the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
KLF5 reversed the impact of miR-1277-5p on CAS cell migration and proliferation
Next, we also investigated whether miR-1277-5p modulated VSMCs proliferation and migration through KLF5. We transfected miR-1277-5p mimics, miR-1277-5p inhibitors, miR-1277-5p + KLF5, miR-1277-5p-in + si-KLF5 in VSMCs. VSMCs migration and proliferation were further detected through the MTT methods, BrdU experiments, and Transwell experiments. The results unveiled that the down-regulation of VSMCs migration and proliferation caused by miR-1277-5p mimics could be partially reversed by overexpressed KLF5. While KLF5 knockdown could enervate the promotion of miR-1277-5p inhibitors on VSMCs migration and proliferation (Fig. 6A-F). These data indicated that miR-1277-5p could target KLF5 to regulate VSMCs proliferation and migration.
miR-1277-5p targeted KLF5 to modulate VSMCs proliferation and migration. A–D Cell proliferation assessed by MTT methods and BrdU experiments in VSMCs transfected with miR-NC, miR-1277-5p mimic, pc-KLF5, or co-transfected with miR-1277-5p mimic and pc-KLF5 (inhibitor-NC, miR-1277-5p inhibitor, si-KLF5, or co-transfected with miR-1277-5p inhibitor and si-KLF5), and treated with ox-LDL for 24 h. E–F Transwell experiments to measure VSMCs migration after transfection and treated with ox-LDL for 24 h. Data presented are the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01
Discussion
AS is the most usual and most serious type of arterial pathological change. It often results in the involvement of critical organs such as the heart and brain, which gravely affects people’s health (Jouni et al. 2016; Kudlaty et al. 2016). In recent years, studies have substantiated that lncRNA performs a diversity of biological functions. lncRNA is broadly expressed in biological cells, primarily through epigenetic modification, transcription regulation, and post-transcriptional regulation to modulate DNA methylation, histone modification or chromatin remodeling, etc., to silence or activate genes (Chen and Carmichael 2010). The imbalance of lncRNA has been corroborated to be concerned with the emergence, progress, and prognosis of AS, and it is expected to become a novel target for diagnosing and treating AS (Wu et al. 2014, 2020; Pan 2017). According to reports, lncRNA LOC285194 is down-regulated in aortic atherosclerotic plaques in the ApoE-/- mouse model and is capable of regulating VSMC apoptosis (Cheng et al. 2020). LncRNA NEXN-AS1 bates the progression of AS through modulating the actin-binding protein NEXN (Hu et al. 2019). LINC01123 is a newly discovered lncRNA with cancer-promoting effects and is engaged in the emergence and progression of multiple tumors. Nevertheless, there is no pertinent report on the specific role of LINC01123 in CAS. In this paper, we substantiated that LINC01123 was patently highly expressed in the serum of CAS patients and ox-LDL induced cells. Also, overexpressed LINC01123 conspicuously promoted VSMCs migration and proliferation, whereas LINC01123 knockdown exerted the opposite effect. These findings indicated that LINC01123 likely facilitated the proliferation and migration of VSMCs, and might be associated with stable plaque formation.
Studies have corroborated that miRNAs exert a key part in cell apoptosis, proliferation, and differentiation. Evidence has recently verified that miRNA is engaged in the emergence and progress of AS. miR-200c is up-regulated in carotid plaques and is available to be served as a biomarker of AS (Magenta et al. 2018). miR-126-5p promotes endothelial cell proliferation and curbs the progression of AS by suppressing Dlk1 (Schober et al. 2014). Up-regulated miR-330-5p is connected to the stability of carotid plaque via targeting Talin-1 (Wei et al. 2019). Additionally, there are reports that lncRNA can act as a molecular sponge of miRNA to adjust human diseases. For example, lncRNA MIAT up-regulates CD47 through sponge miR-149-5p to contribute to AS cell proliferation (Ye et al. 2019). Knockdown of LncRNA TUG1 can inhibit the progression of AS via targeting miR-133a to modulate FGF1 (Zhang et al. 2018a, b). Similarly, the down-regulation of LncRNA HOTTIP suppresses ox-LDL-induced VSMC migration and proliferation through governing the miR-490-3p/HMGB1 axis and PI3K-AKT signal pathway (Guo et al. 2020). In this work, we first revealed the interaction mechanism between LINC01123 and miR-1277-3p in CAS. We found that LINC01123 could work as a molecular sponge of miR-1277-5p in CAS, and miR-1277-5p expression was down-regulated in CAS patients and ox-LDL-induced VSMCs. Additionally, miR-1277-5p could stifle VSMCs migration and proliferation and could weaken the promotion of LINC01123 on VSMCs migration and proliferation. These results indicated that LINC01123 might regulate the formation of fibrous plaque through sponging miR-1277-5p.
