Abstract
Connective tissue growth factor (CTGF) is a downstream mediator of transforming growth factor-beta 1 (TGF-β1) and TGF-β1-induced CTGF expression is regulated through SMAD and mitogen-activated protein kinase (MAPK) signaling pathways. The fine modulation of TGF-β1 signaling is very important to the process of tooth development. However, little is known about the localization of CTGF, MAPK and SMAD in the context of TGF-β1 signaling during odontogenesis. Hence, we aimed to investigate the expression of TGF-β1, CTGF, phosphorylated-SMAD2/3 (p-SMAD2/3) and phosphorylated-ERK1/2 (p-ERK1/2). ICR mice heads of embryonic (E) day 13.5, E14.5, E16.5, postnatal (PN) day 0.5 and PN3.5 were processed for immunohistochemistry. Results revealed that at E13.5, TGF-β1 and CTGF were strongly expressed in dental epithelium (DE) and dental mesenchyme (DM), while p-SMAD2/3 was intensely expressed in the internal side of DE. p-ERK1/2 was not present in DE or DM. At E14.5 and E16.5, strong staining for TGF-β1 and CTGF was detected in enamel knot (EK) and dental papilla (DPL). DPL was intensely stained for p-ERK1/2 but negatively stained for p-SMAD2/3. There was no staining for p-SMAD2/3 and p-ERK1/2 in EK. At PN0.5 and PN3.5, moderate to intense staining for TGF-β1 and CTGF was evident in preameloblasts (PA), secretary ameloblasts (SA) and dental pulp (DP). p-SMAD2/3 was strongly expressed in SA and DP but sparsely localized in PA. p-ERK1/2 was intensely expressed in DP, although negative staining was observed in PA and SA. These data demonstrate that TGF-β1 and CTGF show an identical expression pattern, while p-SMAD2/3 and p-ERK1/2 exhibit differential expression, and indicate that p-SMAD2/3 and p-ERK1/2 might play a regulatory role in TGF-β1 induced CTGF expression during tooth development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Teeth develop as a result of sequential and reciprocal interactions between oral epithelium and mesenchyme. Thickening of the oral ectoderm marks the initial morphological sign of tooth formation, followed by three continuous but distinguishable stages of crown morphogenesis: bud, cap and bell stages. Extensive studies on tooth development have demonstrated that numerous signaling molecules belonging to several different families are associated with epithelial-mesenchymal interactions (Thesleff and Aberg 1999; Jernvall and Thesleff 2000; Shi et al. 2016; Zhang et al. 2017). In particular, transforming growth factor-beta1 (TGF-β1) is a pleiotropic cytokine regulating many developmental and homeostatic processes (Hongo et al. 2016). TGF-β1 is present in bone, cartilage and tooth and has unique functions during embryonic craniofacial morphogenesis (Chai et al. 1994). TGF-β1 exerts its biological effects by binding to a cell surface receptor complex of type I and type II receptors. Upon its ligation, the type II receptor phosphorylates the type I receptor (TGFβRI). TGFβRI kinases phosphorylate SMAD2 and SMAD3 to form a transcriptional complex with SMAD4, which translocates into the nucleus to initiate the transcription of target genes (Yoshimoto et al. 2015). In addition to the signaling via the canonical SMAD pathway, TGF-β1 can also signal by activating the other signaling pathways such as mitogen-activated protein kinase (MAPK) including extracellular signal regulated kinase (ERK1/2), Jun-N-terminal kinase (JNK1/2) and P38 (Javelaud and Mauviel 2005; Zhou et al. 2017). Many studies have demonstrated evidence for crosstalk between the SMAD and MAPK signaling cascades activated by TGF-β1. MAPKs can potentiate, synergize or antagonize the TGF-β1/SMAD pathway, and SMADs have been shown to mediate the activation of MAPKs (Arnott et al. 2008; Mu et al. 2012). Thus, SMADs and MAPKs cooperate to achieve TGF-β1 target gene activation, but this interaction varies in a cell type-dependent manner.
