Abstract
Kinetin-induced programmed death of cortex cells made the roots of Vicia faba ssp. minor (faba bean) seedlings shorter and thicker since the respective cells were not as long but wider then in the untreated seedlings (control). Thus the weight of 2-cm long apical parts of the kinetin-treated roots was greater. Furthermore, apical hooks as well as aerenchyma formation in the roots were observed. The presented studies aimed at revealing the role of ethylene in kinetin-induced cell death. To this end ACC (1-aminocyclopropane-1-carboxylic acid; ethylene precursor) and calcium ion amounts were analysed. Influence of ethylene biosynthesis and reception inhibitors on viability of cells and on the amount and distribution of calcium ions was also assessed. The results of the studies showed that both calcium ions and ethylene were involved in the kinetin-induced cell death. This allowed us to discuss their crucial role in this process and to propose a general scheme of induction of aerenchyma formation pathway resulting from kinetin-induced death of cortex cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytokinins (CKs), N6-substituted adenine analogues, are plant growth hormones including isoprenoid (zeatin, dihydrozeatin and isopentenyladenine; zeatin ribosides), aromatic (benzylaminopurine—BAP and its derivatives, ortho- and meta-topolin—mT and their methoxy-derivatives; van Staden et al. 2008) and furfural (kinetin, N-6-furfuryladenine; Barciszewski et al. 2007) derivatives. In animal cells kinetin (natural CK) controls differentiation, stimulates cell proliferation, activates antioxidant enzymes, triggers dedifferentiation of human leukemia cells as well as limits a human mRNA splicing defect (Cabello et al. 2009). In plants kinetin, which was discovered in DNA and in extracts of root nodules of a multipurpose tree Casuarina equisetifolia (Barciszewski et al. 2007), regulates metabolism and apical dominance (Barciszewski et al. 2007), promotes cell division (Shi and Rashotte 2012) and controls the activity of IAA oxidases (van Staden et al. 2008). In Physcomitrella patens (a moss species), kinetin initiates transcription of rRNA and induces vegetative bud formation, moreover it inhibits a hypersensitive response (HR) and senescence and it can suppress viral- and mercuric chloride-induced necrosis (Barciszewski et al. 2007).
Kinetin, among other CKs, can interact with ethylene via the signalling pathway (Zhang et al. 2013). The ethylene (Iqbal et al. 2013) and the CKs (Hwang et al. 2012) signalling pathways are well known and their two-component receptors are localized in plasma and endoplasmic reticulum (ER) membranes (Caesar et al. 2011; Shi and Rashotte 2012; Iqbal et al. 2013). Ethylene and CKs participate in the control of cell division in quiescent center cells and in the asymmetric elongation of A. thaliana roots (Zhang et al. 2013). The CKs signalling elements (HK4, RR1, RR10 and RR11) employ parts of the ethylene signalling pathway, especially ETR1 and EIN2 receptors (Kushwah et al. 2011). The CKs signalling pathways might control ethylene synthesis (Choi et al. 2011; Shi and Rashotte 2012), however, the latter might also take part in CKs synthesis (Gonçalves et al. 2013). Ethylene and CKs cross-talk depends on HPt (Kunikowska et al. 2013b) proteins through His kinase of ETR1 receptor (Scharein et al. 2008). In the ethylene and the CKs signalling processes calcium ions (Ca2+) play a crucial role. Together with other molecules (e.g. 1,4,5-triphosphate and cyclic ADP-ribose), they are the secondary messengers of cellular signalling processes in abiotic and biotic responses and in developmental processes via Ca2+-specific proteins (calmodulin). ER in plants and animals is the Ca2+ reservoir and the Cyclic Nucleotide Gated Channels (CNGC) in ER membrane are their transport pathways (Stael et al. 2012).
