Abstract
L-3,4-Dihydroxyphenylalanine (L-DOPA) is a compound with strong allelopathic effects on Brassicaceae, Asteraceae, Cucurbitaceae and Hydrophyllaceae species. Although Gramineae are less affected by L-DOPA with respect to their root growth, metabolic routes that protect them from L-DOPA toxicity are poorly understood. We identified a DOPA glucoside in maize (Zea mays L., Gramineae) roots and leaves, but DOPA aglycon was not detectable in maize. Accordingly, when maize seedlings were exposed to L-DOPA solution, DOPA glucoside concentrations increased in maize leaves, suggesting that absorbed L-DOPA is rapidly converted to glucoside conjugate. When DOPA glucoside solution was applied to lettuce seeds (Lactuca sativa; Asteraceae), lettuce radicle growth was less inhibited compared to free L-DOPA. Considering that maize radicle growth is less affected by free L-DOPA, it is likely that maize seedlings protect themselves from toxicity by L-DOPA glucosylation. Interestingly, a developmental stage dependent variation in DOPA glucoside concentration was observed with highest level of metabolite detected in L2 stage maize leaves. As DOPA glucoside also increased in maize during herbivory by the bird cherry-oat aphid (Rhopalosiphum padi L.) and the Graminae generalist armyworm (Mythimna loreyi), as well as in response to treatment with the plant hormones, we propose that DOPA glucoside might be involved in various stress responses and/or defense in maize seedlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants release various secondary metabolites into the soil, which can influence growth and development of neighboring plants, both positively and negatively. These chemical compounds are known as allelochemicals and typically suppress seed germination, reduce growth of roots and other meristems, and inhibit seedling growth. The allelochemicals are chemically diverse, including phenolic compounds, terpenoids, alkaloids and nitrogen-containing chemicals (non-protein amino acids, benzoxazinoids, cyanogenic glycosides), and many other chemical families (Macías et al. 2019).
In gramineous species, including key cereals such as maize, wheat and rye, benzoxazinoid are major secondary metabolites that show both allelopathic and pest protection properties (Niculaes et al. 2018). The key reported benzoxazinoids are 2,4-dihydroxy-(2H)-1,4-benzoxazin-3(4H)-one (DIBOA) and 2,4-dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one (DIMBOA). Notably, high concentrations of benzoxazinoids are produced in young tissues of roots and shoots, where they are glucosylated and stored in vacuoles or exuded by roots (Schulz et al. 2019). It is generally accepted that glucosylation is used to reduce the autotoxicity of benzoxazinoids as the glucosides have reduced chemical reactivity (Sicker et al. 2000).
Velvet bean [Mucuna pruriens (L.) var. utilis] is a tropical legume, cultivated as a cover crop in the tropics (Tarawali et al. 1999) and it was confirmed to exert allelopathic effects (Fujii et al. 1991). The main phytotoxic compound is the non-protein amino acid L-3,4-dihydroxyphenylalanine (L-DOPA). L-DOPA content is as much as 1% and 4 − 7% in the leaves and seeds, respectively, and L-DOPA exuded from the roots of Mucuna spp. showed strong allelochemical activity (Nishihara et al. 2005). A study on the effects of L-DOPA on different plant species revealed a variety of plant responses to L-DOPA (Nishihara et al. 2004). According to this study, Gramineae species were less affected than Leguminosae, Brassicaceae, Asteraceae, Cucurbitaceae and Hydrophyllaceae species in terms of inhibition of radicle growth. Nishihara et al. (2004) reported that only perennial grass of the tested gramineous plants can metabolize L-DOPA to dopamine, therefore metabolism of L-DOPA to dopamine was proposed as one possible detoxification mechanisms of perennial grass. However, self-protection mechanism against L-DOPA toxicity in other Gramineae species are still poorly understood.
In order to gain more information about the L-DOPA tolerance mechanisms in gramineous plants, such as glucosylation that is known to reduce the autotoxicity of benzoxizanoids, we searched for DOPA metabolites in maize seedlings exposed to L-DOPA solution. DOPA glucoside was identified in maize (Zea mays L.) roots and leaves, both with or without exposure to L-DOPA. We further examine the effect of DOPA glucoside on lettuce seeds, known to be sensitive to L-DOPA, as well as report apparent changes in maize DOPA glucoside concentration caused by plant hormone treatments or insect feeding.