One of the targets of TGF-β signaling is a cysteine-rich secretory protein CCN2 (also known as connective tissue growth factor, CTGF). CTGF belongs to CCN family, which consists of CCN1/CYR61, CCN2/CTGF, CCN3/NOV, CCN4/WISP-1, CCN5/WISP-2 and CCN6/WISP-3 (Muromachi et al. 2015). CTGF exhibits diverse cellular functions including extracellular matrix production, angiogenesis, normal growth and development of certain tissues, and cell migration and adhesion (Takeuchi et al. 2009). A study from Shimo et al. (2002) showed that ctgf gene was strongly expressed in preameloblasts, while its expression was markedly downregulated in fully differentiated ameloblasts. However, another study by Yamaai et al. (2005) demonstrated that ctgf was expressed in the preameloblasts and preodontoblasts of first molar and in the ameloblasts and odontoblasts of embryonic day 18 incisor tooth germs. Therefore, the expression of CTGF remains controversial during ameloblasts differentiation. According to a previous report (Pacheco et al. 2008), CTGF and TGF-β signaling components such as TGF-β1, SMAD2/3 and SMAD4 were localized in regions of odontogenic potential, but lack of ctgf did not affect TGF-β1/SMAD signaling or proliferation in the developing tooth, which indicates that CTGF is a downstream mediator of TGF-β1 signaling and may modulate the roles of this pathway during odontogenesis. Interestingly, it was found that treatment of dental epithelial cells with recombinant CTGF stimulated proliferation, whereas treatment with neutralizing antibody inhibited it (Shimo et al. 2002). In sharp contrast, TGF-β1 was reported to inhibit proliferation of enamel organ epithelial cells. Furthermore, attenuation of smad2 gene expression resulted in significant advancement of embryonic tooth development (Ito et al. 2001). It is becoming evident that negative feedback mechanisms may function in parallel with positive feedback resulting in signaling that can turn itself off without any new external signals (Jernvall and Thesleff 2000). Moreover, several studies found that MAPK signaling is involved in mouse tooth and oral development (Cho et al. 2008; Xu et al. 2008). Hence, in order to better understand how TGF-β1 signaling finetunes the process of tooth development, we investigated the expression pattern of TGF-β1, CTGF, phosphorylated-SMAD2/3 (p-SMAD2/3) and phosphorylated-ERK1/2 (p-ERK1/2) in the developing tooth germs.
Materials and methods
Preparation of tissue sections and histological staining
All the experimental procedures were performed according to the guideline of the Animal Ethics Committee, School and Hospital of Stomatology, Wenzhou Medical University. The Adult ICR mice were mated overnight and embryonic (E) 0.5 was designated as the day on which the presence of a vaginal plug was confirmed. At least three embryos and postnatal (PN) mice at each developmental stage (E13.5, E14.5, E16.5, PN0.5 and PN3.5) were used in this study. The samples at each time point were fixed in 10% neutral formalin solution for 48 h, after which the tissue specimens of E16.5, PN0.5 and PN3.5 were decalcified for several days to 2 weeks by immersion in EDTA (10%, pH 7.2, 4 °C) solution, dehydrated using a graded ethanol series and embedded in paraffin. Serial 5 μm longitudinal sections were then cut in the bucco-lingual plane of the tooth.
