Abstract
Arabidopsis TEMPRANILLO 1 (AtTEM1) is a member of the RAV transcription factor subfamily and has plant-specific AP2 and B3 domains. This study demonstrated that AtTEM1 is localized in nuclei. Expression of AtTEM1 was discovered to be high in the flower, stem, rosette, and cauline leaf, but low in the silique and root tissue, and was strongly induced by drought and mannitol treatments. Compared with wild-type plants, the 35S::AtTEM1 plants bolted later and produced more rosette leaves before flowering, whereas, the AtTEM1 mutant plants (tem1) bolted earlier but produced an almost equal number of rosette leaves before flowering. Drought tolerance was significantly reduced in the 35S::AtTEM1 transgenic Arabidopsis plants, but was enhanced in the tem1 plants. Under drought and mannitol stresses, the 35S::AtTEM1 transgenic Arabidopsis exhibited increased relative electrical conductivity (REC), increased malondialdehyde (MDA) content, and decreased proline content compared with the wild type, whereas lower REC and MDA content and higher proline content were found in the tem1 plants. These results suggest that AtTEM1 in Arabidopsis functions as a negative regulator in response to drought and mannitol stresses. Therefore, AtTEM1 transcription factor is proposed to be a versatile regulator reacting negatively to both flowering time and drought stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants cannot escape unfavourable environmental conditions within their typical life cycle (Kang et al. 2011) and are under stresses when their natural environment deteriorates, such as when there is a drought (Achard et al. 2008). Environmental stresses can severely damage the cellular structure of plants and impair their physiological function (Larcher 2003). To survive adverse environmental conditions, plants develop stress tolerance mechanisms to maintain growth and reproduction (Sato et al. 2014). Many stress signals triggered in plants can cause biochemical and physiological changes, resulting from stress-induced modulation of gene expression (Achard et al. 2008).
The B3 transcription factor family is a large group of plant-specific transcription factors involved in controlling the primary and secondary metabolism, growth and developmental programmes, and responses to environmental stimuli (Licausi et al. 2013). In Arabidopsis, these transcription factors are encoded by 118 genes that can be classified into four major subfamilies: LAV (LEAFY COTYLEDON2 [LEC2]–ABSCISIC ACID INSENSITIVE3 [ABI3]–VAL), ARF (AUXIN RESPONSE FACTOR), RAV (RELATED TO ABI3 and VP1) and REM (REPRODUCTIVE MERISTEM) (Swaminathan et al. 2008).
Some RAV subfamily transcription factors have been functionally characterized, including RAV1, RAV1-like, RAV2/TEMPRANILLO2 (TEM2) and TEM1. Overexpression of RAV1 can reduce the number of lateral roots and rosette leaves, indicating that RAV1 acts as a negative regulator during plant development (Hu et al. 2004). TEM transcription factors are involved in controlling the flowering time for photoperiodic induction. TEM genes have a pivotal role in the direct repression of FLOWERING LOCUS T (FT), which links photoperiod and gibberellin pathways to control flowering in Arabidopsis (Osnato et al. 2012). Overexpression of TEM1 or TEM2/RAV2 in Arabidopsis was demonstrated to delay its flowering time for several weeks by directly repressing FT expression (Castillejo and Pelaz 2008). Brassinosteroids (BR) was revealed through cDNA microarray analysis to possibly regulate RAV1 through a BRI1-independent signal pathway in Arabidopsis. RAV1 may function as a negative regulatory component of growth and development (Hu et al. 2004). Furthermore, transgenic cotton coexpressing the Arabidopsis B3-domain transcription factor AtRAV2 and basic leucine zipper (bZIP) AtABI5 is highly resistant to osmotic and drought stress (Mittal et al. 2014). Overexpression of pepper RAV1 (CARAV1) in Arabidopsis can enhance its ABA sensitivity and its resistance to bacterial pathogens, salt and drought stress (Sohn et al. 2006). AtTEM1 is annotated as an AP2 and B3 domain-containing transcription factor. Down-regulation of AtTEM1 can result in early flowering (Castillejo and Pelaz 2008). The present study analyzed the Arabidopsis AtTEM1 spatial mRNA expression pattern and subcellular localization, and its effect on plant drought response. The results demonstrated that AtTEM1 localizes to the cell nucleus and is expressed at low levels in the silique and root tissue, acts as a negative regulator of flowering time and drought derived stress response.
