Abstract
Millets, resilient and nutritionally rich crops, are increasingly recognized for their potential in sustainable agriculture. Ammonium transporter (AMTs) gene family significantly contribute to the absorption and transport of NH4+ form of nitrogen in plants. The information about the structure and function of ammonium transporter genes in millet species is lacking. The millet crops such as pearl millet, proso millet, finger millet, sorghum, foxtail millet and green foxtail millet exhibit genetic variation in AMTs, which can be harnessed to improve NUE. Thus, genomic sequences of the six millet species were used and a total of 53 AMT genes were identified. Further, comprehensive analysis of chromosomal distribution, transmembrane structure prediction, presence of exons and introns, domain and motif organization, phylogeny, and synteny analysis were carried out. The phylogenetic analysis illustrated that millet AMTs belong to two subfamilies AMT1 and AMT2 (AMT2/AMT3/AMT4). Ka/Ks analysis showed that segmental duplications have contributed considerably in the evolution of millet AMTs. Phylogenetic classification of members of Poaceae using the amino acid sequences of AMT1.1 genes confirms the speciation patterns shown by matK gene sequence. Promoter analysis of millet AMTs showed presence of cis-elements related to light response, anaerobic induction, growth hormones, drought stress, biotic stress and several endogenous signals related to plant growth and development. This research provides insights into the structural and functional aspects of ammonium transporter genes in millets, and will serve as a foundation for utilizing AMTs for devising NUE strategies.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is the most commonly required macronutrient for plant growth and development. It is an essential element for the synthesis of biomolecules such as nucleotides, amino acids, proteins, chlorophyll, and several others (Marcos de Leão et al. 2020). Nitrogen in the soil exists in three forms, which include organic nitrogen compounds, ammonium (NH4+), and nitrate (NO3−) ions (Williams and Miller 2001). Plants absorb nitrogen primarily in the ammonium and nitrate forms, and the organic nitrogen compounds need to be converted to these two forms before being taken by the plants. The use of nitrogen by plants involves absorption, assimilation, and remobilization during plant growth and development. In addition to N absorption from the soil, nitrogen use efficiency (NUE) depends on the assimilation of inorganic nitrogen from the soil, and the utilization of nitrogen during the life cycle of a crop plant (Masclaux-Daubresse et al. 2010; Xu et al. 2012). Plants have a preference for ammonium N form over nitrate nitrogen for uptake from the soil due to the direct assimilation of NH4+ into amino acids in plant cells, whereas, NO3− nitrogen has to be reduced to NH4+ before assimilation (Bloom et al. 1992; Jiang et al. 2019; Boschiero et al. 2019). The biological assimilation of nitrogen occurs either through the glutamine synthetase/glutamate synthase pathway (GS/GOGAT) or through glutamate dehydrogenase (GDH), resulting in the synthesis of glutamine which is the substrate for the synthesis of other amino acids via transamination reactions. Ammonium transport is tightly regulated during plant growth and development by the activities of high- and low-affinity ammonium transporters (Loque et al. 2006; Yuan et al. 2007; Kiba and Krapp 2016). Generally, high-affinity ammonium transport is preferred for NH4+ acquisition by plants due to the low ammonium concentration (< 1 mm) in the soil (Hao et al. 2020).
Ammonium transporters (AMTs) involved in the uptake of NH4+ have been identified in varied plant species (Couturier et al. 2007; Yuan et al. 2007; Tang et al. 2020). These AMTs are distributed in the plasma membranes of plant cells and form homo-or heterotrimers complexes for facilitating the passing of NH4+ ions or NH3 through the pore (Shelden et al. 2001; Ludewig et al. 2003). The transport mechanism of plant AMTs could be an NH4+ uniporter, NH4+/H+ symporter, or NH3/H+ co-transporter. Plant AMTs can be divided into the following two subfamilies: the AMT1 subfamily (AMT1 cluster) and the AMT2 subfamily (AMT2/3/4 cluster) (Huang et al. 2022).
The AMT genes were identified both in prokaryotic and eukaryotic organisms (Mcdonald and Ward 2016). The first ammonium transporter genes were identified in Saccharomyces cerevisiae and Arabadopsis thaliana (Marini et al. 1997; Ninnemann et al. 1994). Further, AMT family genes were characterized in several crop species namely; Zea mays (Gu et al. 2013), Glycine max (Kobae et al. 2010), Arabidopsis thaliana (Loqué et al. 2006; Yuan et al. 2007, 2009, 2013; Lanquar et al. 2009; Huang et al. 2015), Lotus japonicas (Guether et al. 2009; Wang et al. 2022), Oryza sativa (Ferreira et al. 2015; Li et al. 2016), Medicago truncatula (Breuillin-Sessoms et al. 2015), Populus trichocarpa (Wu et al. 2015), Triticum aestivum (Duan et al. 2016; Li et al. 2017), Coffea canephora ( Santos et al. 2017), Medicago truncatula (Breuillin-Sessoms et al. 2015), Pinus (Castro-Rodriguez et al. 2016), Solanum lycopersicum (Filiz and Akbudak 2020), and Malus domestica (Huang et al. 2022).