Immunohistochemistry
As reported previously (Li et al. 2014; Teraishi et al. 2008), polyclonal antibodies against TGF-β1, CTGF, phospho-SMAD2/3 (Santa Cruz Biotechnology, TX, USA) and monoclonal antibody against phospho-ERK1/2 (Thr202/Tyr204) (Cell Signaling Technology, Inc, USA) were used. Sections were deparaffinized in xylene and rehydrated in 100, 95, 85, 70% alcohol, then in distilled water and phosphate-buffered saline (PBS). As for the staining of TGF-β1, CTGF, p-SMAD2/3, enzymatic pretreatment with 0.1% (wt/vol) trypsin (Zhongshan, Beijing, China) for 15 min at 37 °C was performed to increase accessibility of antibody to the epitopes. As for the staining of p-ERK1/2, the sections were placed in citric acid solution (10 mM, pH 6.0) and autoclaved at 100 °C for 20 min to recover antigenic sites possibly obscured by the formalin fixation. After washed in PBS, the sections were soaked in 3% hydrogen peroxidase solution (Zhongshan) for 15 min to block endogenous peroxidase activity. Then the sections were blocked with 5% normal goat serum or 5% skim milk for 20 min at room temperature, and followed by incubation of the primary antibodies (at the following dilutions: CTGF, 1:300; TGF-β1, 1:250; p-SMAD2/3, 1:40; p-ERK1/2, 1:100) overnight at 4 °C. Negative controls were obtained by replacing primary antibody with PBS. After three washes in PBS, the sections were incubated with a biotinylated secondary antibody followed by incubation with conjugated streptavidin–peroxidase (Zhongshan) for 20 min, or with polymer helper followed by polyperoxidase-conjugated anti-(mouse/rabbit/goat) IgG for 20 min (Zhongshan). Specific immunostaining was visualized by incubation with 3,3′-diaminobenzidine tetrachloride solution (Zhongshan) for 3 min and the sections were washed with distilled water. Then the sections were counterstained with hematoxylin solution, rinsed in running tap water, dehydrated in a series of ethanol and cleared with xylene. All sections were viewed and photographically recorded using a Nikon Eclipse Microscope (Nikon, Tokyo, Japan).
Assessment
An assessment of the localization of the antibody staining was made, as described by Matias et al. (2003). The intensity of the staining was determined in a semi-quantitative manner. Immunoreactivity of the antibody in the major tissues and cells was given a score (−, negative; ±, weakly positive; +, moderately positive; or ++, strongly positive). As this was a descriptive study, statistical analysis of the data was not undertaken.
Results
Immunohistochemical staining
Tables 1 and 2 summarized the expression of TGF-β1, CTGF, p-SMAD2/3 and p-ERK1/2 during mouse tooth development.
TGF-β1
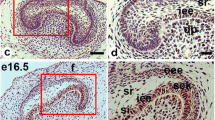
At E13.5 (the bud stage), strong staining for TGF-β1 was detected in oral epithelium (OE), dental epithelium (DE) and dental mesenchyme (DM) (Fig. 1a). As tooth morphogenesis advanced into cap stage (E14.5), moderate to intense staining for TGF-β1 was localized in inner enamel epithelium (IEE), stellate reticulum (SR), outer enamel epithelium (OEE), primary enamel knot (PEK), cervical loop (CL) and dental papilla (DPL) (Fig. 1b). At E16.5 (the early bell stage), intense staining for TGF-β1 was present in IEE, OEE, SR, stratum intermedium (SI), secondary enamel knot (SEK), CL and DPL (Fig. 1c). At PN0.5 (the presecretary stage), weak to moderate staining for TGF-β1 was found in SR and dental pulp (DP), whereas strong reaction with TGF-β1 was observed in SI, OEE, preameloblasts (PA) and odontoblasts (OD) (Fig. 1d–f). At PN3.5 (the secretary stage), intense staining for TGF-β1 was seen in secretary ameloblasts (SA), OD, SI, OEE, SR and DP (Fig. 1g–i).