Materials and methods
Plant material and transformation
Arabidopsis thaliana ecotype Columbia and its transgenic plants were grown at 22 °C, with a day length of 12 h and a light intensity of 70 micromoles of photons per square meter per second, in plastic bowls (φ = 9 cm) containing a steam-sterilized soil mixture (peat moss, perlite, loam soil, and vermiculite: 5:3:2:1, v/v/v/v).
The T-DNA insertion mutants tem1-1 and tem1-2 were obtained from the Arabidopsis Biological Resource Center and were identified as homozygous using the polymerase chain reaction (PCR) with the primers SALK-F: (5′-AGACTTGACCCTACTCCTCTGA-3′) and SALK-R: (5′-GTCGTCGTTGTCGGCTTT-3′).
To construct plasmids for generating AtTEM1-overexpressing Arabidopsis plants, the DNA fragment containing the AtTEM1 coding region was cloned into the polylinker sites of the plant expression vector pCAMBIA1301m under the control of the CaMV 35S promoter by using the primers TEM1-35-F: (5ʹ-CCCGGGACCCATTTCTTCTTCTTT-3ʹ) and TEM1-35-R: (5ʹ-CAGCTGGGAGGAATTAGATTATTAGAAC-3ʹ). The plasmid pCAMBIA1301m: AtTEM1 was introduced into Agrobacterium tumefaciens strain GV3101 and transformed into Arabidopsis using the floral dip method (Clough and Bent 1998). Transformants were selected by planting the seeds of the transformed plants in Murashige and Skoog (MS) medium containing 50 mg L−1 hygromycin B (Murashige and Skoog 1962). After 2 weeks, the hygromycin B-resistant seedlings were transplanted into soil.
Spatial expression pattern of AtTEM1 in Arabidopsis thaliana
Total RNA was extracted from roots, stems, rosette leaves, cauline leaves, inflorescences, and pods throughout the growth period. The Applied Biosystems Veriti™ 96-well thermal cycler was used for performing reverse transcription PCR (RT-PCR).
Transient expression of AtTEM1 in onion epidermal cells
For the GFPS65T:AtTEM1 construction, the AtTEM1 coding region was amplified through PCR by using the primers TEM1-G-F (5ʹ-GCTCTAGAATGGAATACAGCTGTGTAGACG-3ʹ) and TEM1-G-R (5ʹ-GGATCCCGTCACAAGATGTTGATAATCGCC-3ʹ) to introduce XbaI at the 5ʹ end and BamHI at the 3ʹ end. The resulting fragment was digested with XbaI and BamHI and ligated in frame at the 3ʹ end of a green fluorescent protein (GFP) linearized with XbaI and BamHI. The Agrobacterium tumefaciens-mediated transfection method was used for the transient expression of GFPS65T:AtTEM1 in the epidermal cells of the onion (Allium cepa L.) (Eady et al. 2000; Sun et al. 2007). The subcellular localization of the GFP fusion construct in the onion epidermal cells was monitored using an upright fluorescence microscope (Olympus BX51 Clone).
Treatment for growth development
Arabidopsis thaliana ecotype Columbia and the transgenic plants were germinated on 1/2 MS agar for 1 week and then transplanted into soil at 22 °C under long day (LD) conditions (16 h) under a normal watering regime.
Drought and mannitol stress treatments
For the drought tolerance treatment, Arabidopsis seeds were germinated on 1/2 MS agar for 1 week, after which the seedlings were transplanted into soil and grown under a normal watering regime for 3 weeks. Watering was then withholded for 14 days. When wild-type (WT) plants exhibited the lethal effects of dehydration, watering was resumed, and the plants were allowed to grow for a further 7 dYS. The survival rate was scored. All experiments were repeated at least three times.