This paper highlights an interesting fact that kinetin via Ca2+ ions might cooperate with ethylene to control programmed death of cortex cells induced by kinetin in roots of Vicia faba ssp. minor seedlings (Kunikowska et al. 2013b). It is established that kinetin might cooperate with ethylene to induce death and aerenchyma formation according to the RFI scale (Byczkowska et al. 2013). The cross-talk between ethylene and CKs during PCD processes was also suggested by Carimi et al. (2003), who observed that in suspension cultures during BAP-induced death of cells, ethylene amount was elevated. Thus the present paper is aimed to explain whether aerenchyma formation in cortex of faba bean roots, being an effect of kinetin-induced cell death, depends on ethylene. To confirm our thesis, morpho-anatomical, cytological and spectrophotometrical characterization of faba bean roots was carried out.
Materials and methods
Plant material, treatment and analyses
Vicia faba ssp. minor seeds were germinated for 3 days in darkness at 23 ± 1 °C in Petri dishes (15 cm in diameter and 3-cm heigh) on blotting paper moistened with distilled water (2 ml per seed, humidity 85–93 %). Then 6 of 3-day-old V. faba ssp. minor seedlings with almost equal length (2.5 ± 0.2 cm) of roots were transferred to glass containers (8 cm in diameter and 4-cm heigh). They were treated with 10 ml of an aqueous solution of 46 μM kinetin (Kin; Sigma) with or without aminooxyacetic acid (AOA), cobalt chloride (CoCl2) or 2,5-norbornadiene (NBD) at 1 and 10 μM concentrations. Another set of seedlings was treated with or without 5 μM lanthanum nitrate (LaNO3). Water treatment was the contol (Ctrl). The seedlings were used for morpho-anatomical analyses. Length and width of whole roots, weight of 2-cm apical parts of roots, width and length of cells and aerenchyma formation were assessed. Next cytological (fluorescently measured cell death levels in planta, Ca2+ ion amounts and cell wall thickeness) as well as spectrophotometrical (ACC content) studies were carried out.
Growth parameters and values of indices of cell viability were compared using the Student’s t test while the results of analytic measurements were compared using the U Mann–Whitney test applying MS Excel software.
Estimation of cell death levels in planta and analyses of cell wall thickness and aerenchyma formation
First, length and width of whole roots as well as weight of their 2-cm long apical parts were measured. To detect cell death in 2-cm long apical parts of living roots, between the 4th and the 20th mm from their apex (Fig. 1), they were stained (5 min) with 1 % acridine orange (AO; Sigma) and 1 % ethidium bromide (EB; Sigma) in 0.01 M Na phosphate (pH 7.4; PHB) buffer and fixed (15 min) in 1 % in glutardialdehyde at the PHB. Then handmade 200–300 µm longitudinal sections were washed two times and analysed under the blue (B2A filter) or white light of Optiphot-2 fluorescence microscope (Nikon). Relative fluorescence intensity (RFI) of AO and EB was measured on the basis of fluorescence photographs of 170–210 nuclei from three independent experiments using the Scn Image software. The numbers of living (green; ≤0.34 a.u. of RFI), dying (green-yellow, yellow and yellow-orange nuclei; range from 0.35 to 0.45 a.u. of RFI) and dead (orange-red and red; ≥0.46 a.u. of RFI) cells were estimated according to the RFI scale (Byczkowska et al. 2013). To estimate width and length of 200–300 cortex cells of each of three independent experiments the photographs taken under the white light were analysed using Scn Image software.
Handmade (150–250 µm) cross sections of the apical fragments of roots (fixed for 60 min with 2.5 % gultardialdehyde in PHB) of the seedlings treated with kinetin for 72 h and of those postincubated without kinetin for the next 3 (total of 6 days) and then next 4 days (total of 7 days) were made. Next they were used for morpho-anatomical observations of aerenchyma formation and measurements of size of aerenchyma spaces under the white light of Optiphot-2 microscope (Nikon). To show the aerenchyma spaces more precisely cross sections (obtained as above) of the roots from the seedlings postincubated for 7 days without kinetin were additionally stained with calcofluor white (Fluostain; Sigma) and analysed under UV (UV2A filter) light of Optiphot-2 epi-fluorescence microscope (Nikon).