Methods
Plants and growth conditions
Maize seeds (Zea mays, cv. honey bantam 610, Sakata Seed Co., Yokohama, Japan) were planted at 1 cm depth in plastic pots (5.0 cm long, 5.0 cm wide, 5.6 cm high), containing cultivation soil Naeichiban (Ranpoku Ltd., Niigata, Japan), and the pots were placed in a Biotron LH-300 cabinet (Nihon-ika Co. Ltd., Osaka, Japan) under the following conditions: 18/6 h day/night cycle, 150 μmol photons m−2 s−1 light intensity, 28 °C, and ambient humidity. Plants were watered daily. We used the ‘leaf-over’ method (OMAFRA Publication 75A, Guide to Weed Control) to distinguish between different growth stages. A leaf was counted when it had emerged from the whorl and started to arch over with the next leaf visible in the whorl but standing up straight. For example, at the two-leaf stage (L2), the plant had two leaves with the third leaf standing up straight. Unless otherwise noted, all plants used for experiments were at the L3 stage.
Insects
Mythimna loreyi were obtained from colonies maintained at Okayama University, which had originally been collected in a paddy field in Kurashiki (Okayama prefecture, Japan). M. loreyi larvae were kept on an artificial pinto bean diet until experiments. Rhopalosiphum padi L. were provided by the Zennoh Agricultural Research and Development Center (Hiratsuka), which were maintained with continuous supply of young wheat seedlings before the experiment.
Stable isotope labelling
A maize seedling at the L3 stage was cut at and the cut surface was submerged in a test rube containing 2 mL of 200 mg/L L-DOPA (phenyl-d3) (Sigma-Aldrich, St. Louis, MO, USA) solution for 3 days. The control plants were submerged in water. DOPA glucoside in the maize seedlings was analyzed by LC/MS.
DOPA and DOPA glucoside analysis
Approximately 100 mg of maize tissue was weighted in 2 mL tubes and ground in 200 μL of 50% methanol with 3 mm steel balls using a μT-12 beads crusher shaker (Taitec Co., Saitama, Japan). The extracts were centrifuged at 10,000 × g for 5 min and the supernatants were derivatized using 6-aminoquinolyl-N-hydroxysuccinimidyl (AQC) reagent, a highly reactive amine derivatizing reagent. AQC was synthesized as described by Cohen and Michaud (1993). Plant extracts (10 μL) were mixed with 65 μL of 200 mM borate buffer (pH 8.8) and 5 μL of 1 μmol/mL taurine (internal standard, Fujifilm Wako Pure Chemical, Osaka, Japan). The reaction was initiated by adding of 20 μL of 3 mg/mL AQC reagent in acetonitrile, followed by immediate mixing and incubation for 10 min at 55 °C. Five microliters of each sample was analyzed by LC/MS. The amounts of DOPA glucoside and amino acids were calculated using calibration curves obtained from purified DOPA glucoside and commercially available standards. L-DOPA, tyrosine, and phenylalanine standards were purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan).
LC/MS
Each sample was injected into a Mightysil RP-18 GP II column (50 × 2.0 mm, Kanto Chemical, Tokyo, Japan), followed by separation using a Waters Aquity system (Waters, Milford, MA, USA). Solvent A was water containing 0.1% formic acid and solvent B was acetonitrile containing 0.1% formic acid. The gradient was 0–3 min, 1% B, 3–12 min, linear gradient to 40% B, 12–13 min, linear gradient to 99% B, 13–17 min, 99% B, 17–18 min, linear gradient to 1% B, 18–22 min, 1% B. The flow rate was 0.2 mL/min, and column temperature was 40 °C. Mass spectra were recorded on a Synapt G2 HDMS instrument (Waters, Milford, MA, USA). Mass spectrometer (MS) data acquisition was performed using the following conditions: ESI-positive ionization mode, 2.0 kV capillary voltage, 30 V cone voltage, 550 °C desolvation temperature, 90 L/h desolvation gas flow, and 120 °C source temperature. Mass spectra were scanned for m/z 150–800 in MS mode and m/z 100–800 in MS/MS mode.