Expression of TGF-β1 during mouse tooth development. The boxed regions in d and g are enlarged in e and f, and h and i, respectively. a At E13.5, TGF-β1 is strongly expressed in oral epithelium (OE), dental epithelium (DE) and dental mesenchyme (DM). b At E14.5, moderate to intense staining for TGF-β1 is detected in inner enamel epithelium (IEE) (black arrow), stellate reticulum (SR), outer enamel epithelium (OEE), primary enamel knot (PEK) (purple arrow), cervical loop (CL) and dental papilla (DPL). c At E16.5, TGF-β1 is intensely expressed in IEE, OEE, SR, stratum intermedium (SI), secondary enamel knot (SEK) (purple arrow), CL and DPL. d–f At PN0.5, weak to moderate staining for TGF-β1 is observed in SR and dental pulp (DP), whereas TGF-β1 is strongly expressed in SI, OEE, preameloblasts (PA) and odontoblasts (OD). g–i At PN3.5, strong TGF-β1 is localized in secretary ameloblasts (SA), OD, SI, OEE, SR and DP. Scale bar 50 μm. (Color figure online)
p-SMAD2/3
At E13.5, strong p-SMAD2/3 was localized in the internal part of the tooth bud and OE, while weak to negative staining for p-SMAD2/3 was seen in DM (Fig. 2a). At E14.5, PEK, DPL and CL were not positive for p-SMAD2/3, whereas SR, IEE and OEE exhibited sparse staining for p-SMAD2/3 (Fig. 2b). At E16.5, weak staining was found in SR, while CL at one side was strongly positive for p-SMAD2/3. There was no staining in SEK, SI, IEE, OEE and DPL (Fig. 2c). At PN0.5, p-SMAD2/3 was strongly expressed in OEE, SR, SI and DP, whereas very faint and negative staining was found in PA and OD respectively (Fig. 2d–f). At PN3.5, strong reaction with p-SMAD2/3 was evident in SI, SA and DP, while SR and OEE showed weak staining for p-SMAD2/3. Similar to PN0.5, OD was negative for p-SMAD2/3 (Fig. 2g–i).
Expression of p-SMAD2/3 during mouse tooth development. The boxed regions in d and g are enlarged in e and f, and h and i, respectively. a At E13.5, p-SMAD2/3 is strongly expressed in the internal side of the tooth bud and OE, while weak to negative staining is seen in DM. b At E14.5, p-SMAD2/3 is not present in PEK (purple arrow), DPL and CL, whereas SR, IEE (black arrow) and OEE exhibited sparse staining for p-SMAD2/3. c At E16.5, there is no staining in SEK (purple arrow), SI, IEE, OEE and DPL, while SR and CL at one side are weakly and strongly stained for p-SMAD2/3 respectively. d–f At PN0.5, p-SMAD2/3 is strongly expressed in OEE, SR, SI and DP, whereas sparse and negative staining is found in PA and OD respectively. g–i At PN3.5, p-SMAD2/3 is intensely expressed in SI, SA and DP but weakly expressed in SR and OEE. OD is not reactive with p-SMAD2/3. Scale bar 50 μm. (Color figure online)
CTGF
The expression pattern of CTGF and TGF-β1 largely overlapped throughout the stages of odontogenesis. At E13.5, OE, DE and DM exhibited strong staining for CTGF (Fig. 3a). At E14.5, strong CTGF was widely localized in PEK, IEE, OEE, SR, CL and DPL (Fig. 3b). At E16.5, strong reaction with CTGF was evident in IEE, OEE, SR, SI, SEK, DPL and CL (Fig. 3c). At PN0.5, intense staining for CTGF was visible in OEE, SR, SI, PA, OD and DP (Fig. 3d–f). At PN3.5, there was strong staining for CTGF in OEE, SR, SI, SA, OD and DP (Fig. 3g–i).
Expression of CTGF during mouse tooth development. The boxed regions in d and g are enlarged in e and f, and h and i, respectively. a At E13.5, OE, DE and DM show strong staining for CTGF. b At E14.5, intense CTGF is widely localized in PEK (purple arrow), IEE (black arrow), OEE, SR, CL and DPL. c At E16.5, CTGF is strongly expressed in IEE, OEE, SR, SI, SEK (purple arrow), DPL and CL. d–f At PN0.5, CTGF is intensely expressed in OEE, SR, SI, PA, OD and DP. g–i At PN3.5, there is strong reaction with CTGF in OEE, SR, SI, SA, OD and DP. Scale bar 50 μm. (Color figure online)
p-ERK1/2
At E13.5, strong p-ERK1/2 was observed in OE, while negative staining was seen in DE and DM (Fig. 4a). At E14.5, SR and DPL showed moderate to strong reaction with p-ERK1/2, while negative staining was found in PEK, IEE, OEE and CL (Fig. 4b). At E16.5, moderate to intense staining for p-ERK1/2 was present in SR and DPL, whereas there was no staining in SEK, CL, IEE, OEE and SI (Fig. 4c). At PN0.5, SR, OEE, DP and some cells in the layer of SI exhibited strong reaction with p-ERK1/2, while no staining was found in PA and OD (Fig. 4d–f). At PN3.5, SR and DP showed strong staining for p-ERK1/2, whereas negative staining was present in SA and OD. Moreover, OEE and SI exhibited weak to negative staining for p-ERK1/2 (Fig. 4g–i).