Cytological analyses of cortex wall thickness were carried out in apical parts of the roots of seedlings treated with kinetin for 72 h using the ImageJ software in two repetitions from two independent experiments.
Estimation of ACC and calcium ion amounts
ACC was isolated from 2-cm long apical parts of roots of the seedlings treated with kinetin for 0–96 h by their homogenization in cold 80 % methanol with butylated hydroxytoluene (BHT; 2 mg l−1) and separated onto the Dowex H+ x 50 W (Sigma) in 3 M HCl (Kaźmierczak and Kaźmierczak 2007). ACC amount in the samples was determined after its conversion by ACC deaminase to α-ketobutyrate (Lim et al. 2012) using the extracts from 2-cm long apical fragments of roots of the non-treated faba bean seedlings in 10 mM Tris–HCl pH 7.4 buffer as the source of deaminase. α-ketobutyrate was determined spectrophotometrically at 540 nm after its conversion to phenylhydrazone with 2,4-dinitrophenyl-hydrazine. The amount of α-ketobutyrate (μM/g of FW) was determined using a standard curve with known amount of α-ketobutyrate (Sigma-Aldrich).
Ca2+ ions were estimated fluorescently and reflectometrically. Fluorescence was measured in cortex cells of the apical parts of faba bean roots of the untreated (0 h) seedlings and those treated with kinetin for 1–96 h or with kinetin and AOA, CoCl2 or NBD for 48 and 72 h. Longitudinal handmade (about 300–400 µm), sections from 2-cm long apical parts of roots (between the 4th and the 20th mm from apex; Fig. 1) fixed with 2 % glutardialdehyde for 1 h in PHB were made. Then they were stained with 100 μM chlorotetracycline (CTC) and photographed under B2A filter of a fluorescence microscope. Measurement of green fluorescence intensity of Ca2+-CTC complexes (cytophotometry) was used to estimate Ca2+ ion amounts and their distribution (in a.u.) using the Scn Image software (Kunikowska et al. 2013a). Additionally, Ca2+ ion amounts were measured in extracts (obtained as above) from the untreated (0 h) and 1–96 h kinetin-treated roots using RQflex 10 plus system according to the manufacturer (Merck) procedure. Ca2+ ions reacted quantitatively with glyoxal bis(2-hydroxyanil) to form a red complex in the presence of hydrogen peroxide.
Results
Effect of kinetin on root and cortex cell morphology as well as on cell death
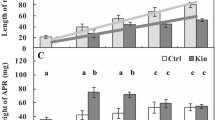
Morphological analyses of 72-h-kinetin treated seedlings (Fig. 1b) showed that the roots were shorter by about 60 % (P < 0.05), thicker by about 45 % (P < 0.05), while the weight of 2-cm apical parts of roots was twofold greater (P < 0.05) compared to the untreated roots (Ctrl; Fig. 1a), moreover an apical hook (Fig. 1b) appeared. Morpho-anatomical analyses of cross sections of the roots under the white (Fig. 2a–d) and fluorescence (Fig. 2e) light of microscope as well as surface (Fig. 2a′–c′) and profile plots (Fig. 2a″–c″) showed formation of aerenchyma spaces. The areas of these spaces in the untreated seedlings were about 164 µm2 and after 72-h-kinetin treatment they were greater by about 140 µm2. In the seedlings post-incubated for the next 3 and next 4 days, the aerenchyma spaces in the roots were greater by about 500 and 1100 µm2 (Fig. 2d, e), respectively. Cytological analyses showed that cellular walls of root cortex cells of V. faba ssp. minor seedlings (Fig. 1a) treated with kinetin (Fig. 1b) for 72 h were thicker by about 20 % compared to Ctrl (Fig. 3a). Profile areas of the cortex cells (CPA) were similar to the untreated ones (Fig. 3b). However, in the kinetin-treated material the length (L; Fig. 3c, c′, c″) of cortex cells was significantly smaller (by about 30 %; P < 0.05), while their width (W; Fig. 3c, c′, c″) was significantly greater (by about 65 %; P < 0.05) compared to Ctrl. Staining of the cortex cell nuclei with AO and EB showed that kinetin induced cell death (Fig. 4a–h). RFI values and appearance of greater condensation of nuclear chromatin (Fig. 4a–h) revealed that, after 24, 48, 72 and 96 h of treatment with kinetin, the numbers of dying cells were about 17.6 ± 1.2, 39.0 ± 3.9, 50.0 ± 2.4 and 42.0 ± 2.1 %, respectively.