To estimate mannitol stress tolerance, Arabidopsis seeds were placed on 1/2 MS agar plates containing 400 mM mannitol for the osmosis germination treatment. For seedling mannitol stress, WT and AtTEM1-transgenic 4-week-old Arabidopsis seedlings were planted in a soilless environment containing 400 mM mannitol and cultured under light for 12 h at 22 °C.
Measurements of REC, MDA and proline content
For the measurement of relative electrical conductivity (REC), malondialdehyde (MDA) and proline content, four-week-old seedlings were used for drought treatment, and the rosette leaves of the transgenic and WT plants during the treatment stage were harvested for the assays. The leaf REC was measured using the method described by Yu et al. (2006). MDA content was measured according to the method of Kuk et al. (2003). Free proline content was measured in acidic extracts and quantified spectrophotometrically using the acid–ninhydrin reagent with proline as a standard (Bates et al. 1973). The treatment was repeated for three times and each sample was assayed with three replicates, the standard deviations were calculated (Cheng et al. 2012).
Real-time PCR analysis
In the real-time PCR analysis treatment, Arabidopsis seeds were germinated on 1/2 MS agar for 1 week. The seedlings were then transplanted into soil and grown under a normal watering regime for 3 weeks. Watering was then withholded for 24 h and the total RNA was extracted from rosette leaves.
The expression of AtTEM1 and drought-responsive genes was analyzed by real-time PCR using the following primers: FT-F (5′-CAACCCTCACCTCCGAGAATAT-3′) and FT-R (5′-TGCCAAAGGTTGTTCCAGTTGT-3′); RD22-F (5′-TAGGAGTCGGTAAAGGCGGT-3′) and RD22-R (5′-CATCGGTGCGTTCTTCTTAGC-3′); P5CS-F (5′-CCAGCTGAGCCCAACAGTGACC-3′) and P5CS-R (5′-CAGTCGGGCAGCCAGGCTATCATTATC-3′); PDH-F (5′-TCACAACCACTGAGCTAAAGTGAGA-3′) and PDH-R (5′-CGATGACGCTGTATCTTGTGATG-3′); ACTIN-F (5′-GATTTGGCATCACACTTTCTACAATG-3′) and ACTIN-R (5′-GTTCCACCACTGAGCACAATG-3′); AtATM3-F (5′-TGCTCGGACATTTTTGAAATC-3′) and AtATM3-R (5′-GTCCATAGCTGCGCATATCTC-3′). The total RNA from WT and transgenic plants was heated to 65 °C for 7 min and subjected to a reverse transcription reaction using RT-AMV transcriptase (Roche) with oligo(dT) for 1 h at 42 °C. PCR was performed using ExTaq DNA polymerase (TaKaRa, Shiga, Japan).
SYBR Green I-based real-time PCR and a melting curve analysis assay were employed using the CFX96 Touch Real-Time Q-PCR system. The 10-μL PCR mixture included 1 μL of RT product, 2× SYBR® Premix DimerEraser, and 1 μL of primer (SYBR® Premix DimerEraser™-Perfect Real Time TaKaRa). The reactions were performed in a 96-well optical plate and entailed an initial step at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, 55 °C for 30 s, and 72 °C for 20 s. After the PCR reaction, a melting curve analysis was increased. Numerical analysis was performed using the 2−ΔΔCt method (Livak and Schmittgen 2001). Data on the threshold cycle (Ct) were determined using default threshold settings and is defined as the fractional cycle number at which fluorescence reaches at fixed threshold (Livak and Schmittgen 2001).
Statistical analysis
Data were presented as mean ± standard deviation from three independent biological replicates for all assays, the statistically significant differences between the transgenic and WT plants in figures were determined with Student’s t test using SPSS 19.0 software (IBM Corporation, Chicago, IL, USA), values of P < 0.05 were considered statistically significant.