Expression of p-ERK1/2 during mouse tooth development. The boxed regions in d and g are enlarged in e and f, and h and i, respectively. a At E13.5, OE is strongly stained for p-ERK1/2, while negative staining is observed in DE and DM. b At E14.5, SR and DPL show moderate to strong staining for p-ERK1/2, whereas negative staining is seen in PEK (purple arrow), IEE (black arrow), OEE and CL. c At E16.5, moderate to intense staining for p-ERK1/2 is detectable in SR and DPL, while there is no staining in SEK (purple arrow), CL, IEE, OEE and SI. d–f At PN0.5, SR, OEE, DP and some cells in the layer of SI show strong reaction with p-ERK1/2, whereas no staining is found in PA and OD. g–i At PN3.5, SR and DP are intensely stained for p-ERK1/2, while no staining is seen in SA and OD. OEE and SI show weak to negative staining for p-ERK1/2. Scale bar 50 μm. (Color figure online)
Discussion
In the present study, we investigated the immunolocalization of CTGF and TGF-β1 signaling cascades components during murine tooth development from E13.5 to PN3.5. At E13.5, both TGF-β1 and CTGF were intensely expressed in OE, DE and DM, while OE was strongly stained for p-SMAD2/3 and p-ERK1/2. These results indicate that odontogenic potential resides in OE, and that TGF-β1 and CTGF play critical roles in regulating epithelial-mesenchymal interaction during tooth morphogenesis. At E14.5 and E16.5, increased staining for p-ERK1/2 was observed in dental papilla compared with dental mesenchyme at E13.5, whereas there was no staining for p-SMAD2/3 in the dental papilla. The dynamic changes of p-ERK1/2 expression might be involved in the shift of odontogenic potential from epithelium to mesenchyme. In addition, enamel knot (EK), a transient structure, consists of primary enamel knots (PEK) and secondary enamel knots (SEK) (Vaahtokari et al. 1996). Signals from the PEK at the cap stage induce the dental papilla cells, and signals from the SEK at the bell stage induce the terminal differentiation of odontoblasts at the cusp tips (Thesleff et al. 2001). Our study showing that both TGF-β1 and CTGF were strongly localized in PEK, SEK and dental papilla supports the view that EKs as signaling centers link tooth morphogenesis and odontoblast differentiation (Thesleff et al. 2001). Unfortunately, here, EKs were not stained for p-SMAD2/3 and p-ERK1/2. Further studies are required to determine the ability of SMAD2/3 and ERK1/2 to modify TGF-β1 induced CTGF expression in EKs. Additionally, we found only one side of cervical loop at E16.5 exhibited the strong immunoreactivity for p-SMAD2/3. Similar expression pattern for α3 and γ2 subunits laminin-5 were observed in developing incisors (Yoshiba et al. 2000). Hence, the asymmetrical expression of p-SMAD2/3 may result from the different developmental origins between labial and lingual cervical loops.