Cortex cross sections of the control (a) roots of seedlings, after 3 day-treatment with kinetin (b) and after the following 4- (c) and 7-day (d, e) culture without kinetin with intercellular (a) and aerenchyma (b–e) spaces (indicated by arrows) under the white (a–d) and fluorescence light (e) and aerenchyma surface (a′–c′) as well as line plot profiles (a″–c″). Values placed in a″–c″ describe the area of intercellular and aerenchyma space profiles. Scale bars are 100 μm (for all micrographs)
Wall thickness (a) and profile area (PA; b) as well as length and width (c) of cortex cells of 2-cm long apical parts of the untreated (control; Ctrl, c′) and kinetin-treated (c″) V. faba ssp. minor seedling roots. Error bars represent the SE of the mean of two independent experiments. P < 0.05 indicates statistically significant differences between the results. Scale bar on c′ and c″ is 50 µm
Fluorescence micrographs of nuclei in the living cells (a, e) and those at early (b, f) or late phase of dying (c, g) as well as in the dead cells (d, h) of the root cortex in the untreated (a, e) and kinetin-treated (b–d, f–h) seedlings. Scale bar is 10 μm and is applied to all figures (colour online only)
ACC content and calcium ion amounts after kinetin treatment
Spectrophotometrical analyses showed that the amount of ACC, expressed as the amount of α-ketobutyrate, was greater by about 8.0 µg (P < 0.05) after 3-h-kinetin-treatment than in the untreated seedlings (0 h). After the next 6 and 12 h the amount of ACC was greater on average by about 4.0 µg g−1 FW, while after the 24 and 72 h by about 13.5 µg g−1 FW than in the untreated material (0 h; Fig. 5).
ACC content, expressed as the amount of α-ketobutyrate, in 2-cm long apical parts of untreated (0 h) and kinetin-treated (1–96 h) V. faba ssp. minor seedling roots. Error bars represent the SE of the mean of four independent experiments in two repetitions. P < 0.05 indicates statistically significant differences between the results
Cytophotometrical determination of Ca2+ ions fluorescence after staining with CTC showed that the complexes of Ca2+-CTC in the cells of the untreated seedlings (0 h) were localized in small cytoplasmic vesicles (Fig. 6a), and their amount, expressed as total fluorescence intensity (TFI), was about 12,000 a.u. (Fig. 7a). In the roots treated with kinetin for 3–48 h the amount of Ca2+ ions was greater (P < 0.05) on average by about 5000 a.u. (Fig. 7a), while after 96 h this parameter was similar to the untreated roots (0 h; Fig. 7a). Micrographs (Fig. 6b–e) also showed that after treatment with kinetin, Ca2+ ions left vesicles and spread in cytosol. This was manifested by empty (Ca2+-free) vesicles (Fig. 6b, d) and lower mean fluorescence intensity (MFI; Fig. 7b) of Ca2+-CTC complexes.
