Abstract
Many studies have shown genotypic differences in Cadmium (Cd) accumulation among rice cultivars, and concentrations in shoots and grains are generally higher in indica rice cultivars than in japonica rice cultivars, but the mechanism remains unknown. The main objective of this study was to investigate differences in heavy metal accumulation between rice subspecies through the analysis of 46 indica cultivars and 30 japonica cultivars. At the seedling stage, the mean Cd concentrations in the shoots of indica subspecies were significantly higher than those in japonica subspecies (1.22-fold), but this pattern was not observed in the roots. At the filling stage, the mean Cd concentrations in the shoots and spikes of indica subspecies were 1.66- and 2.14-fold higher than the respective concentrations in japonica subspecies. At the harvest stage, the mean Cd concentrations in the shoots and brown rice of indica subspecies were 1.61- and 2.27-fold higher than the respective concentrations in japonica subspecies. These results indicate that root-to-shoot and shoot-to-grain translocation, rather than Cd absorption in the roots, may be the key processes that determine the differences in Cd accumulation among rice subspecies. Gene expression analysis revealed that overall, the expression levels of the Cd transporter gene OsNramp1 notably increased (22.46-fold), but the expression levels of OsHMA2, OsHMA3 and OsNRAMP5 were not significantly changed at the seedling stage in the 76 cultivars exposed to Cd; the expression levels of OsNramp1 were positively correlated with the Cd concentrations in spikes at the filling stage. In addition, a significant difference was observed in the expression levels of OsNramp1 between the indica and japonica subspecies, which may explain the higher Cd concentrations in roots but lower Cd concentrations in spikes and brown rice for the japonica subspecies. Together, these results demonstrate that OsNramp1 may be the most important gene among the four selected genes in the promotion of Cd uptake by roots and transfer of Cd into spikes and eventually into brown rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd), a heavy metal pollutant that enters the human body persistently through enrichment in the food chain, is highly toxic with chronic intake. Cd toxicity is particularly problematic for people in developing countries, where contamination of agricultural soil with heavy metals is more serious because of the overuse of phosphate fertilizers, sewage sludge, wastewater irrigation and drainage in agriculture (Kikuchi et al. 2007; Valipour 2014, 2015a, b; Zhou et al. 2015). Rice is an important staple food for nearly half of the world’s population (Valipour et al. 2015) and is also a major source of Cd uptake. The demand for rice has grown in recent decades, and the global production and consumption of rice have increased approximately threefold from 1960 to 2011 (Ishikawa et al. 2012). Therefore, it is essential to produce rice with low Cd concentrations to reduce the potential risk that Cd poses to human health.
In recent years, many studies have shown genotypic differences in Cd accumulation among rice cultivars (Watanabe et al. 2004; Arao and Ishikawa 2006; Liu et al. 2007). Morishita et al. (1987) reported that the Cd concentrations in brown rice ranged from 2.1 to 27.0 ppb among 28 japonica varieties and from 4.1 to 55.5 ppb among 23 indica varieties under normal soil conditions. Through a study of 52 cultivars grown in soil containing a Cd concentration of 100 mg kg−1, Liu et al. (2005) revealed that the Cd concentrations in straw and brown rice were significantly higher in cultivars with indica consanguinity than in those with japonica consanguinity and that the Cd concentrations in brown rice ranged from 0.22 to 2.86 mg kg−1. In addition, higher Cd but lower Zn concentrations were also observed in the grains of super rice cultivars compared with the grains of common hybrid rice cultivars (Shi et al. 2009).
Many studies have evaluated the potential of Cd uptake in an attempt to explain the variations in Cd accumulation among rice cultivars, but we found that this may not be the case. For example, using the high-Cd accumulation indica cultivar ‘Habataki’ and the low-Cd accumulation japonica cultivar ‘Sasanishiki’, Uraguchi et al. (2009) found that root-to-shoot Cd translocation via the xylem, rather than Cd absorption in roots, is the major process determining shoot and grain Cd accumulation in rice. In addition, Zhou et al. (2015) found that heavy metal accumulation in 32 hybrid brown rice cultivars is related to the ability of rice to transfer heavy metals in the husk and straw rather than the root. Moreover, some findings have revealed that the nodes are the central organ for Cd transport from the xylem to the phloem and play a key role in the transport of Cd from the soil to grains at the grain-filling stage (Tanaka et al. 2007; Fujimaki et al. 2010).