Results
Spatial expression of AtTEM1 in Arabidopsis thaliana
Semi-quantitive RT-PCR analysis was performed to determine the spatial expression pattern of AtTEM1. As presented in Fig. 1, AtTEM1 was expressed at high levels in the flower, stem, rosette and cauline leaf, but relatively low levels in the silique and root tissue.
Sub-cellular localization of AtTEM1 protein
Arabidopsis AtTEM1 is in the RAV family of transcription factors. The main characteristic of RAV members is the presence of two different DNA-binding domains: a B3 and an AP2 domain. The RAV family members have thus been classified as members of either the B3 super-family or the AP2/EREBP (APETALA2) family of transcription factors. By using Clustal v2.1 multiple amino acid alignment and SMART, the putative AP2 and B3 transcriptional activation domains at the N-terminus and the C-terminus were identified. Additionally, the putative nuclear localization signal (NLS) and the B3 repression domain (BRD) were further identified (Fig. S1).
To determine whether the AtTEM1 protein localizes in the nucleus, a CaMV35S::GFP:AtTEM1 fusion construct was introduced into onion epidermal cells through the Agrobacterium tumefaciens-mediated transfection method (Varagona et al. 1992). Compared with the free GFP which was randomly localized in the cytoplasm (Fig. 2d), the GFP: AtTEM1 was observed predominantly in the nucleus (Fig. 2f, h), indicating that AtTEM1 is a nuclear-localized protein.
Subcellular localization of AtTEM1 in onion epidermal cells. a, b Onion epidermal cells in visible and ultraviolet light (UV). c, d 35S::GFP65T observed in the cytoplasm. e, f 35S::GFP65T:AtTEM1 observed in the nucleus. g, h 35S::GFP65T:AtTEM1 observed in the nucleus at 2× amplification. Scale bars on panel indicate 50 and 100 μm
AtTEM1 negatively affects flowering time
To determine the role of AtTEM1 in plant growth and development, reverse genetic analyses were performed. Under LD conditions, tem1 mutants flowered earlier than WT plants, whereas 35S::AtTEM1 plants flowered much later than the WT plants. Whereas the AtTEM1 mutant plants tem1-1 and tem1-2 had smaller rosettes size, but the 35S::AtTEM1 transgenic plants were indistinguishable from the WT plants (Fig. 3a, b). The 35S::AtTEM1 plants bolted 1.2 weeks later than the WT plants (Fig. 3c, P < 0.05) and produced at least 3 extra rosette leaves before flowering (Fig. 3d, P < 0.05); however, the tem1 plant bolted 1.1 weeks earlier than the WT plants (Fig. 3c, P < 0.05) but produced nearly the same number of rosette leaves (Fig. 3d, P < 0.05) before flowering. The expression of FT in the tem1 and WT plants increased under LD conditions, and the expression of FT in the tem1 was much higher than that in the WT plants; whereas the expression of FT in the 35S::AtTEM1 was almost stable (Fig. 3e). These results confirm that AtTEM1 acts redundantly to repress FT under LD conditions.
Phenotypes and FT expression of 35S::AtTEM1, tem1 and WT Arabidopsis under LD conditions. a, b 35S::AtTEM1, tem1 and WT grown under LD conditions for 4 weeks, observed from the top (a) and from front (b); c FT expression; d leaf numbers; and e bolting time of WT and transgenic plants. Data marked with asterisk mean the statistically significant differences between the transgenic and WT plants (*P < 0.05; **P < 0.01; ***P < 0.001)
Seasonal changes in the day length affect the flowering time of numerous plant species. The flowering of Arabidopsis is accelerated by exposure to LD conditions (Castillejo and Pelaz 2008). Compared with the WT plants, the 35S::AtTEM1 transgenic plants exhibited late-flowering phenotype (Fig. 3), whereas the tem1 plants were early flowering as reported previously (Castillejo and Pelaz 2008).