Relative amount of calcium ions in the root cortex cells of 2-cm long apical parts of V. faba ssp. minor seedlings cultured without (0) and with (1–96 h) kinetin expressed as total (a) and mean (b) fluorescence intensity (FI). Error bars represent the SE of the mean of three independent experiments. P < 0.05 indicates statistically significant differences between the results
Analyses showed that the mean values of FI were about 13,000, 8000 and 11,000 a.u. following 0–3-, 6–24- and 48–96-h treatments, respectively (Fig. 7b). It was also found that depletion of the total cellular Ca2+ ion amounts after 96 h (Fig. 7a) of treatment with kinetin was correlated with restoration of small vesicles filled with Ca2+-CTC complexes (Fig. 6f).
Effect of AOA, CoCl2 and NBD on the number of dying cells and calcium ion amounts
Studies of the role of ethylene during kinetin-induced cell death were carried out with inhibitors of ethylene synthesis (AOA and CoCl2) and reception (NBD) at 1 and 10 µM concentrations. Cytophotometrical determination of fluorescence intensity showed that the inhibitors alone at both concentrations did not induce cell death in root cortex cells (control). Following 72-h treatment with kinetin and AOA (Fig. 8c, d) or CoCl2 (Fig. 8e, f) the significant (P < 0.05) reduction of the number of dying cortex cells (Fig. 8a, b) was observed, by about 40 and 95 %, respectively. After 72-h with NBD treatment the number of dying cells (Fig. 8g, h) was significantly (P < 0.05) greater, by about 15 % (1 µM) to 25 % (10 µM; Fig. 8a, b).
The number of living, dying and dead cells in the root cortex of V. faba ssp. minor seedlings treated with kinetin for 72 h (a, b) as well as with kinetin and 1 μM (c) or 10 μM (d) AOA and 1 μM (e) or 10 μM (f) CoCl2 and 1 μM (g) or 10 μM (h) NBD. Error bars represent the SE of the mean of three independent experiments. P < 0.05 indicates statistically significant differences between the results
Moreover, FI of Ca2+-CTC complexes showed that the relative amount of Ca2+ ions after 48- and 72-h treatments with both concentrations (1 or 10 µM) of AOA (Fig. 9a) or CoCl2 (Fig. 9b) together with kinetin was significantly (P < 0.05) lower compared to the kinetin-treated variant (drop line, Fig. 9a, b). Whereas, the relative amount of Ca2+ ions after 48- and 72-h of treatments (Fig. 9c) with both concentrations of NBD together with kinetin was not statistically different (P > 0.05) compared to the kinetin-treated variant (drop line, Fig. 9c).
Relative amount (expressed as a total fluorescence intensity) of calcium ions in the root cortex cells of 2-cm long apical parts of V. faba ssp. minor seedlings treated for 48 and 72 h with kinetin supplemented with AOA (a), CoCl2 (b) and NBD (c) at 1 and 10 μM concentration. Dotted lines indicate the levels of calcium ions in apical parts of roots of the kinetin-treated series. Error bars represent the SE of the mean of three independent experiments. P < 0.05 indicates statistically significant differences
Discussion
It has been proposed that kinetin triggers a vacuolar type of death (Kunikowska et al. 2013a). It is characterized with (1) nuclear membrane invagination with elimination of pores from nuclear envelope (Domínguez and Cejudo 2012), (2) greater nuclear volume, (3) chromatin condensation (Doniak et al. 2014), (4) formation of micronuclei and DNA degradation without DNA ladder formation, (5) lower number of 2C and 4C nuclei accompanied by appearance of fraction of “hypoploid” cells, (6) changes in cellular dehydrogenase activities, (7) lower number of mitochondria and (8) changes in conductivity of the culture media (Kunikowska et al. 2013a).