DOPA glucoside purification
Approximately 1.0 kg of fresh maize leaves were used for extraction with 5 L of 50% methanol. The volume of the extraction liquid was reduced to approximately 50 mL by rotary evaporation. The viscous extract was suspended in 150 mL water and was extracted thrice using 150 mL of ethyl acetate. The aqueous layer was reduced to approximately 20 mL through rotary evaporation and was loaded on a DOWEX 50 column chromatography (H+ type, 200–400 mesh, 4.7 × 17 cm, Wako Pure Chemical Industries, Osaka), which was washed with 2 L of water. The amine fraction eluted from the DOWEX 50 column using 2 L of 2 M ammonium hydroxide was evaporated in vacuo, and dissolved in 75% acetonitrile/water. A portion of the amine fraction (20 µL) was injected into an Asahipak NH2-50 4E column (250 × 4.6 mm, 5 μm, Shodex, Showa Denko K.K., Tokyo, Japan) and was separated using a PU715 pump (GL Science, Tokyo, Japan) with an isocratic mobile phase of acetonitrile/water containing 50 mM ammonium formate (75/25) and a flow rate of 1 mL/min. The DOPA glucoside was isolated at Rt = 33.0 min. High performance liquid chromatography (HPLC) was performed at 40 °C and was monitored using a UV702 UV/VIS detector (GL Science, Tokyo, Japan). The target peaks were collected by hand and samples from multiple HPLC runs were pooled and dried under vacuum. This process was repeated until approximately 5 mg of the target compound was obtained. For nuclear magnetic resonance (NMR) structural determination, 5 mg of the compound purified from maize tissue was dissolved in 500 µL of deuterated methanol (CD3OD) and was analyzed using a JEOL ECZ-600 spectrometer at 600 MHz (JEOL, Tokyo, Japan). Chemical shifts are shown on a δ (ppm) scale with tetramethylsilane as an internal standard. DOPA glucoside: 1H-NMR (600 MHz, CD3OD) β-glucose: δ 4.92 (1H, d, J = 6.6 Hz), 3.94 (1H, d, J = 10.8), 3.72 (1H, m) 3.54 (3H, m), 3.42 (1H, m), DOPA: 7.10 (1H, d, J = 1.2 Hz), 6.88 (1H, dd, J = 8.4, 1.2), 6.85 (1H, d, J = 8.4 Hz), 3.80 (1H, dd, J = 7.8, 4.8), 3.17 (1H, dd, J = 15, 4.8), 2.99 (1H, dd, J = 15, 7.8). 13C-NMR (125 MHz, CD3OD): δ 168.7, 144.9, 144.5, 126.7, 123.7, 117.0, 115.7, 101.1, 76.0, 75.1, 72.6, 69.2, 60.2, 55.4, 35.3. These spectral data were consistent with those reported by Sivakumar et al. (2009).
After NMR analysis, 1 mg of DOPA glucoside was hydrolyzed with 5% hydrochloric acid/methanol (1 mL) for 4 h at 80 °C. The solution was neutralized with sodium carbonate and was separated by thin layer chromatography (TLC, silica gel 60 F254, Merck, Darmstadt, Germany) using butanol/acetic acid/water (4:1:5 upper layer) as the eluent. The sugar moiety was identified as glucose by comparing its Rf value with that of the reference sample. Spots were detected by spraying with 10% vanillin in sulfuric acid followed by heating.
Effect of L-DOPA and DOPA glucoside radicle growth on maize and lettuce seeds
Five seeds of maize or lettuce (Lactuca sativa, cv. Melbourne MT) were placed on a filter paper (No. 2, 90 mm, Advantec, Tokyo, Japan) in 90-mm diameter Petri dishes containing 4 mL of distilled water (control), L-DOPA solution (10, 50, or 200 mg/L) or DOPA glucoside solution (10, 50, or 200 mg/L). Each dish was placed in a Biotron LH-300 cabinet at 25°C under dark conditions for three days. Radicle length of each plant was measured, and DOPA, DOPA glucoside and other amino acids were extracted from the roots of each plant using 50% methanol, followed by LC/MS analyses.
Herbivory treatments
In the herbivory treatments, three second-instar larvae of M. loreyi were starved for at least 3 h and then placed on the leaves of a maize seedling at the L3 stage, which was then maintained in a plastic container for two days. The area within 0.5 cm around the feeding wounds were used for analyses. The control plant was put in a container without larvae. For aphid feeding treatments, ten wingless adult aphids (R. padi) were placed on the leaves of a maize seedling at the L3 stage, which was then maintained in a plastic container for four days, and the leaves were subsequently collected for analyses. The control plant was placed in a container without aphids.
Plant hormone treatments
For the plant hormone treatments, leaves of a maize seedling at the L3 stage were sprayed with 1 mM jasmonic acid (JA), salicylic acid (SA), or 1-aminocyclopropane-1-carboxylic acid (ACC, an ethylene metabolic precursor) in distilled water four times during two days and the control plants were treated with distilled water. Leaves were collected for analysis 48 h after the first spraying. For the measurement of DOPA glucoside at each growth stage, maize leaves at the L2 to L5 stages were sprayed with 1 mM JA in water four times during two days.