AtTEM1 negatively regulates drought tolerance in Arabidopsis
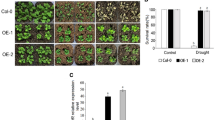
To determine the possible involvement of AtTEM1 in drought and mannitol stress responses, the transpirational water loss rates among the 35S::AtTEM1, tem1 and WT plants were first compared. The entire aerial shoots were cut from 4-week-old soil-grown plants; and placed on filter paper at ambient temperature; their weights were measured to monitor their water loss over time. After 2 h, 35S::AtTEM1 plants exhibited a moderate but statistically significant increase in water loss compared with the WT and tem1 plants. The water loss rate of the 35S::AtTEM1 transgenic plants was thus higher than that of the WT and tem1 plants (Fig. 4b), resulting in accelerated plant wilting. Drought treatment was then performed by withholding water for 14 days and then re-watered for further 7 days. After withholding water, the 4-week-old WT and the 35S::AtTEM1 transgenic plants were discovered to exhibit significant dehydration and wilting whereas most of the tem1 plants was still alive (Fig. 4a). After re-watering, the tem1 plants were almost recovered, whereas the 35S::AtTEM1 plants were almost dead. The same result was obtained from the mannitol treatment of 2-week-old plant seedlings (Fig. 5a) and the seed germination (Fig. 5c). After treatment, only 3.7% of the WT plants and 4.1% (P < 0.05) of the 35S::AtTEM1 plants were survived, but 51.7% (P < 0.001) of the tem1 plants exhibited almost normal growth (Fig. 5b). Under 400 mM mannitol treatment, 58.8% (P < 0.001) of tem1 seeds germinated, while 13.9% of the WT and 15.9% (P < 0.05) of the 35S::AtTEM1 seeds germinated under the stress (Fig. 5d).
Phenotypic and biochemical index changes in 35S::AtTEM1, tem1 and WT Arabidopsis under drought treatments. a Four-week-old plants before drought treatment (upper), after 14 days drought treatment (middle) and after re-watering for 7 days (bottom); b water loss rates of aerial shoots of 4-week-old plants after being exposed to ambient temperature. c REC; d MDA; e proline content
Next, whether the physiological indicators changed within the period after the drought was investigated. The REC of electrolyte leakage can be used as an indicator of cell membrane penetrability. By contrast, proline enrichment in stressed plants is a general response to various abiotic stresses and serves as effective indicator of stress tolerance (Akram et al. 2007). MDA, a product of lipid peroxidation, is associated with the oxidative degradation of cell membrane lipids and its abundance serves as an indicator of cell membrane damage.
The REC, MDA content, and proline content revealed that there were no significant differences between the transgenic lines and WT plants before mannitol stress. After the treatment, the REC and MDA content of the tem1 plants were significantly lower in than those of the WT and 35S::AtTEM1 plants (Fig. 4c, d). While the highest proline accumulation was observed in the tem1 plants (Fig. 4e). Despite having an enhanced wilting phenotype than the WT plants (Fig. 4a), the 35S::AtTEM1 transgenic plants were not revealed to have significantly different levels of physiological indicators (Fig. 4c–e).
AtTEM1 negatively affects drought-responsive gene expression
Real-time PCR analysis of AtTEM1 gene expression demonstrated that AtTEM1 responded rapidly to the drought stress (Fig. 6a, P < 0.05). According to the FT expression and growth phenotype results, AtTEM1 may result in changes in FT expression (Fig. 3c, P < 0.05). Because 35S::AtTEM1 transgenic and tem1 plants had different responses to abnormal drought stress, the expression of drought-responsive genes, namely PDH, RD22, and P5CS, was investigated. Consistent with their drought tolerant phenotype, these genes were much more strongly induced in tem1 plants than in the 35S::AtTEM1 transgenic and WT plants (Fig. 6; P < 0.05).