The present studies are focused on the phenomenon briefly reported earlier by Kunikowska et al. (2013a), that kinetin-induced PCD of faba bean root cortex cells led to elimination of these cells and formation of aerenchyma. It is obvious that aerenchyma formation after kinetin treatment was the effect of elimination of protoplasts and then nuclei (van Doorn et al. 2011) and/or cellular walls of dying cells (Drew et al. 2000; Joshi and Kumar 2012). Aerenchyma spaces were greater every day which was reflected by its greater profile area and by the fact that walls of the remaining cells became thicker. Thickening of the cell walls allowed us to classify cell death as vacuolar (van Doorn et al. 2011).
It is known that ethylene is the secondary messenger in aerenchyma formation during hypoxia (Drew et al. 2000) or drought (Jaramillo et al. 2013) stress. This fact was also confirmed in roots by ACC treatment of faba bean seedlings (Byczkowska et al. 2013). After kinetin treatment the roots were shorter and thicker, the weight of their 2-cm long apical parts was greater compared to Ctrl. Moreover, formation of an exaggerated root apical hook was observed. All of these facts indicated “ethylene triple response”, a typical symptom of ethylene action (Iqbal et al. 2013) and suggested that ethylene was involved in the PCD induced by kinetin (Drew et al. 2000). Greater ACC amount in the kinetin-treated roots as well as the impact of ethylene synthesis (AOA or CoCl2) and reception (NBD) inhibitors on kinetin-induced cell death also pointed to this phenomenon. Application of the inhibitors without kinetin did not induce cell death, whereas application of AOA or CoCl2 with kinetin decreased the number of dying cells; only NBD did not influence cell death induced by kinetin.
Also Ca2+ ions, which in plants are stored in ER lumen and in cellular walls (Bergner and Huber 2008), are known as critical molecules involved in regulation of many cellular processes including cell death (Stael et al. 2012). Our studies with the use of fluorescence microscopy and RQflex (Supplementary Fig. 1) showed that kinetin treatment increased the amount of Ca2+ ions in the root cortex cells of faba bean seedlings. The enhanced amount of cytosolic Ca2+ ions might be connected with their transport from ER via membrane CNG Channels (Stael et al. 2012) because 5 µM LaNO3 (inhibitor of ER-Ca2+ channels) applied to the seedlings culture medium totally inhibited kinetin-induced cell death (Supplementary Fig. 2). Ca2+ ions may be transported to the dying cells from the other cells through ER in plasmodesmata which join together plant cells into a cellular domain. Moreover, fluorescence micrographs showed that Ca2+ ions were spread in cytosol which was reflected in the mean fluorescence intensity of Ca2+-CTC complexes. During kinetin-induced cell death Ca2+-containing vesicles partially disappeared and Ca2+-free vacuoles emerged instead. Participation of Ca2+ ions in the kinetin-induced death of cortex cells analysed in relation to their interaction with ethylene showed that treatment with kinetin combined with AOA and CoCl2 partially or completely inhibited the cell death process and simultaneously the amount of Ca2+ ions was lower than in the roots treated only with kinetin. In the roots treated with kinetin and NBD, in which cell death was not inhibited, the amount of Ca2+ ions was similar to the kinetin-treated ones. These results showed that Ca2+ ions mediated kinetin-induced cell death. This indicates that they can also modulate cell death via control of ethylene synthesis and/or ethylene signalling pathways (Iqbal et al. 2013), thus ethylene might be the messenger in kinetin-induced cell death process. It also seems that kinetin and ethylene could regulate this process by a positive and/or negative feedback.