Root Cd uptake is the first process that contributes to the transport of Cd ultimately into grains, and several genes related to this process have been cloned. OsIRT1 and OsIRT2 are involved in the influx of Cd2+ and Fe2+, and lower levels of available iron might induce OsIRTs, which can contribute to Cd uptake by the roots (Ishimaru et al. 2006; Nakanishi et al. 2006). In addition, transporters belonging to the natural resistance-associated macrophage protein (Nramp) family also mediate Cd transport. In rice, OsNramp1, which localizes to the plasma membrane, is an iron transporter with Cd-influx activity, and OsNramp5, which is also found in the plasma membrane, contributes to Mn, Cd and Fe transport (Takahashi et al. 2011; Ishimaru et al. 2012; Sasaki et al. 2012). After the genes involved in root Cd uptake were described, OsHMA2 was revealed to play a role in Zn and Cd loading and to participate in root-to-shoot translocation through the phloem (Satoh-Nagasawa et al. 2012; Takahashi et al. 2012). Whereas, OsHMA3 regulates xylem Cd transport in rice by mediating the vacuolar sequestration of Cd in root cells (Ueno et al. 2010; Miyadate et al. 2011). Subsequently, OsLCT1 is involved in Cd transport from the phloem to grains, and OsLCT1 expression is higher in leaf blades and nodes during reproductive stages (Uraguchi et al. 2011). In addition, OsHMA2 in the nodes also plays an important role in the preferential distribution Zn as well as Cd through the phloem to developing tissues, and a high expression level was also detected in the nodes at the reproductive stage (Yamaji et al. 2013). Among these genes, OsHMA3 was identified from a quantitative trait locus (QTL) for Cd accumulation, which indicates that this gene may be responsible for the difference in Cd accumulation between cultivars. However, Takahashi et al. used several cultivars with the same OsHMA3 sequence to suggest that a higher expression of OsNramp1 in the roots could lead to increased Cd accumulation among these cultivars (Takahashi et al. 2011). In addition to the genes involved in the long-distance Cd transport processes described above, rice genes related to Cd tolerance have been reported, such as OsCDT1 (Kuramata et al. 2009), OsLCD (Shimo et al. 2011), and OsPDR5/ABCG43 (Oda et al. 2011).
Despite these physiological and molecular findings, there is little explanation for the differences in Cd accumulation between the indica and japonica rice subspecies. The main objectives of this study were (1) to investigate the differences in Cd content in rice tissues between indica and japonica subspecies at different stages, (2) to investigate any correlations between the expression levels of Cd transporter genes and Cd content in rice tissues, and (3) to identify useful Cd transporter germplasm resources among different rice varieties and search for possible methods for non-transgenic low-Cd accumulation breeding.
Materials and methods
Rice plant materials and identification of indica–japonica characteristics
Based on differences in Cd accumulation between indica and japonica cultivars, we selected 76 rice cultivars with typical genetic backgrounds. The indica and japonica cultivars were studied according to Cheng’s index method for glume hair, leaf hair, length between the first and second rachis internodes, glume color at the heading stage, phenol reaction and grain length/width ratio (Cheng 1988). Using Nipponbare as a control, the materials were scored and classified as indica (H), indica—clinous (H′), japonica—clinous (K′), and japonica (K). The score and classification of each cultivar are shown in Table S1. All of the seeds were sterilized in 10 % H2O2 solution for 30 min, washed with deionized water, and germinated under moist sand conditions. After 15 days, seedlings of the selected 76 rice cultivars were transplanted for hydroponic growth and paddy soil experiments.
Hydroponic crop experiment
The seedlings of each cultivar used for hydroponic culture were transferred to six opaque plastic barrels (5.0 L) containing a liquid culture medium recommended by the International Rice Research Institute (IRRI). Each barrel had five holes in the lid, and we planted three seedlings per hole. After 7 days of pre-culture, the Cd treatment was started. The source of Cd was CdSO4, and we set two Cd treatment levels: 0 (control) and 8.9 μM. Each cultivar under the two treatments was planted in three barrels to obtain three replications. The culture medium was renewed every 3 days, and the pH was adjusted to 5.0 with HCl and NaOH. After 15 days of treatment, we collected three rice plants from each of three barrels corresponding to each cultivar and each treatment, and the roots of the collected nine plants of each cultivar and treatment were then obtained and mixed to obtain a sample for gene expression analysis. The other rice plants remaining in the barrels were collected to measure physiological indicators, such as the Cd, Mn, Cu, Zn, and Fe concentrations. The length and dry weight were measured for the two treatments. SPAD values were also measured before collection.