Herbivore bioassays
To increase endogenous DOPA glucoside concentration, a maize seedling at the L3 stage was cut at the stem and the cut surface was submerged in a test tube containing 2 mL of 50 or 200 mg/L L-DOPA solution. The control plants were submerged in water. The concentrations of L-DOPA and DOPA glucoside in the maize seedlings were analyzed using LC/MS on days 1, 2, 3, and 4 after the start of submergence. One day after the start of submergence, three adult aphids were placed in a tube containing a maize seedling in L-DOPA solution, which was then covered with a nylon net. Aphid nymphs were counted for three days.
An M. loreyi larva was placed in a 120-mL plastic cup containing maize leaves. The leaves used for feeding were submerged in 50 or 200 mg/L DOPA glucoside solution for one day before feeding. Larval weights were measured on days 1, 3, and 5 after the start of feeding.
Statistical analysis
Statistical analyses were performed with Microsoft Excel (for Student’s t tests; Microsoft Corporation, Redmont, WA, USA) or JMP statistical software (for Tukey–Kramer tests; SAS Institute Inc., Cary, NC, USA).
Results
Identification of DOPA glucoside accumulation in maize leaves and roots
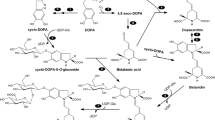
We searched for DOPA and related metabolites, based on selective derivatization of primary and secondary amino groups by AQC, in maize leaves submerged in 200 mg/L L-DOPA solution for 3 days. We found a previously unknown peak of m/z 530 at Rt = 6.6 min in positive ionization mode. This unknown peak was also detected in untreated leaves (Fig. 1), however, it was more abundant in L-DOPA-treated than in untreated leaves. The m/z value was consistent with that of the [M + H]+ ion of DOPA glycoside derivatized with AQC. The MS/MS spectrum of the precursor ion at m/z 530 yielded a product ion at m/z 368 [(M + H)−162]+, corresponding to the loss of a hexose group (Supplementary Fig. 1). To the best of our knowledge, DOPA glycoside has not yet been reported in maize. Therefore, we purified DOPA glycoside from maize leaves for structural identification. 1H- and 13C-NMR spectral data of the purified DOPA glycoside were similar to those of DOPA-3- β-O-glucoside or DOPA-4-β-O-glucoside reported by Sivakumar et al. (2009) (Supplementary Fig. 2). However, we were unable to determine whether the glycosidic linkage position was O-3 or O-4 because of insufficient resolution in the HMBC (Supplementary Fig. 3). Using TLC, the sugar moiety of the purified DOPA glycoside was identified as glucose. LC/MS analysis showed that the purified DOPA glucoside was identical to the natural maize metabolite. DOPA in its free state was not detected in maize (Fig. 1).
a Total ion chromatogram (TIC) of the untreated maize leaf extracts derivatized with AQC. b Extracted ion chromatogram (EIC) of derivatized DOPA glucoside (m/z 530) in maize leaf extract and structure of DOPA glucoside. c EIC of m/z 368 of untreated maize leaf extract. d EIC of derivatized L-DOPA standard (m/z 368)
Figure 2a shows the concentration of DOPA glucoside in maize leaves and roots without L-DOPA treatment. When maize leaves were submerged in L-DOPA solution, the DOPA glucoside concentration increased (Fig. 2b). We also analyzed maize leaves submerged in 200 mg/L L-DOPA (phenyl-d3) solution for 3 days. LC/MS analysis showed the accumulation of d3-labeled DOPA glucoside in response to the uptake of isotope labeled L-DOPA solution (Supplementary Fig. 4). All evidence so far suggested that absorbed L-DOPA is metabolized to a glucoside conjugate but physiological function of this conversion was not yet clear.
a Concentrations of DOPA glucoside in untreated maize leaves and roots. The results are expressed as means ± SEM (n = 4). b Concentrations of DOPA glucoside in maize leaves submerged in 0, 50, or 200 mg/L L-DOPA solution. Results are expressed as means ± SEM (n = 3). Different letters indicate statistically significant differences between treatments according to a Tukey–Kramer test (P < 0.05)
Effects of L-DOPA and DOPA glucoside on maize and lettuce radicle growth
To examine the effect of DOPA glucoside on radicle growth, lettuce and maize seeds were treated with purified DOPA glucoside (Fig. 3a). Susceptibility to phytotoxicity of L-DOPA varies among plant species, with maize showing resistance, but lettuce being more sensitive to L-DOPA than maize (Hachinohe et al. 2004). In maize seeds, radicle growth was not inhibited by L-DOPA treatment, but was slightly promoted by treatment with 50 or 200 mg/L DOPA glucoside. In lettuce, L-DOPA or DOPA glucoside inhibited radicle growth, however, the extent of suppression by DOPA glucoside was lower than by L-DOPA. These results show that the toxicity of L-DOPA can be reduced by glucosylation that rapidly occurs in the maize plant (Fig. 3b).