KLF5 is a multifunctional transcription factor with a zinc-finger structure. It acts vitally in cardiovascular, cerebrovascular, cancer, and other diseases, and takes part in the modulation of cell proliferation, migration, and apoptosis (Nagai et al. 2005; Tang et al. 2018). Research evidence has shown that KLF5 is highly expressed in VSMCs and participates in the phenotypic transformation of smooth muscle cells and the vascular remodeling process (Koritschoner et al. 1997). Recent studies have evidenced that KLF5 plays pivotally in the tissue remodeling of cardiovascular diseases, such as atherosclerosis, vascular restenosis, and cardiac hypertrophy (Ma et al. 2017). Scholars have previously justified that KLF5 expression is tightly connected to the proliferation of smooth muscle cells in cardiovascular diseases (Zheng et al. 2011). Moreover, there is evidence that miRNA can affect disease progression by targeting KLF5. For example, miR-145-5p promotes the differentiation of gastric cancer cells by directly targeting KLF5 (Zhou et al. 2019). miR-152 dampers the malignant progression of AS via down-regulating KLF5 (Wang et al. 2019). miR-9 stifles VSMCs migration and proliferation through targeting KLF5 (Lu et al. 2019). However, the association between KLF5 and miR-1277-5p or LINC01123 has not been elucidated in CAS. In this work, we attested that KLF5 was the downstream target of miR-1277-5p, and LINC01123 modulated KLF5 expression through competitive binding with miR-1277-5p. Additionally, KLF5 could greatly reverse the suppressive impact of miR-1277-5p on VSMCs proliferation and migration. Those indicated that LINC01123 might modulate KLF5 expression through sponge miR-1277-5p to promote the formation of fibrous plaque.
Collectively, we attested that LINC01123 was overtly up-regulated in CAS patient serum and ox-LDL-induced VSMCs. Overexpressed LINC01123 promoted VSMCs migration and proliferation, and LINC01123 knockdown curbed VSMCs migration and proliferation. Mechanistically, we proved that LINC01123 modulated KLF5 expression through sponge miR-1277-5p to promote the formation of fibrous plaque. Our results indicate that the LINC01123/miR-1277-5p/KLF5 axis is a vital factor in the formation of fibrous plaque, event associated with stable plaque formation.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Chen LL, Carmichael GG (2010) Decoding the function of nuclear long non-coding RNAs. Curr Opin Cell Biol 22(3):357–364
Cheng Q, Zhang M, Zhang M, Ning L, Chen D (2020) Long non-coding RNA LOC285194 regulates vascular smooth muscle cell apoptosis in atherosclerosis. Bioengineered 11(1):53–60
Di Gregoli K, Mohamad Anuar NN, Bianco R et al (2017) MicroRNA-181b controls atherosclerosis and aneurysms through regulation of TIMP-3 and elastin. Circ Res 120(1):49–65
Gepner AD, Young R, Delaney JA et al (2017) Comparison of carotid plaque score and coronary artery calcium score for predicting cardiovascular disease events: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 6(2):e005179
Guo X, Liu Y, Zheng X, Han Y, Cheng J (2020) HOTTIP knockdown inhibits cell proliferation and migration via regulating miR-490-3p/HMGB1 axis and PI3K-AKT signaling pathway in ox-LDL-induced VSMCs. Life Sci 248:117445
Hankey GJ. Stroke (2017) Lancet 389(10069):641–654
Hansson GK, Hermansson A (2011) The immune system in atherosclerosis. Nat Immunol 12(3):204–212
Hu YW, Guo FX, Xu YJ et al (2019) Long noncoding RNA NEXN-AS1 mitigates atherosclerosis by regulating the actin-binding protein NEXN. J Clin Invest 129(3):1115–1128
Hua Q, Jin M, Mi B et al (2019) LINC01123, a c-Myc-activated long non-coding RNA, promotes proliferation and aerobic glycolysis of non-small cell lung cancer through miR-199a-5p/c-Myc axis. J Hematol Oncol 12(1):91
Jono S, McKee MD, Murry CE et al (2000) Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87(7):E10–E17
Jouni H, Askew JW, Crusan DJ, Miller TD, Gibbons RJ (2016) Temporal trends of single-photon emission computed tomography myocardial perfusion imaging in patients without prior coronary artery disease: a 22-year experience at a tertiary academic medical center. Am Heart J 176:127–133
Koritschoner NP, Bocco JL, Panzetta-Dutari GM, Dumur CI, Flury A, Patrito LC (1997) A novel human zinc finger protein that interacts with the core promoter element of a TATA box-less gene. J Biol Chem 272(14):9573–9580
Kudlaty EA, Kendrick DE, Allemang MT, Kashyap VS, Wong VL (2016) Upper extremity steal syndrome is associated with atherosclerotic burden and access configuration. Ann Vasc Surg 35:82–87