Field experiment and experiment site
Fuyang, located in the developed Yangtze River Delta region, is a district of Hangzhou City, northwestern Zhejiang Province, China. The site chosen for this field experiment was tested to determine the Cd, Mn, Cu, Zn and Fe concentrations (µg g−1 dry weight), which were 0.433, 31.03, 150.0, 449.4 and 21,031.3, respectively. The heavy metal contamination in the environment mainly comes from the heavy metal waste water produced by metal mines, non-ferrous metal smelting and processing. In addition, the pH of the tested soil was 6.3, and the contents of the organic matter, total N, available N, instant P and instant K in the plow layer were 34.6, 2.53, 139, 16 and 140 g kg−1, respectively. According to a randomized complete block design, each cultivar was planted in five lines with six plants per line, and three replicates of each cultivar were planted. At the filling stage (15 days after heading) and at the harvest stage, three rice plants from each plot were collected and mixed to obtain a sample. The samples were then separated into two tissue types (shoots and spike for the filling stage; shoots and brown rice for the harvest stage) to measure the heavy metal concentration. Flag leaves and node I (uppermost node connecting to the panicle and flag leaf) were collected for gene expression analysis of Cd-related transporter genes at the filling stage.
Chemical analysis
All of the samples from the hydroponic experiment and field experiment that were used to test the Cd concentrations were cleaned with deionized water and placed in a 105 °C drying oven for 1 h. Then, the samples were placed in a 60 °C drying oven for approximately 1 week. Rice tissues were digested using the nitric–perchloric acid digestion method (Hseu 2004), and the heavy metals concentrations were determined by inductively coupled plasma optical emission spectrometry (ICP-OES). A standard reference material (GBW(E)081581, China Standard Materials Research Center, Beijing, China) was used to ensure the accuracy of metal analyses. In addition, we set up a standard curve every 25 samples, and the correlation coefficient of the calibration curves was 0.9999–1.0000.
Gene expression analysis
The total RNA from different tissues and different cultivars was extracted using the RNAprep Pure Plant Kit (Tiangen Biotech, Qiagen, Beijing), and cDNA was synthesized using ReverTra Ace qPCR RT Master Mix with the gDNA Remover kit (Toyobo, Shanghai, China). Two microliters of cDNA were used for real-time PCR (qRT-PCR), which was performed with SYBR Premix Ex Taq™ II (TaKaRa, Kyoto, Japan) using gene-specific primers, and each reaction was repeated three times. The sequences of the oligonucleotide primers (Life Technologies, Shanghai, China) that were used for qRT-PCR, which were tested to ensure their suitability for all of the cultivars, are listed in Table S2, and qRT-PCR was performed using an ABI 7900 instrument. All of the expression levels were normalized to that of eEF-1a (eukaryotic elongation factor 1-alpha).
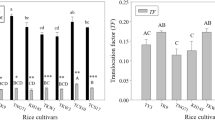
Translocation factor
The translocation factor (TF), defined as the ratio of metal concentration in shoot to root (TFrs), can be used to investigate plant translocation capability and the redistribution of metals throughout plant tissues in different rice cultivars (Zhou et al. 2013).
Statistical analysis
Statistical analyses were performed using Microsoft Excel 2007 and SPSS v17.0. A paired-samples t test was used for the analysis of the hydroponic crop experiment to measure the differences in plant growth between the cultivars under the control and Cd treatments. The differences in the metal concentration and gene expression level between indica and japonica subspecies were determined with one-way ANOVA, and correlations between the metal concentrations in rice tissues, gene expression levels and indica–japonica index were examined with Pearson’s correlation coefficient. Statistical significance was defined as p < 0.05 (significant) and p < 0.01 (highly significant).