a Changes in radicle length of maize and lettuce seeds treated with 10, 50, 200 mg/L L-DOPA or DOPA glucoside for three days. Results are expressed as mean ± SD (n = 5). Different letters show statistically significant differences between treatments according to a Tukey–Kramer test (P < 0.05). b Concentrations of DOPA glucoside, phenylalanine, and tyrosine in the maize seeds treated with L-DOPA and DOPA glucoside for three days. Results are expressed as means ± SEM (n = 5). Asterisks indicate statistically significant differences compared to control (H2O) by Student's t-test (*P < 0.05, **P < 0.01)
Detoxification by glycosidation has been reported for secondary metabolites including benzoxazinoids, but promotion of root growth observed after application of DOPA glucoside is more difficult to explain (Fig. 3a). To get a clue as to why maize radicle growth was slightly promoted by DOPA glucoside, we analyzed DOPA and other amino acids in maize radicles subjected to L-DOPA and DOPA glucoside treatments (Fig. 3b). The concentration of DOPA glucoside in maize radicles increased due to L-DOPA treatment but did not change after DOPA glucoside treatment. As before, DOPA in its free state was not detected in maize radicles treated with L-DOPA or DOPA glucoside. We found the concentration of phenylalanine and tyrosine increased in maize radicles exposed to 50 or 200 mg/L DOPA glucoside (Fig. 3b).
DOPA glucoside formation in maize leaves induced by insect feeding and plant hormone treatment
As DOPA has been reported to be associated with insecticidal properties of legume seeds (Rehr et al. 1973), and other monocot metabolites such as benzoxazinoids function both in allelopathy and pest protection, we investigated whether DOPA glucoside accumulation would be induced by insect feeding. The level of DOPA glucoside was increased by caterpillars (M. loreyi; Fig. 4a) and aphids (R. padi; Fig. 4b) feeding. Because many maize defense responses are regulated by plant hormones, we also investigated whether DOPA glucoside accumulation would be affected by treatment with plant hormones related to stress and defense responses. DOPA glucoside levels increased after treatment with JA, SA, and ACC, and there was no significant difference among the three hormones (Fig. 5a). The concentration of DOPA glucoside was the highest in L2 stage leaves and decreased with plant ages (Fig. 5b). In order to know the amount of DOPA glucoside induction at each growth stage, maize leaves at each stage were treated with JA used as a representative hormone. The amount of DOPA glucoside induced by 1 mM JA decreased with plant age. In L5 stage leaves, DOPA glucoside was not detected even after JA treatment.
Induction of DOPA glucoside formation in maize leaves treated with M. loreyi feeding (a) and R. padi feeding (b). a Third instar larvae of M. loreyi (n = 3) were fed with maize leaves of L3 stage plants for two days. The control plant was placed in a container without larvae. b Five adult aphids were applied to the maize plant of L3 stage for four days. A maize plant without aphid treatment was used as a control. After each treatment, maize leaves were analyzed by LC/MS. Results are expressed as means ± SEM (n = 5). Asterisks indicate statistically significant differences to control according to Student's t-test (*P < 0.05)
a Induction of DOPA glucoside formation in leaves sprayed with water (control), 1 mM JA, SA, and ACC four times during two days. Maize leaves were extracted two days after the first spraying. (b) DOPA glucoside levels in maize leaves at different growth stages (L2–L5) were analyzed following JA or water (control) spray. After each treatment, maize leaves were analyzed by LC/MS. Results are expressed as mean ± SEM (n = 5). Significant differences to control in a Student's t-test are indicated by an asterisk (*P < 0.05, **P < 0.01)
DOPA glucoside effects on insects
To examine the effects of DOPA glucoside on insects, we prepared maize leaves with higher DOPA glucoside accumulation than what occurs naturally. Figure 2b shows that submergence of cut maize seedlings in 50 or 200 mg/L L-DOPA solution elevated the endogenous leaf levels of DOPA glucoside to more than two-fold, compared to untreated maize leaves at the L3 stage. These leaves were used to examine the direct effects of DOPA glucoside on R. padi and M. loreyi. Despite several efforts, reproduction of R. padi and growth of M. loreyi larvae was not significantly affected by increased DOPA glucoside levels (Supplementary Fig. 5). No visual changes were observed in aphids and caterpillars used in the DOPA glucoside experiment.