Amelogenesis is a dynamics process that includes the presecretary, secretary and maturation stages (Porto et al. 2009). TGF-β1 is reported to be involved in the differentiation of ameloblasts and the secretion of enamel matrix (D’Souza et al. 1990). A study from Yamaai et al. (2005) revealed that ctgf was expressed in the preameloblasts of upper first molar and in the ameloblasts of incisor at E18 tooth germs. In line with these data, here, CTGF and TGF-β1 were both strongly expressed in preameloblasts and secretary ameloblasts. Moreover, our study showed that p-SMAD2/3 was weak to negative in preameloblasts but strongly expressed in secretary ameloblasts, suggesting that the expression of p-SMAD2/3 is stage-specific and canonical TGF-β1-SMAD2/3-CTGF signaling pathway might be involved in amelogenesis. A study from Cho et al. (2008) found strong activation of ERK was detected in the inner dental epithelium and secretary ameloblasts. Nevertheless, it has been found that high expression of p-ERK is a pathological mechanism leading to enamel developmental defects, Costello Syndrome, characterized by hypo-mineralized and disorganized enamel. Interestingly, MEK inhibitor not only rescued the density and patterning of enamel, but also reduced the p-ERK levels to control (Goodwin et al. 2014). Combined with the mentioned results, our study demonstrating the absence of p-ERK1/2 in preameloblasts and secretary ameloblasts indicates that negative staining or low levels of p-ERK1/2 may be necessary for the differentiation of ameloblasts. Taken together, p-SMAD2/3 and p-ERK1/2 exhibited differential expression pattern, elucidation of which will advance our understanding of the complexity of TGF-β1-mediated CTGF production during enamel formation.
Odontoblast is a monolayer of cells at the periphery of the dental pulp and responsible for primary, secondary and tertiary reactionary dentinogenesis (Simon et al. 2009). Dental pulp cells, under appropriate conditions, may differentiate into odontoblast-like cells, deposit mineralized tissue and subsequently form reparative dentin (Yongchaitrakul and Pavasant 2007; Lian et al. 2016; Du et al. 2016). TGF-β1 is known to be implicated in the differentiation of odontoblast-like cells and in dental pulp tissue repair (Sloan and Smith 1999). A study from Hwang et al. (2008) revealed that more intense TGF-β receptor I and SMAD2/3 immunoreactivity was observed in the odontoblast-like cells from the newly formed reparative dentin compared with that in the odontoblast layer of the normal dentin. In addition, it has been documented that matrix metalloproteinase-3 stimulates CTGF expression and secretion independently of the protease activity and dependently on dynamin-related endocytosis, which is involved in cell migration in human dental pulp cells (Muromachi et al. 2012). In accord with these reports, our study demonstrated that strong reaction with TGF-β1, CTGF and p-SMAD2/3 was localized in dental pulp, indicating that p-SMAD2/3 might be involved in TGF-β1 induced CTGF production in dental pulp cells. Besides, it is worth mentioning that, here, intense staining for p-ERK1/2 was also present in dental pulp. Therefore, a more detailed analysis of the MAPK (ERK1/2) and SMAD2/3 pathways, their interactions as well as their independent functions is required to fully understand how CTGF is regulated by TGF-β1 in dental pulp cells.
In conclusion, this is the first report to systematically investigate the expression of CTGF, p-ERK1/2 and p-SMAD2/3 in the context of TGF-β1 signaling during odontogenesis. TGF-β1 and CTGF showed an identical expression pattern, while p-SMAD2/3 and p-ERK1/2 exhibited differential expression. In light of the limitations of our study, further transgene and conditional knockout mouse models should be used to explore the roles of CTGF and TGF-β1 signaling cascades in tooth development.