Results
Effects of Cd treatment on plant growth among rice cultivars and types at the seedling stage
After 15 days of Cd treatment, shoot growth was inhibited and chlorophyll content (SPAD) was reduced compared with controls in all cultivars, and significant differences (p < 0.01) were observed (Table S3). The reduction in shoot length ranged from 11.90 to 57.24 %, with an average of 28.50 %. In accordance with the shoot length, the overall shoot dry weight per plant under Cd treatment decreased 29.69 % (significantly). “Xiangzaoxian 7” decreased the most (18.95 %) in SPAD, showing an approximately threefold greater reduction than average (5.57 %), and “Laolongxu” decreased the least (0.14 %). However, in terms of root growth, 42 rice cultivars under Cd treatment showed increased root length compared with the controls (ranging from 0.32 to 29.50 %), and the other 34 rice cultivars showed decreases (ranging from −16.11 to −0.27 %). The average root length increased by 2.21 %. The root dry weight per plant changed significantly (p < 0.01) between cultivars under control treatment and Cd treatment (23 varieties decreased, and the others increased). The total dry weight per plant increased by 8.92 %.
The root dry weight of the overall japonica rice subspecies under Cd treatment increased significantly (p < 0.01) more than that of the indica rice subspecies, and the SPAD of overall japonica rice subspecies under Cd treatment decreased significantly (p < 0.05) more than that of the indica rice subspecies. No significant differences were found between the two subspecies in root length, shoot length or shoot dry weight.
Variations in root Cd uptake and root-to-shoot Cd translocation among rice cultivars and subspecies at the seedling stage
Because Cd is originally absorbed into the plants through the roots, the Cd uptake by roots may be the key step determining the variation in Cd accumulation among rice cultivars. Cd concentrations in roots and shoots among the selected 76 cultivars were first measured in the hydroponic crop experiment under Cd treatment (Table S4). Correlation coefficient analysis showed that the Cd concentrations in shoots were significantly positively correlated with the concentrations in roots, and the TFrs and correlation coefficient were 0.32 and 0.70, respectively (Fig. 1). Notably, the Cd concentration in shoots was much more closely correlated with the TFrs than the Cd concentration in roots, which was much more obvious between the indica and japonica rice subspecies.
By comparing the differences in Cd concentrations between two subspecies of rice tissues (Fig. 2a), we found that the mean Cd concentrations in shoots for the indica rice varieties were significantly higher than for the japonica rice varieties (p < 0.01), although the mean Cd concentration in roots for the indica varieties was less than that for the japonica varieties (p = 0.079). This finding shows that the high Cd concentration in the roots of japonica did not result in a high Cd concentration in shoots compared with indica, which indicates subspecies differentiation in root-to-shoot translocation. Similar results were also found for Cd accumulation in rice tissues between the two subspecies (Fig. 2b).
The percentage of Cd accumulation in rice tissues revealed that most indica varieties accumulate less than 70 % of their Cd in roots, which is less than most japonica varieties accumulate (Fig. 2c). In addition, the mean percentages of Cd accumulation in shoots obtained for the indica and japonica subspecies were 33.40 % and 23.26 %, respectively. This difference clearly shows that most indica varieties have greater root-to-shoot Cd translocation abilities than japonica varieties, which results in the over-accumulation of Cd in shoots.
Expression analysis of Cd transporter genes in cultivars at the seedling stage in the presence and absence of Cd
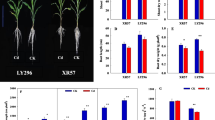
The expression of OsNramp1 in rice roots in all of the cultivars under Cd treatment was highly upregulated with a mean increase of 22.24-fold, ranging from 2.94 to 115.51-fold, compared with the level observed in control (Fig. 3). However, the changes in the expression levels of OsHMA2, OsHMA3, and OsNRAMP5 in the presence of Cd differed among the cultivars (Fig. S1). In addition, the expression level changes of these three genes were not as obvious as those of OsNramp1 compared with the expression obtained with the control treatment, showing mean increases or decreases of 1.59-, 1.04- and 0.88-fold, respectively.