Discussion
We identified a DOPA glucoside in maize leaves and roots. DOPA glucoside has been reported as a compound involved in the browning of broad bean (Vicia faba) (Nagasawa et al. 1961; Andrew and Pridham 1965), but it was found here from maize for the first time. In our experiments, radicle growth inhibition by purified DOPA glucoside was weaker than that by L-DOPA. Therefore, maize seedlings are thought to protect themselves from the toxicity of L-DOPA by its glucosylation. In plants, many secondary metabolites are stored as an inactive glucoside form in vacuoles to avoid toxicity to the plant itself (Shitana and Yazaki 2020; Wari et al. 2022). Phytotoxicity of L-DOPA is thought to occur due to oxidative damage caused by reactive oxygen species generated in the melanin synthesis pathway (Hachinohe and Matsumoto 2005, 2007; Matsumoto 2011). In the early stage of melanogenesis, oxidation of L-DOPA to DOPA ortho-quinone is catalyzed by cytosolic polyphenol oxidase (PPO) or autooxidation. Offen et al. (2001) reported that methylation of the 3-OH group by catechol-O-methyltransferase decreased L-DOPA toxicity to nerve cells in vitro. Glucosylation of the catechol structure may inhibit the formation of the toxic DOPA ortho-quinone. Matsumoto (2011) also described that susceptibility of plants to phytotoxicity of L-DOPA may differ between species due to differential activity of PPOs. Plant species with high PPO activity introduce more L-DOPA into melanin biosynthesis and generate larger amounts of toxic reactive oxygen species, whereas species with lower PPO activity metabolize L-DOPA to other non-toxic metabolites and introduce less L-DOPA to melanin synthesis. The former ones are thus sensitive to L-DOPA toxicity and the latter are resistant. We propose that glucosylation may also contribute to reduction in L-DOPA toxicity.
In the present study, DOPA glucoside slightly promoted maize radicle growth. L-DOPA incorporated in maize tissues as a glucoside may be converted to aromatic amino acids and promote root growth as a nitrogen source. Hachinohe et al. (2004) confirmed the metabolism of L-DOPA to tyrosine and phenylalanine in barnyard grass and lettuce by using 14C-L-DOPA. However, the concentrations of these aromatic amino acids did not increase during exposure to L-DOPA. Whether these amino acids are increased by L-DOPA exposure depends on the plant species. In cucumber roots treated with L-DOPA, the amounts of tyrosine and phenylalanine increase (Nakajima et al. 1999). Growth of cucumber seedlings were inhibited immediately after L-DOPA treatment, but recovered rapidly thereafter, and the inhibition did not last. Nakajima et al. (1999) speculated that L-DOPA absorbed in the cucumber seedlings was detoxified by being converted to aromatic amino acids, and growth was recovered. Considering that growth of lettuce radicle is still inhibited by L-DOPA, although L-DOPA is converted to tyrosine and phenylalanine in lettuce (Hachinohe et al. 2004), L-DOPA may not be detoxified enough because of low conversion of L-DOPA to aromatic amino acids.
In the current study, the increase in tyrosine and phenylalanine levels in maize after DOPA glucoside treatment exceeded that after L-DOPA treatment. It is tempting to speculate that glycosylation of L-DOPA may be promoting its conversion to aromatic amino acids, although the possible mechanisms remain elusive. Tyrosine and phenylalanine are precursors of various compounds, such as phenylpropanoids, flavonoids, lignin, and tannins, which are involved in plant growth. For example, poplars fertilized with phenylalanine show increased root growth (Jiao et al. 2018).
L-DOPA is present in velvet bean (Mucuna pruriens) seeds at high concentrations and it is proposed to serve as a chemical barrier to insect attack (Rehr et al. 1973). Furthermore, L-DOPA concentration was found to be highly correlated with winter wheat resistance to grain aphids (Sitobion avenae) (Ciepiela and Sempruch 1999). In the current study, DOPA glucoside levels in maize leaves increased by caterpillar and aphid feeding, however, no effect of DOPA glucoside on insect growth was observed. We assume that DOPA glucoside may not be toxic to insects. For instance, catecholamines that serve as precursors for quinone sclerotizing agents of insect cuticles or tanned structures are often stored in the hemolymph as glucose conjugates (Brunet 1963; Hopkins et al. 1995). We also cannot exclude that basal levels of DOPA glucoside in maize exert maximal toxicity and elevated levels have no further impact on the insects. DOPA glucoside-free maize will be necessary to test this alternative hypothesis.