References
Arnott JA, Zhang X, Sanjay A, Owen TA, Smock SL, Rehman S, DeLong WG, Safadi FF, Popoff SN (2008) Molecular requirements for induction of CTGF expression by TGF-beta1 in primary osteoblasts. Bone 42:871–885. doi: 10.1016/j.bone.2008.01.006
Chai Y, Mah A, Crohin C, Groff S, Bringas P Jr, Le T, Santos V, Slavkin HC (1994) Specific transforming growth factor-beta subtypes regulate embryonic mouse Meckel’s cartilage and tooth development. Dev Biol 162:85–103
Cho KW, Cho SW, Lee JM, Lee MJ, Gang HS, Jung HS (2008) Expression of phosphorylated forms of ERK, MEK, PTEN and PI3K in mouse oral development. Gene Expr Patterns 8:284–290. doi: 10.1016/j.gep.2007.12.001
D’Souza RN, Happonen RP, Ritter NM, Butler WT (1990) Temporal and spatial patterns of transforming growth factor-beta 1 expression in developing rat molars. Arch Oral Biol 35:957–965
Du J, Wang Q, Yang P, Wang X (2016) FHL2 mediates tooth development and human dental pulp cell differentiation into odontoblasts, partially by interacting with Runx2. J Mol Histol 47:195–202. doi: 10.1007/s10735-016-9655-6
Goodwin AF, Tidyman WE, Jheon AH, Sharir A, Zheng X, Charles C, Fagin JA, McMahon M, Diekwisch TG, Ganss B, Rauen KA, Klein OD (2014) Abnormal Ras signaling in Costello syndrome (CS) negatively regulates enamel formation. Hum Mol Genet 23:682–692. doi: 10.1093/hmg/ddt455
Hongo S, Yamamoto T, Yamashiro K, Shimoe M, Tomikawa K, Ugawa Y, Kochi S, Ideguchi H, Maeda H, Takashiba S (2016) Smad2 overexpression enhances adhesion of gingival epithelial cells. Arch Oral Biol 71:46–53. doi: 10.1016/j.archoralbio.2016.06.025
Hwang YC, Hwang IN, Oh WM, Park JC, Lee DS, Son HH (2008) Influence of TGF-beta1 on the expression of BSP, DSP, TGF-beta1 receptor I and Smad proteins during reparative dentinogenesis. J Mol Histol 39:153–160
Ito Y, Zhao J, Mogharei A, Shuler CF, Weinstein M, Deng C, Chai Y (2001) Antagonistic effects of Smad2 versus Smad7 are sensitive to their expression level during tooth development. J Biol Chem 276:44163–44172
Javelaud D, Mauviel A (2005) Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene 24:5742–5750
Jernvall J, Thesleff I (2000) Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 92:19–29
Li S, Ge S, Yang P (2014) Immunohistochemical localization of connective tissue growth factor, transforming growth factor-beta1 and phosphorylated-smad2/3 in the developing periodontium of rats. J Periodontal Res 49:624–633. doi: 10.1111/jre.12143
Lian M, Zhang Y, Shen Q, Xing J, Lu X, Huang D, Cao P, Shen S, Zheng K, Zhang J, Chen J, Wang Y, Feng G, Feng X (2016) JAB1 accelerates odontogenic differentiation of dental pulp stem cells. J Mol Histol 47:317–324. doi: 10.1007/s10735-016-9672-5
Matias MA, Li H, Young WG, Bartold PM (2003) Immunohistochemical localization of fibromodulin in the periodontium during cementogenesis and root formation in the rat molar. J Periodontal Res 38:502–507
Mu Y, Gudey SK, Landström M (2012) Non-Smad signaling pathways. Cell Tissue Res 347:11–20. doi: 10.1007/s00441-011-1201-y
Muromachi K, Kamio N, Narita T, Annen-Kamio M, Sugiya H, Matsushima K (2012) MMP-3 provokes CTGF/CCN2 production independently of protease activity and dependently on dynamin-related endocytosis, which contributes to human dental pulp cell migration. J Cell Biochem 113:1348–1358. doi: 10.1002/jcb.24007
Muromachi K, Kamio N, Matsuki-Fukushima M, Nishimura H, Tani-Ishii N, Sugiya H, Matsushima K (2015) CCN2/CTGF expression via cellular uptake of BMP-1 is associated with reparative dentinogenesis. Oral Dis 21:778–784. doi: 10.1111/odi.12347
Pacheco MS, Reis AH, Aguiar DP, Lyons KM, Abreu JG (2008) Dynamic analysis of the expression of the TGF-β/smad2 pathway and CTGF during early steps of tooth development. Cells Tissues Organs 187:199–210