Expression of AtTEM1 and drought-inducible genes in WT, 35S::AtTEM1 and tem1. a Time course expression level of AtTEM1 under drought treatment; b–d expression level of drought responsive gene RD22 (b), PDH (c) and P5CS (d) under drought treatment in WT, 35S::AtTEM1 and tem1 plants. Columns marked with asterisk indicate the statically significant differences between the transgenic and WT plants (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
AtTEM1 is an AP2 and B3 domain-containing transcription factor. Down regulation of AtTEM1 could result in early flowering (Castillejo and Pelaz 2008). In the present study, over-expression of AtTEM1 in Arabidopsis was demonstrated to result in late flowering as was reported previously (Castillejo and Pelaz 2008). This study also demonstrated that AtTEM1 is localized in the nucleus and acts as a negative regulator for flowering time and drought stress response.
AtTEM1 is a versatile regulator of growth and drought stress
In this study, Arabidopsis AtTEM1 was demonstrated to be a nuclear localization transcription factor. AtTEM1 expression was high in the flower, stem, rosette and cauline leaf, but low in the silique and root tissue. The 35S::AtTEM1 plants bolted later than the WT plants and produced extra rosette leaves before flowering; however, the tem1 plants bolted earlier and produced nearly the same number of rosette leaves before flowering. These results suggested that AtTEM1 transcription factor negatively regulates flowering time.
AtTEM1 was also discovered to act negatively on plants under drought stress conditions, more transpirational water loss was observed in the 35S:AtTEM1 transgenic plants compared with the tem1 and WT plants (Fig. 4b), and a higher rate of growth resumption was observed in the tem1 after drought treatment (Fig. 4a). Abiotic stress conditions affect the expression of RAVs transcription factors in various species (Fu et al. 2014). Expression of BnaRAV-1 in Brassica napus was induced by treatment with cold, NaCl or polyethylene glycol (PEG) (Zhuang et al. 2011). In present study, the expression of AtTEM1 transcription factor was induced by drought (Fig. 6a, P < 0.05). PDH, RD22, and P5CS were reported drought-responsive genes (Yamaguchi-Shinozaki and Shinozaki 1993; Kiyosue et al. 1996). The expression of these drought-responsive genes was strongly induced in tem1 plants than in the WT and 35S:AtTEM1 transgenic plants, suggesting that AtTEM1 may negatively regulate the expression of these drought-responsive genes. Under drought and mannitol stresses, increased REC and MDA content and decreased proline content were identified in the 35S:AtTEM1 plants, while lower REC and MDA content and increased proline content were found in the tem1 plants (Fig. 4c–e). These results suggested that AtTEM1 in Arabidopsis functions as a negative regulator in response to drought and mannitol stresses.
In this study, delayed flowering was discovered in 35S::AtTEM1 transgenic plants. By contrast, early flowering was revealed in tem1 mutant plants. We also discovered that 35S::AtTEM1 transgenic plants were less drought tolerant than WT plants, whereas tem1 mutant plants were much more drought tolerant. Based on these results, AtTEM1 transcription factor is proposed to be a versatile regulator reacting negatively to both flowering time and drought stress.