In addition to L-DOPA, catecholamines such as dopamine, epinephrine, and norepinephrine have been found in many plant species and their biosynthesis is regulated by stress (Kulma and Szopa 2007). For example, dopamine levels decrease during heat stress in the subantarctic crucifer Pringlea antiscorbutica (Hennion and Martin-Tanguy 2000), and dopamine, epinephrine and norepinephrine levels increase in potato leaves 5 min after wounding (Szopa et al. 2001). Other stressful conditions such as drought, abscisic acid treatment and UV treatment, significantly increase dopamine levels in potato plants (Swiedrych et al. 2004). In the present study, DOPA glucoside concentrations in maize leaves were also increased due to plant hormone treatments. The physiological role of endogenous DOPA glucoside in maize seedlings (not treated with L-DOPA) is currently unknown, however, DOPA glucoside may be involved in the early growth stress responses. Further research is required to elucidate potential correlations between stress protection and DOPA glucoside.
References
Andrews RS, Pridham JB (1965) Structure of a Dopa glucoside from Vicia faba. Nature 205:1213–1214
Brunet PCJ (1963) Tyrosine metabolism in insects. Ann N Y Acad Sci 15:1020–1034
Ciepiela AP, Sempruch C (1999) Effect of L-3,4-dihydroxyphenylalanine, ornithine and γ-aminobutyric acid on winter wheat resistance to grain aphid. J Appl Entomol 123:285–288. https://doi.org/10.1046/j.1439-0418.1999.00356.x
Cohen SA, Michaud DP (1993) Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem 211:279–287. https://doi.org/10.1006/abio.1993.1270
Fujii Y, Shibuya T, Yasuda T (1991) L-3,4-Dihydroxyphenylalanine as an allelochemical candidate from Mucuna pruriens (L.) DC. var. utilis. Agric Biol Chem 55:617–618. https://doi.org/10.1271/bbb1961.55.617
Hachinohe M, Matsumoto H (2005) Involvement of reactive oxygen species generated from melanin synthesis pathway in phytotoxicity of L-DOPA. J Chem Ecol 31:237–246. https://doi.org/10.1007/s10886-005-1338-9
Hachinohe M, Matsumoto H (2007) Mechanism of Selective phytotoxicity of l-3,4-dihydroxyphenylalanine (L-DOPA) in barnyardglass and lettuce. J Chem Ecol 33:1919–1926. https://doi.org/10.1007/s10886-007-9359-1
Hachinohe M, Sunohara Y, Matsumoto H (2004) Absorption, translocation and metabolism of L-DOPA in barnyardgrass and lettuce: their involvement in species-selective phytotoxic action. Plant Growth Regul 43:237–243. https://doi.org/10.1023/B:GROW.0000045996.72922.1b
Hennion F, Martin-Tanguy J (2000) Amines of the subantarctic crucifer Pringlea antiscorbutica are responsive to temperature conditions. Physiol Plant 109:232–243. https://doi.org/10.1034/j.1399-3054.2000.100303.x
Hopkins TL, Morgan TD, Mueller DD, Tomer KB, Kramer KJ (1995) Identification of catecholamine β-glucosides in the hemolymph of the tobacco hornworm, Manduca sexta (L.), during development. Insect Biochem Mol Biol 25:29–37. https://doi.org/10.1016/0965-1748(94)00043-H
Jiao Y, Chen Y, Ma C, Qin J, Nguyen THN, Liu D, Gan H, Ding S, Luo Z (2018) Phenylalanine as a nitrogen source induces root growth and nitrogen-use efficiency in Populus × canescens. Tree Physiol 38:66–82. https://doi.org/10.1093/treephys/tpx109
Kulma A, Szopa J (2007) Catecholamines are active compounds in plants. Plant Sci 172:433–440. https://doi.org/10.1016/j.plantsci.2006.10.013
Macías FA, Mejías FJR, Molinillo JMG (2019) Recent advances in allelopathy for weed control: from knowledge to applications. Pest Manag Sci 75:2413–2436. https://doi.org/10.1002/ps.5355
Matsumoto H (2011) The mechanisms of phytotoxic action and selectivity of non-protein aromatic amino acids L-DOPA and m-tyrosine. J Pestic Sci 36:1–8. https://doi.org/10.1584/jpestics.R10-15
Nagasawa T, Takagi H, Kawakami K, Suzuki T, Sahashi Y (1961) Studies on the browning compounds of broad bean Vicia faba L. Agr Biol Chem 25:441