Expression levels of OsNramp1 in rice roots grown in the absence (control) and presence of 1 ppm CdSO4 for 15 days at the seedling stage. The expression was determined by quantitative RT-PCR, and expression level of eEF-1a was used as internal control. Error bars present ±SD of three technological replicates
After the 76 rice varieties were grouped into indica and japonica subspecies, the mean expression levels of OsHMA2, OsHMA3, OsNramp1, and OsNramp5 increased 1.47-, 1.02-, 14.12-, and 0.80-fold in the indica subspecies and 1.77-, 1.04-, 34.69-, and 1.00-fold in the japonica subspecies, respectively, compared with the levels obtained in each cultivar under the control treatment. Notably, OsNramp1, which is responsible for root Cd uptake, was markedly more upregulated in the roots of japonica subspecies than those of indica subspecies in the presence of Cd, which may have resulted in the higher Cd concentrations observed in the roots of japonica cultivars.
Heavy metal accumulation difference between rice subspecies at the filling stage
The filling stage is an essential stage for transporting nutrients to the spike and thus may also be an important stage for transporting heavy metals during grain-filling. In accordance with the results of the hydroponic crop experiment under Cd treatment, Cd concentrations in the shoots and spikes of indica subspecies were significantly higher than in the japonica subspecies (p < 0.05 and p < 0.01) at the filling stage (Table S4). In addition, the mean concentrations of Cd in the shoots and spikes of the indica subspecies were 1.66- and 2.14-fold higher than those in the japonica subspecies, respectively. Cd is a non-essential metal required for the growth of rice plants and varies greatly in both subspecies. The coefficients of variation were 93.89 and 93.22 % in shoots and spikes for the indica subspecies, respectively, and 68.53 and 56.99 % for the japonica subspecies.
Gene expression analysis of the variation in Cd accumulation among rice cultivars and subspecies in the field experiment
In flag leaves at the filling stage, a highly positive correlation was obtained for the 76 studied cultivars between the expression level of OsNramp1 and the Cd concentration in spikes (Fig. 4a), which suggests that higher OsNramp1 expression levels are associated with higher Cd accumulation in spikes. In addition, the overall expression levels observed in the indica cultivars were significantly higher than those found in the japonica cultivars (p = 0.028), with mean levels of 0.0023 and 0.0012, respectively. This finding is consistent with the result that the Cd concentrations in spikes of the indica subspecies were approximately two-fold higher than those of the japonica subspecies. Moreover, a highly positive correlation was also detected between the OsNramp1 expression levels in flag leaves at the filling stage and the Cd concentrations in brown rice at the harvest stage (Fig. 4b), which indicated that OsNramp1 plays an important role in transporting Cd into spikes during the grain-filling stage and eventually contributes to the higher accumulation of Cd in brown rice.
The expression of OsHMA2 in flag leaves and nodes was positively correlated with the Cd concentration in spikes in the 76 studied cultivars (Figs. S2A, B). In addition, the expression levels of OsHMA2 in flag leaves were significantly positively correlated with those in nodes (p < 0.01), and a significant difference (p < 0.01) was detected between the two rice tissues, with mean levels of 0.0018 and 0.0236, respectively. These results indicate that at this stage, nodes rather than flag leaves may be major sites for the OsHMA2-regulated transport of Cd into spikes, and higher OsHMA2 expression levels may contribute to higher Cd accumulation in spikes. However, no significant difference was found in the expression levels of OsHMA2 between the indica and japonica subspecies (p = 0.336), which indicates that OsHMA2 may not contribute to the differentiation of Cd accumulation between the two rice subspecies.
Heavy metal accumulation mechanism between rice subspecies at the harvest stage
At the harvest stage, Cd concentrations varied dramatically in shoots and in brown rice, especially for the indica subspecies (Table S4). An analysis of the Cd concentrations in brown rice revealed that the japonica rice cultivar “Changbai 9” had the lowest concentration (0.033 µg g−1) and the indica rice cultivar “Yangjiannuo” had the highest concentration (1.290 µg g−1). The highest value was approximately 40-fold higher than that of “Changbai 9”. The Cd concentrations in the brown rice and shoots in the indica subspecies were significantly higher than in the japonica subspecies (p < 0.01 and p < 0.05, respectively). In addition, the mean concentrations of Cd in the shoots and brown rice of the indica subspecies were 1.61- and 2.27-fold higher than those in the japonica subspecies, respectively. The mean Cd concentrations in brown rice belonging to the indica subspecies and japonica subspecies were 0.36 and 0.16 µg g−1, respectively. Compared with the contaminant limits in rice set by international food safety standards (Cd < 0.4 µg g−1), 17 indica cultivars exceeded the standard, whereas none of the japonica cultivars exceeded the limit (Fig. 5). A correlation coefficient analysis was performed to investigate the relationship between the Cd concentrations in brown rice and the indica–japonica characteristics using the Cheng index method. The results revealed that Cd concentrations in brown rice were significantly negatively linearly correlated with Cheng’s index (Fig. 6).