It seems possible that in the faba bean roots kinetin modified by phosphoribosyl transferase to adequate monophospates (Kunikowska et al. 2013a) activates ER and/or plasma membrane (Iqbal et al. 2013) specific cytokinin receptors (Caesar et al. 2011) and then cellular and ER membrane Ca2+ ion channels (CNGC; Jammes et al. 2011). It activates ethylene synthesis by Ca2+-sensitive ACC synthases and ACC oxidases (Iqbal et al. 2013) leading to increase in concentration of ethylene which activates ethylene specific receptors (ETR1,2; ERS1,2 and EIN4; Iqbal et al. 2013). Then through small GTP-dependent Ran proteins, molecular signals go downstream to CTR1 protein kinase (Iqbal et al. 2013) and activate ethylene-dependent MAP kinases and EIN2 (nuclear membrane receptor; Iqbal et al. 2013). Then primary (EIN3 and EIL1) and secondary (ERF1 and EREBP1) transcription factors are activated (Trobacher 2009; Iqbal et al. 2013) and via Ca2+ ions it leads to death of faba bean cortex cells and formation of aerenchyma according to the scheme:
References
Barciszewski J, Massino F, Clark BFC (2007) Kinetin—a multiactive molecule. Int J Biol Macromol 40:182–192
Bergner A, Huber RM (2008) Regulation of the endoplasmic reticulum Ca2+-store in cancer. Anti-Cancer Agents Med Chem 8:705–709
Byczkowska A, Kunikowska A, Kaźmierczak A (2013) Determination of ACC-induced cell programmed death in roots of Vicia faba ssp. minor seedlings by acridine orange and ethidium bromide staining. Protoplasma 250:121–128
Cabello CM, Bair WB III, Ley S, Lamore SD, Azimian S, Wondrak GT (2009) The experimental chemotherapeutic N6-furfuryladenosine (kinetin-riboside) induces rapid ATP depletion, genotoxic stress, and CDKN1A (p21) upregulation in human cancer cell lines. Biochem Pharmacol 77:1125–1138
Caesar K, Thamm AMK, Witthöft J, Elgass K, Huppenberger P, Grefen C, Horak J, Harter K (2011) Evidence for the localization of the Arabidopsis cytokinin receptors AHK3 and AHK4 in the endoplasmic reticulum. J Exp Bot 62:5571–5580
Carimi F, Zottini M, Formentin E, Terzi M, Schiavo FL (2003) Cytokinins: new apoptotic inducers in plants. Planta 216:413–421
Choi J, Choi D, Lee S, Ryu C, Hwang I (2011) Cytokinins and plant immunity: old foes or new friends? Trends Plant Sci 16:388–394
Domínguez F, Cejudo FJ (2012) A comparison between nuclear dismantling during plant and animal programmed cell death. Plant Sci 197:114–121
Doniak M, Barciszewska MZ, Kaźmierczak J, Kaźmierczak A (2014) The crucial elements of the ‘last step’ of programmed cell death induced by kinetin in root cortex of V. faba ssp. minor seedlings. Plant Cell Rep 33:2063–2076
Drew MC, He CJ, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5:123–127
Gonçalves CX, Tiecher A, Chaves FC, Nora L, Zhengguo L, Latché A, Pech J-C, Rombaldi CV (2013) Putative role of cytokinin in differential ethylene response of twolines of antisense ACC oxidase cantaloupe melons. Postharvest Biol Technol 86:511–519
Hwang I, Sheen J, Müller B (2012) Cytokinin signaling networks. Annu Rev Plant Biol 63:353–380
Iqbal N, Trivellini A, Masood A, Ferrante A, Khan NA (2013) Current understanding on ethylene signaling in plants: the influence of nutrient availability. Plant Physiol Biochem 73:128–138
Jammes F, Hu HC, Villiers F, Bouten R, Kwak JM (2011) Calcium-permeable channels in plant cells. FEBS J 278:4262–4276
Jaramillo RE, Nord EA, Chimungu JG, Brown KM, Lynch JP (2013) Root cortical burden influences drought tolerance in maize. Ann Bot-London 112:429–437
Joshi R, Kumar P (2012) Lysigenous aerenchyma formation involves non-apoptotic programmed cell death in rice (Oryza sativa L.) roots. Physiol Mol Biol Plants 18:1–9