Nakajima N, Hiradate S, Fujii Y (1999) Characteristics of growth inhibitory effect of L-3, 4-dihydroxyphenylalanine (L-DOPA) on cucumber seedlings. J Weed Sci Tech (in Japanese) 44:132–138. https://doi.org/10.3719/weed.44.132
Niculaes C, Abramov A, Hannemann L, Frey M (2019) Plant Protection by Benzoxazinoids—Recent Insights into Biosynthesis and Function. Agronomy 8:143. https://doi.org/10.3390/agronomy8080143
Nishihara E, Parvez MM, Araya H, Fujii Y (2004) Germination growth response of different plant species to the allelochemical L-3,4-dihydroxyphenylalanine (L-DOPA). Plant Growth Regul 42:181–189. https://doi.org/10.1023/B:GROW.0000017483.76365.27
Nishihara E, Parvez M, Araya H, Kawashima S, Fujii Y (2005) L-3-(3,4-Dihydroxyphenyl)alanine (L-DOPA), an allelochemical exuded from velvetbean (Mucuna pruriens) roots. Plant Growth Regul 45:113–120. https://doi.org/10.1007/s10725-005-0610-x
Offen D, Panet H, Galili-Mosberg R, Melamed E (2001) Catechol-O-methyltransferase decreases levodopa toxicity in vitro. Clin Neuropharmacol 24:27–30
Rehr SS, Janzen DH, Feeny PP (1973) L-DOPA in legume seeds: a chemical barrier to insect attack. Science 181:81–82
Schulz M, Schütz V, Bigler L, Sicker D, Laschke L (2019) Conversions of benzoxazinoids and downstream metabolites by soil microorganisms. Front Ecol Evol 7:238. https://doi.org/10.3389/fevo.2019.00238
Shitana S, Yazaki K (2020) Dynamism of vacuoles toward survival strategy in plants. Biochim Biophys Acta Biomembr 1862:183127. https://doi.org/10.1016/j.bbamem.2019.183127
Sicker D, Frey M, Schulz M, Gierl A (2000) Role of natural benzoxazinones in the survival strategy of plants. Int Rev Cytol 198:319–346. https://doi.org/10.1016/S0074-7696(00)98008-2
Sivakumar R, Ponrasu T, Divakar S (2009) Syntheses of dopa glycosides using glucosidases. Glycoconj J 26:199–209. https://doi.org/10.1007/s10719-008-9176-y
Swiedrych A, Lorenc-Kukula K, Skirycz A, Szopa J (2004) The catecholamine biosynthesis route in potato is affected by stress. Plant Physiol Biochem 42:593–600. https://doi.org/10.1016/j.plaphy.2004.07.002
Szopa J, Wilczynski G, Fiehn O, Wenczel, a., and Willmitzer, L. (2001) Identification and quantification of catecholamines in potato plants (Solanum tuberosum) by GC–MS. Phytochemistry 58:315–320. https://doi.org/10.1016/S0031-9422(01)00232-1
Tarawali G, Manyong VM, Carsky RJ, Vissoh PV, Osei-Bonsu P, Galiba M (1999) Adoption of improved fallows in West Africa: lessons from mucuna and stylo case studies. Agrofor Syst 47:93–122. https://doi.org/10.1023/A:1006270122255
Wari D, Aboshi T, Shinya T, Galis I (2022) Integrated view of plant metabolic defense with particular focus on chewing herbivores. J Integr Plant Biol 64:449–475. https://doi.org/10.1111/jipb.13204
Acknowledgements
We thank Mr. Hitomi Miyazaki (Zennoh Agricultural Research and Development Center) for providing R. padi. This study was supported by the Joint Research Program of the Institute of Plant Science and Resources at Okayama University, by a Grant-in-Aid for Scientific Research (No. 18K14397 to T.A., No. 21H02196 to I.G.) from the Japan Society for the Promotion of Sciences.
Funding
Japan Society for the Promotion of Science,18K14397,Takako Aboshi, 21H02196, Ivan Galis,Joint Research Program of the Institute of Plant Science and Resources at Okayama University, 3026, Takako Aboshi
Author information
Authors and Affiliations
Contributions
TA, KI and IG contributed to the study’s design. Data collection and analysis were performed by TA and KI with the aid of TS and TM. The first draft of the manuscript was written by TA with the aid of IG. All authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Additional information
Communicated by Zhong-Hua Chen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aboshi, T., Ittou, K., Galis, I. et al. Glucosylation prevents autotoxicity of stress inducible DOPA in maize seedlings. Plant Growth Regul 101, 159–167 (2023). https://doi.org/10.1007/s10725-023-01009-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01009-w