Moreover, Cd concentrations in brown rice were also found to be positively correlated with those in spikes at the filling stage (p < 0.01, r = 0.867), which suggests that decreased Cd concentrations in spikes at the filling stage can ultimately decrease Cd concentrations in brown rice.
Discussion
As Cd is initially taken up by the roots, and many studies have evaluated the potential of Cd uptake in an attempt to explain the variations in Cd concentrations in aerial parts among ecotypes, cultivars, and relatives (Uraguchi et al. 2009). In this study, the processes of Cd uptake and translocation in the rice tissues of 76 rice varieties at three different stages were characterized to investigate the mechanism underlying the differences in Cd accumulation among rice cultivars and subspecies. At the seedling stage, although the Cd concentrations in the shoots in the indica subspecies were 1.23-fold higher than those in the japonica subspecies, no significant differences in the root Cd concentration were observed between the two subspecies. Moreover, the TFrs values of the indica subspecies were significantly higher than those of the japonica subspecies, which results in high Cd concentrations in the shoots of indica subspecies. When grown in soils, the Cd concentrations in the shoots and spikes in the indica subspecies were 1.66- and 2.14-fold higher, respectively, than those in the japonica subspecies at the filling stage, and the Cd concentrations in the shoots and brown rice in the indica subspecies were 1.61- and 2.27-fold higher, respectively, than those in the japonica subspecies at the harvest stage. The difference was greater between the two rice subspecies in the spikes and brown rice compared to the shoots at the filling and harvest stages, respectively, which shows a higher translocation ability in transporting Cd from shoots to spikes and to grains in the indica subspecies. All these results indicate that Cd accumulation in grains is more closely correlated with the root-to-shoot and shoot-to-grain translocation abilities than with Cd uptake by roots. And it may not be appropriate to select low-Cd accumulation cultivars with the detection of the Cd concentrations in roots.
Based on previous research, OsHMA2, OsHMA3, OsNramp1 and OsNramp5 are thought to be the main genes that regulate the uptake and translocation of Cd in roots. However, we found that after long-term Cd treatment, the expression of OsHMA2, OsHMA3 and OsNramp5 in the roots of most of the studied cultivars did not clearly differ from that found in the absence of Cd. Only the transcript levels of OsNramp1 were upregulated significantly in the roots of the investigated cultivars in the presence of Cd. In addition, the expression of OsNramp1 in the roots of the japonica subspecies was 34.69-fold higher after long-term Cd treatment compared with the expression observed in the absence of Cd, and this value is approximately 2.46-fold higher than the upregulation observed in the indica subspecies, a finding that may explain the overall higher Cd concentration in the roots of japonica cultivars. These results indicate that among the four selected genes, OsNramp1 may be more closely correlated with the regulation of Cd accumulation in the roots at the seedling stage. A gene expression analysis at the filling stage also showed that higher expression levels of OsNramp1 generally resulted in higher Cd concentrations in the spikes of the 76 investigated cultivars, and the significantly higher expression levels of OsNramp1 observed in the indica subspecies may have promoted the overall higher Cd concentrations in the spikes of indica subspecies. The above-mentioned results indicate that OsNramp1 can contribute to Cd uptake by roots at the seedling stage and promote the transport of Cd into spikes at the filling stage and eventually into brown rice. Moreover, it is likely that at least one additional gene that contributes to the higher translocation ratio of Cd from roots to shoots at the seedling stage, which results in overall higher Cd concentrations in the shoots of indica subspecies, has not been identified.
According to the study by Takahashi et al. (2011), the difference of a 406-bp deletion of the putative promoter region of OsNramp1 exists between different cultivars, which do regulate the expression of OsNramp1, and eventually regulate the difference in Cd accumulation among cultivars. In addition, the mutation of OsHMA3 can also regulate the accumulation of Cd in rice plants. In this study, we found the cultivar “Xiangzaoxian 7”, with extremely high root-to-shoot Cd translocation in the hydroponic experiment, and the cDNA sequence of OsHMA3 in “Xiangzaoxian 7” was consistent with that of the high-Cd translocation cultivar “Cho-ko-koku,” which has a 53-aa deletion and 33-aa substitution of OsHMA3 compared with the low-Cd translocation cultivar “AK 63” (Miyadate et al. 2011).