Kaźmierczak A, Kaźmierczak JM (2007) The level of endogenous 1-aminocyclopropane-1-carboxylic acid in gametophytes of Anemia phyllitidis is increased during GA3-induced antheridia formation. Acta Physiol Plant 29:211–216
Kunikowska A, Byczkowska A, Kaźmierczak A (2013a) Kinetin induces cell death in root cortex cells of Vicia faba ssp. minor seedlings. Protoplasma 250:851–861
Kunikowska A, Byczkowska A, Doniak M, Kaźmierczak A (2013b) Cytokinins résumé: their signaling and role in programmed cell death in plants. Plant Cell Rep 32:771–780
Kushwah S, Jones AM, Laxmi A (2011) Cytokinin interplay with ethylene, auxin, and glucose signaling controls Arabidopsis seedling root directional growth. Plant Physiol 156:1851–1866
Lim J-H, An C-H, Kim Y-H, Jung B-K, Kim S-D (2012) Isolation of auxin- and -aminocyclopropane-1-carboxylic acid deaminase-producing bacterium and its effect on pepper growth under saline stress. J Korean Soc Appl Biol Chem 55:607–612
Scharein B, Voet-van-Vormizeele J, Harter K, Groth G (2008) Ethylene signaling: identification of a putative ETR1–AHP1 phosphorelay complex by fluorescence spectroscopy. Anal Biochem 377:72–76
Shi X, Rashotte AM (2012) Advances in upstream players of cytokinin phosphorelay: receptors and histidine phosphotransfer proteins. Plant Cell Rep 31:789–799
Stael S, Wurzinger B, Mair A, Mehlmer N, Vothknecht UC, Teige M (2012) Plant organellar calcium signalling: an emerging field. J Exp Bot 63:1525–1542
Trobacher CP (2009) Ethylene and programmed cell death in plants. Botany 87:757–769
van Doorn WG, Beers EP, Dangl JL, Franklin-Tong VE, Gallois P, Hara-Nishimura I, Jones AM, Kawai-Yamada M, Lam E, Mundy J, Mur LAJ, Petersen M, Smertenko A, Taliansky M, van Breusegem F, Wolpert T, Woltering E, Zhivotovsky B, Bozhkov PV (2011) Morphological classification of plant cell deaths. Cell Death Differ 18:1241–1246
van Staden J, Zazimalova E, George EF (2008) Plant growth regulators II: cytokinins, their analogues and antagonists. In: George EF, Hall MA, de Clerk G-J (eds) Plant propagation by tissue culture, 3rd edn. Springer, Dordrecht, pp 205–226
Zhang W, Swarup R, Bennett M, Schaller GE, Kieber JJ (2013) Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr Biol 23:1979–1989
Acknowledgments
We thank Mrs. M. Fronczak for her help in preparing this manuscript in English. This work was partially supported by Grant from the University of Łódź, No. 545/487, 545/764 and 545/502.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Amount of calcium ions expressed in µg g−1 FW measured using the RQflex equipment (Merck) in homogenates from 2-cm long apical parts V. faba ssp. minor seedling roots cultured without (0) and with kientin for 1, 2, 3, 6, 12, 18, 24, 48, 72, 96 h. Error bars represent the SE of the mean of three experiments. P < 0.05 indicates statistically significant differences between the results (TIFF 1944 kb)
Supplementary Fig. 2
Effect of La(NO3)2 on the number of living, dying and dead cells in the root cortex cells V. faba ssp. minor seedlings treated for 48, 72, 96 h without (A) and with kinetin (B) mixed with 5 μM La(NO3)2 (C,D). Error bars represent the SE of the mean of three experiments. P < 0.05 indicates statistically significant differences between the results (TIFF 3324 kb)
Rights and permissions
About this article
Cite this article
Doniak, M., Byczkowska, A. & Kaźmierczak, A. Kinetin-induced programmed death of cortex cells is mediated by ethylene and calcium ions in roots of Vicia faba ssp. minor . Plant Growth Regul 78, 335–343 (2016). https://doi.org/10.1007/s10725-015-0096-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0096-0