References
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P (2008) The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20:2117–2129
Akram NA, Shahbaz M, Ashraf M (2007) Relationship of photosynthetic capacity and proline accumulation with the growth of differently adapted populations of two potential grasses (Cynodon dactylon (L.) Pers. and Cenchrus ciliaris L.) to drought stress. Pak J Bot 39:777–786
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Castillejo C, Pelaz S (2008) The balance between CONSTANS and TEMPRANILLO activities determines FT expression to trigger flowering. Curr Bio 18:1338–1343
Cheng MC, Hsieh EJ, Chen JH, Chen HY, Lin TP (2012) Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiol 158:363–375
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Eady C, Weld R, Lister C (2000) Agrobacterium tumefaciens-mediated transformation and transgenic-plant regeneration of onion (Allium cepa L.). Plant Cell Pep 19:376–381
Fu M, Kang HK, Son SH, Kim SK, Nam KH (2014) A subset of RAV transcription factors modulates drought and salt stress responses aba-independently in Arabidopsis. Plant Cell Physiol 55(11):1892–1904
Hu YX, Wang YH, Liu XF, LI JY (2004) Arabidopsis RAV1 is down-regulated by brassinosteroid and may act as a negative regulator during plant development. Cell Res 14:8–15
Kang HG, Kim J, Kim B, Jeong H, Choi SH, Kim EK, Lee HY, Lim PO (2011) Overexpression of FTL1/DDF1, an AP2 transcription factor, enhances tolerance to cold, drought, and heat stresses in Arabidopsis thaliana. Plant Sci 180:634–641
Kiyosue T, Yoshiba Y, Yamaguchi-Shinozaki K, Shinozaki K (1996) A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 8:1323–1335
Kuk YI, Shin JS, Burgos NR, Hwang TE, Han O, Cho BH, Jung S, Guh JO (2003) Antioxidative enzymes offer protection from chilling damage in rice plants. Crop Sci 43:2109–2117
Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functional groups. Springer, New York
Licausi F, Ohme-Takagi M, Perata P (2013) APETALA2/ethylene responsive factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol 199:639–649
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25:402–408
Mittal A, Gampala SS, Ritchie GL, Payton P, Burke JJ, Rock CD (2014) Related to ABA-insensitive3 (ABI3)/viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotech J 12:578–589
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Osnato M, Castillejo C, Matías-Hernández L, Pelaz S (2012) TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis. Nat Commun 3(3):199–202
Sato H, Mizoi J, Tanaka H, Maruyama K, Qin F, Osakabe Y, Morimoto K, Ohori T, Kusakabe K, Nagata M (2014) Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stress-induced gene expression by forming a heat stress-specific transcriptional complex with NF-Y subunits. Plant Cell 26:4954–4973
Sohn KH, Lee SC, Jung HW, Hong JK, Hwang BK (2006) Expression and functional roles of the pepper pathogen-induced transcription factor RAV1 in bacterial disease resistance, and drought and salt stress tolerance. Plant Mol Biol 61:897–915
Sun W, Cao Z, Li Y, Zhao Y, Zhang H (2007) A simple and effective method for protein subcellular localization using Agrobacterium-mediated transformation of onion epidermal cells. Biologia 62:529–532
Swaminathan K, Peterson K, Jack T (2008) The plant B3 superfamily. Trends Plant Sci 13:647–655
Varagona MJ, Schmidt RJ, Raikhel NV (1992) Nuclear localization signal (s) required for nuclear targeting of the maize regulatory protein Opaque-2. Plant Cell 4:1213–1227
Yamaguchi-Shinozaki K, Shinozaki K (1993) The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of rd22, a gene responsive to dehydration stress in Arabidopsis thaliana. MGG 238:17–25
Yu X, Peng YH, Zhang MH, Shao YJ, Su WA, Tang ZC (2006) Water relations and an expression analysis of plasma membrane intrinsic proteins in sensitive and tolerant rice during chilling and recovery. Cell Res 16:599–608
Zhuang J, Sun CC, Zhou XR, Xiong AS, Zhang J (2011) Isolation and characterization of an AP2/ERF-RAV transcription factor BnaRAV-1-HY15 in Brassica napus L. HuYou15. Mol Biol Rep 38:3921–3928
Acknowledgements
This study was supported by The Key Science and Technology Project of Hunan Province (2014WK2004), The National Natural Science Foundation of China (31300216), The Natural Science Foundation of Hunan Province (2015JJ3081), and Supported by Hunan Provincial Innovation Foundation for Postgraduate (CX2015B241).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, G., Liu, A., Zhou, X. et al. Arabidopsis TEMPRANILLO1 transcription factor AtTEM1 negatively regulates drought tolerance. Plant Growth Regul 83, 119–127 (2017). https://doi.org/10.1007/s10725-017-0288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-017-0288-x