Using the above-described results, we may identify methods to reduce the potential risk that Cd poses to agricultural and even human health. On the one hand, using marker-assisted selection, useful alleles of Cd transporter genes and their promoters, particularly those with obvious effects, such as OsNramp1, as was identified in various cultivars in this study, could be bred into particular cultivars to promote low Cd accumulation. On the other hand, identifying cultivars with relatively higher Cd concentrations in shoots and lower Cd concentrations in brown rice may be an effective approach for simultaneously reducing Cd concentrations in paddy soils and reducing human health risks from Cd. And we have selected several cultivars with these characteristics, as is shown in Table S4 (e.g., Liutiaozi, Nonghu 6, and Mubanggu).
Abbreviations
- Cd:
-
Cadmium
- CK:
-
Control
- TFrs :
-
Translocation factor of root to shoot
References
Arao T, Ishikawa S (2006) Genotypic differences in cadmium concentration and distribution of soybean and rice. JARQ 40:21–30. doi:10.6090/jarq.40.21
Cheng KS (1988) A statistical evaluation of the classification of rice cultivars into hsien and keng subspecies. Rice Genet Newslett 4:46–48
Fujimaki S, Suzui N, Ishioka NS, Kawachi N, Ito S, Chino M, Nakamura S (2010) Tracing cadmium from culture to spikelet: noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol 152:1796–1806. doi:10.1104/pp.109.151035
Hseu ZY (2004) Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour Technol 95:53–59. doi:10.1016/j.biortech.2004.02.008
Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, Hase Y, Arao T, Nishizawa NK, Nakanishi H (2012) Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci USA 109:19166–19171. doi:10.1073/pnas.1211132109
Ishimaru Y, Suzuki M, Tsukamoto T et al (2006) Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 45:335–346. doi:10.1111/j.1365-313X.2005.02624.x
Ishimaru Y, Takahashi R, Bashir K et al. (2012) Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci Rep 2:286. doi:10.1038/srep00286
Kikuchi T, Okazaki M, Toyota K, Motobayashi T, Kato M (2007) The input–output balance of cadmium in a paddy field of Tokyo. Chemosphere 67:920–927. doi:10.1016/j.chemosphere.2006.11.018
Kuramata M, Masuya S, Takahashi Y, Kitagawa E, Inoue C, Ishikawa S, Youssefian S, Kusano T (2009) Novel cysteine-rich peptides from Digitaria ciliaris and Oryza sativa enhance tolerance to cadmium by limiting its cellular accumulation. Plant Cell Physiol 50:106–117. doi:10.1093/pcp/pcn175
Liu J, Zhu Q, Zhang Z, Xu J, Yang J, Wong MH (2005) Variations in cadmium accumulation among rice cultivars and types and the selection of cultivars for reducing cadmium in the diet. J Sci Food Agric 85:147–153. doi:10.1002/jsfa.1973
Liu J, Qian M, Cai G, Yang J, Zhu Q (2007) Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J Hazard Mater 143:443–447. doi:10.1016/j.jhazmat.2006.09.057
Miyadate H, Adachi S, Hiraizumi A et al (2011) OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189:190–199. doi:10.1111/j.1469-8137.2010.03459.x
Morishita T, Fumoto N, Yoshizawa T, Kagawa K (1987) Varietal differences in cadmium levels of rice grains of japonica, indica, javanica, and hybrid varieties produced in the same plot of a field. Soil Sci Plant Nutr 33:629–637. doi:10.1080/00380768.1987.10557611
Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK (2006) Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr 52:464–469. doi:10.1111/j.1747-0765.2006.00055.x
Oda K, Otani M, Uraguchi S, Akihiro T, Fujiwara T (2011) Rice ABCG43 is Cd inducible and confers Cd tolerance on yeast. Biosci Biotechnol Biochem 75:1211–1213. doi:10.1271/bbb.110193
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24:2155–2167. doi:10.1105/tpc.112.096925
Satoh-Nagasawa N, Mori M, Nakazawa N, Kawamoto T, Nagato Y, Sakurai K, Takahashi H, Watanabe A, Akagi H (2012) Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol 53:213–224. doi:10.1093/pcp/pcr166