Porto IM, Merzel J, de Sousa FB, Bachmann L, Cury JA, Line SR, Gerlach RF (2009) Enamel mineralization in the absence of maturation stage ameloblasts. Arch Oral Biol 54:313–321. doi: 10.1016/j.archoralbio.2009.01.007
Shi L, Li L, Wang D, Li S, Chen Z, An Z (2016) Spatiotemporal expression of caveolin-1 and EMMPRIN during mouse tooth development. J Mol Histol 47:337–344. doi: 10.1007/s10735-016-9675-2
Shimo T, Wu C, Billings PC, Piddington R, Rosenbloom J, Pacifici M, Koyama E (2002) Expression, gene regulation, and roles of Fisp12/CTGF in developing tooth germs. Dev Dyn 224:267–278
Simon S, Smith AJ, Lumley PJ, Berdal A, Smith G, Finney S, Cooper PR (2009) Molecular characterization of young and mature odontoblasts. Bone 45:693–703. doi: 10.1016/j.bone.2009.06.018
Sloan AJ, Smith AJ (1999) Stimulation of the dentine-pulp complex of rat incisor teeth by transforming growth factor-beta isoforms 1–3 in vitro. Arch Oral Biol 44:149–156
Takeuchi H, Kubota S, Murakashi E, Fukada T, Hashimoto S, Takigawa M, Numabe Y (2009) Effect of transforming growth factor-beta1 on expression of the connective tissue growth factor (CCN2/CTGF) gene in normal human gingival fibroblasts and periodontal ligament cells. J Periodontal Res 44:161–169. doi: 10.1111/j.1600-0765.2008.01093.x
Teraishi T, Miura K, Imaki J (2008) An optimized immunohistochemical method for detection of phosphorylated mitogen-activated protein kinases. J Immunol Methods 330:34–43
Thesleff I, Aberg T (1999) Molecular regulation of tooth development. Bone 25:123–125
Thesleff I, Keränen S, Jernvall J (2001) Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv Dent Res 15:14–18
Vaahtokari A, Aberg T, Jernvall J, Keränen S, Thesleff I (1996) The enamel knot as a signaling center in the developing mouse tooth. Mech Dev 54:39–43
Xu X, Han J, Ito Y, Bringas P Jr, Deng C, Chai Y (2008) Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev Cell 15:322–329. doi: 10.1016/j.devcel.2008.06.004
Yamaai T, Nakanishi T, Asano M, Nawachi K, Yoshimichi G, Ohyama K, Komori T, Sugimoto T, Takigawa M (2005) Gene expression of connective tissue growth factor (CTGF/CCN2) in calcifying tissues of normal and cbfa1-null mutant mice in late stage of embryonic development. J Bone Miner Metab 23:280–288
Yongchaitrakul T, Pavasant P (2007) Transforming growth factor-beta1 up-regulates the expression of nerve growth factor through mitogen-activated protein kinase signaling pathways in dental pulp cells. Eur J Oral Sci 115:57–63
Yoshiba K, Yoshiba N, Aberdam D, Meneguzzi G, Perrin-Schmitt F, Stoetzel C, Ruch JV, Lesot H (2000) Differential expression of laminin-5 subunits during incisor and molar development in the mouse. Int J Dev Bio 44:337–340
Yoshimoto T, Fujita T, Kajiya M, Matsuda S, Ouhara K, Shiba H, Kurihara H (2015) Involvement of smad2 and Erk/Akt cascade in TGF-β1-induced apoptosis in human gingival epithelial cells. Cytokine 75:165–173. doi: 10.1016/j.cyto.2015.03.011
Zhang H, Jani P, Liang T, Lu Y, Qin C (2017) Inactivation of bone morphogenetic protein 1 (Bmp1) and tolloid-like 1 (Tll1) in cells expressing type I collagen leads to dental and periodontal defects in mice. J Mol Histol 48:83–98. doi: 10.1007/s10735-016-9708-x
Zhou T, Guo S, Zhang Y, Weng Y, Wang L, Ma J (2017) GATA4 regulates osteoblastic differentiation and bone remodeling via p38-mediated signaling. J Mol Histol 48:187–197. doi: 10.1007/s10735-017-9719-2
Acknowledgements
This study was supported by the Zhejiang Province Natural Science Foundation (Grant No: LY16H140005) and Wenzhou Science and Technology Planning Project (Grant No: Y20150077).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Li, S., Pan, Y. Differential expression of transforming growth factor-beta1, connective tissue growth factor, phosphorylated-SMAD2/3 and phosphorylated-ERK1/2 during mouse tooth development. J Mol Hist 48, 347–355 (2017). https://doi.org/10.1007/s10735-017-9733-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-017-9733-4