Lu X, Ma ST, Zhou B, Lı T (2019) MiR-9 promotes the phenotypic switch of vascular smooth muscle cells by targeting KLF5. Turk J Med Sci 49(3):928–938
Ma D, Zheng B, Suzuki T et al (2017) Inhibition of KLF5-Myo9b-RhoA pathway-mediated podosome formation in macrophages ameliorates abdominal aortic aneurysm. Circ Res 120(5):799–815
Ma D, Zheng B, Liu H-L et al (2020) Klf5 down-regulation induces vascular senescence through eIF5a depletion and mitochondrial fission. PLoS Biol 18(8):e3000808
Magenta A, Sileno S, D’Agostino M et al (2018) Atherosclerotic plaque instability in carotid arteries: miR-200c as a promising biomarker. Clin Sci (Lond) 132(22):2423–2436
Nagai R, Suzuki T, Aizawa K, Shindo T, Manabe I (2005) Significance of the transcription factor KLF5 in cardiovascular remodeling. J Thromb Haemost 3(8):1569–1576
Pan JX (2017) LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur Rev Med Pharmacol Sci 21(2):322–328
Schober A, Nazari-Jahantigh M, Wei Y et al (2014) MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 20(4):368–376
Shang T, Zhou X, Chen W (2020) LINC01123 promotes progression of colorectal cancer via miR-625-5p/LASP1 axis. Cancer Biother Radiopharm. https://doi.org/10.1089/cbr.2020.3740
Small EM, Frost RJ, Olson EN (2010) MicroRNAs add a new dimension to cardiovascular disease. Circulation 121(8):1022–1032
Su Y, Yuan J, Zhang F et al (2019) MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis 10(5):365
Tang J, Li Y, Sang Y et al (2018) LncRNA PVT1 regulates triple-negative breast cancer through KLF5/beta-catenin signaling. Oncogene 37(34):4723–4734
Wang W, Zhang Y, Wang L et al (2019) mircroRNA-152 prevents the malignant progression of atherosclerosis via down-regulation of KLF5. Biomed Pharmacother 109:2409–2414
Wei X, Sun Y, Han T et al (2019) Upregulation of miR-330-5p is associated with carotid plaque’s stability by targeting Talin-1 in symptomatic carotid stenosis patients. BMC Cardiovasc Disord 19(1):149
Wu G, Cai J, Han Y et al (2014) LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 130(17):1452–1465
Wu Y, Zhang F, Li X et al (2020) Systematic analysis of lncRNA expression profiles and atherosclerosis-associated lncRNA-mRNA network revealing functional lncRNAs in carotid atherosclerotic rabbit models. Funct Integr Genomics 20(1):103–115
Yahagi K, Kolodgie FD, Lutter C et al (2017) Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol 37(2):191–204
Yang Y, Wu J, Zhou H, Liu W, Wang J, Zhang Q (2020) STAT1-induced upregulation of lncRNA LINC01123 predicts poor prognosis and promotes the progression of endometrial cancer through miR-516b/KIF4A. Cell Cycle 19(12):1502–1516
Ye ZM, Yang S, Xia YP et al (2019) LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis 10(2):138
Zhang Z, Salisbury D, Sallam T (2018) Long noncoding RNAs in atherosclerosis. J Am Coll Cardiol 72(19):2380–2390
Zhang L, Cheng H, Yue Y, Li S, Zhang D, He R (2018b) TUG1 knockdown ameliorates atherosclerosis via up-regulating the expression of miR-133a target gene FGF1. Cardiovasc Pathol 33:6–15
Zheng B, Han M, Shu YN et al (2011) HDAC2 phosphorylation-dependent Klf5 deacetylation and RARα acetylation induced by RAR agonist switch the transcription regulatory programs of p21 in VSMCs. Cell Res 21(10):1487–1508
Zhou T, Chen S, Mao X (2019) miR-145-5p affects the differentiation of gastric cancer by targeting KLF5 directly. J Cell Physiol 234(5):7634–7644
Acknowledgements
Not applicable.
Funding
Provincial Key R&D Program of Hainan (Grant No. ZDYF2019196), National Natural Science Foundation of China (81960227), Natural Science Foundation of Guangdong Province (2017A030313622) and Guangzhou Provincial Program of Science and Technology (201804010446).
Author information
Authors and Affiliations
Contributions
GW and MG conceived and designed the study. YZ and GZ analyzed the data. GZ and YG contributed to the literature review. GW and MG and YG wrote the manuscript. GW and MG reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The experiment was often approved by the Ethics Committee of the Hainan Provincial Hospital of Chinese Medicine, and all patients participating in this study provided written informed consent following the “Helsinki Declaration”.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weng, G., Gu, M., Zhang, Y. et al. LINC01123 promotes cell proliferation and migration via regulating miR-1277-5p/KLF5 axis in ox-LDL-induced vascular smooth muscle cells. J Mol Histol 52, 943–953 (2021). https://doi.org/10.1007/s10735-021-10010-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-021-10010-4