Shi J, Li L, Pan G (2009) Variation of grain Cd and Zn concentrations of 110 hybrid rice cultivars grown in a low-Cd paddy soil. J Environ Sci 21:168–172. doi:10.1016/S1001-0742(08)62246-9
Shimo H, Ishimaru Y, An G, Yamakawa T, Nakanishi H, Nishizawa NK (2011) Low cadmium (LCD), a novel gene related to cadmium tolerance and accumulation in rice. J Exp Bot 62:5727–5734. doi:10.1093/jxb/err300
Takahashi R, Ishimaru Y, Senoura T, Shimo H, Ishikawa S, Arao T, Nakanishi H, Nishizawa NK (2011) The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J Exp Bot 62:4843–4850. doi:10.1093/jxb/err136
Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanishi H (2012) The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ 35:1948–1957. doi:10.1111/j.1365-3040.2012.02527.x
Tanaka K, Fujimaki S, Fujiwara T, Yoneyama T, Hayashi H (2007) Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.). Soil Sci Plant Nutr 53:72–77. doi:10.1111/j.1747-0765.2007.00116.x
Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA 107:16500–16505. doi:10.1073/pnas.1005396107
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688. doi:10.1093/jxb/erp119
Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, Yoshida A, Kyozuka J, Ishikawa S, Fujiwara T (2011) Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci USA 108:20959–20964. doi:10.1073/pnas.1116531109
Valipour M (2014) Future of the area equipped for irrigation. Arch Agron Soil Sci 60:1641–1660. doi:10.1080/03650340.2014.905675
Valipour M (2015a) Future of agricultural water management in Africa. Arch Agron Soil Sci 61:907–927. doi:10.1080/03650340.2014.961433
Valipour M (2015b) A comprehensive study on irrigation management in Asia and Oceania. Arch Agron Soil Sci 61:1247–1271. doi:10.1080/03650340.2014.905675
Valipour M, Ziatabar Ahmadi M, Raeini-Sarjaz M, Gholami Sefidkouhi MA, Shahnazari A, Fazlola R, Darzi-Naftchali A (2015) Agricultural water management in the world during past half century. Arch Agron Soil Sci 61:657–678. doi:10.1080/03650340.2014.944903
Watanabe T, Shimbo S, Nakatsuka H, Koizumi A, Higashikawa K, Matsuda-Inoguchi N, Ikeda M (2004) Gender-related difference, geographical variation and time trend in dietary cadmium intake in Japan. Sci Total Environ 329:17–27. doi:10.1016/j.scitotenv.2004.03.010
Yamaji N, Xia J, Mitani-Ueno N, Yokosho K, Feng Ma J (2013) Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol 162:927–939. doi:10.1104/pp.113.216564
Zhou H, Zeng M, Zhou X, Liao BH, Liu J, Lei M, Zhong QY, Zeng H (2013) Assessment of heavy metal contamination and bioaccumulation in soybean plants from mining and smelting areas of southern Hunan Province, China. Environ Toxicol Chem 32:2719–2727. doi:10.1002/etc.2389
Zhou H, Zeng M, Zhou X, Liao B, Peng P, Hu M, Zhu W, Wu Y, Zou Z (2015) Heavy metal translocation and accumulation in iron plaques and plant tissues for 32 hybrid rice (Oryza sativa L.) cultivars. Plant Soil 386:317–329. doi:10.1007/s11104-014-2268-5
Acknowledgments
This work was supported by a Special Fund for Agro-Scientific Research in the Public Interest (No. 201403015), the National Natural Science Foundation of China (No. 31571616) and Funds for Science and Technology Innovation Project from the Chinese Academy of Agricultural Sciences. We gratefully acknowledge Dr. Da-Li Zeng, China National Rice Research Institute, China, for supplying seeds of the 76 selected cultivars.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Quan Zhou and Guo-Sheng Shao have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, Q., Shao, GS., Zhang, YX. et al. The difference of cadmium accumulation between the indica and japonica subspecies and the mechanism of it. Plant Growth Regul 81, 523–532 (2017). https://doi.org/10.1007/s10725-016-0229-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0229-0