Millets, a group of small-seeded grains, have gained recognition as a pivotal component in achieving global food security and contribute to agricultural sustainability. Millet species include pearl millet (Pennisetum glaucum), finger millet (Eleusine coracana), green foxtail millet (Setaria viridis), foxtail millet (Setaria italica), great millet (Sorghum bicolor), proso millet (Panicum miliaceum), kodo millet (Paspalum scrobiculatum), Japanese barnyard millet (Echinocloa esculenta), Indian barnyard millet (Echinocloa frumentacea), and little millet (Panicum sumatrense), among others (Goron and Raizada 2015). Millets are hardy, resilient crops that thrive in diverse agro-climatic conditions, making them an essential resource for enhancing agricultural sustainability. Their exceptional nutritional profile, including high levels of protein, fiber, and essential micronutrients, placed millets as key contributors to improving food security, especially in regions grappling with malnutrition and food scarcity (Ceasar 2023). Improving the NUE of the cereals is essential to enhance yields under low-nutrient soils and conserve the fertility of the soils (Baligar et al. 2001; Bariya and Ahish 2014; Naeem et al. 2017; Nieves-Cordones et al. 2020). Several investigations have been conducted during the last decade by the wider scientific community, employing various molecular genetic tools to study and improve the NUE of crop plants. These include the utilization of genome-wide association study (GWAS) approach, molecular marker-assisted breeding (MAB), nutrient transporter characterization and functional genomics approaches. These investigations have been reported for model crop plants to improve NUE (Hawkesford 2012; Avin-Wittenberg et al. 2018). However, genome-based and forward genetic research may not be easy for millets with limited genomic resources.
Complete annotated genome sequence information is lacking for many millet species, which limits the understanding of gene sequences involved in determining the NUE traits in these crops. Nevertheless, the genome sequences of six millets, namely; Setaria viridis, Setaria italica, Eleusine coracana, Sorghum bicolor, Pennisetum glaucum and Panicum miliaceum are available for sequence analyses. These genome sequences provide a valuable resource to understand the structural and functional domains of genes coding for plant productivity, consequently enabling us to identify the AMT genes in the millet genome. Scanty reports for the AMT gene family characterization in millets are available in the literature, with limited coverage of genome sequence databases. Inadequate analysis of two AMTs in S. bicolor (Koegel et al. 2013), followed by an EcAMT1 study with other nutrient transporters (Maharajan et al. 2022), and a brief report about S. italica AMTs (SiAMT1.1 and SiAMT1.3) phylogeny and level of expression (Ahmad et al. 2018; Ceasar et al. 2023). Whereas, S. viridis, P. glaucum and P. miliaceum AMTs have not been taken into account for defining the structural and functional domains of this gene family.
After discussing all the above, in this study, six millet species are taken into consideration. We performed detailed analyses of the sequence characteristics, gene structures, chromosome distribution, motif compositions, and evolutionary relationships of millet AMT genes. In this context, the characterization of AMT genes from these six species and their comparative analysis to study and improve the NUE in millets may be helpful for further research.
Material and methods
Identification of AMT genes in different species
The genomic sequences, protein sequences, coding sequences (CDS) and genomic feature files (GFF) of six millet crops (S. viridis, S. italica, E. corocana, S. bicolor, P. miliaceum, and P. glaucum) obtained from Phytozome database (https://phytozome-next.jgi.doe.gov/ accessed on June1, 2023), National Genomics Data Center (NGDC) (https://ngdc.cncb.ac.cn/gwh/ accessed on June 1 2023) and the International Pearl Millet Genome Sequencing Consortium (IPMGSC) (https://cegsb.icrisat.org/ipmgsc/index.html accessed on June 1 2023). The Hidden Markov model (HMM) of all the conserved protein domain file Pfam-A.hmm was downloaded from InterPro (https://www.ebi.ac.uk/interpro/download/pfam/ accessed on 5 June, 2023). A simple HMM search of the TBtools software (Chen et al. 2020) was used to obtain ammonium transporters in different species. Pfam Id of ammonium transporter (Ammonium_transp—PF00909) was used for this study. Proteins with e-values of less than 5E-40 were included in further analyses. Different splicing variants of one gene and the incomplete genes were discarded. We searched for the ammonium-domain in all of the collected proteins using Interproscan (http://www.ebi.ac.uk/ Tools/pfa/iprscan/ accessed on June 6, 2023) and SMART software (Letunic et al. 2012).
Physicochemical properties and transmembrane structure analysis of AMT proteins
The theoretical molecular weight (kDa) and isoelectric point (pI) of millet AMTs were calculated using the ExPASy server (http://web.expasy.org/compute_pi/ accessed on June 6, 2023) (Gasteiger et al. 2003). The evaluation of the grand average of hydropathicity (GRAVY) of all identified proteins was measured through the GRAVY calculator (https://www.gravy-calculator.de/ accessed on June 6, 2023) (Gasteiger 2003). Predictions of subcellular localization of the concerned proteins were verified with the help of Plant-mPloc tool (http://www.csbio.sjtu.edu.cn/cgi-bin/PlantmPLoc.cgi/ accessed on June 6, 2023) (Chou and Shen 2010). The TMHMM server v. 2.0 (https://services.healthtech.dtu.dk/services/TMHMM-2.0/ accessed on June 6, 2023) (Krogh et al. 2001) was used for the prediction of transmembrane helices in AMT proteins. Individually, the physical locations of millet AMTs genes were obtained from the millet database (S. viridis, S. italica, E. coracana, S. bicolor, P. miliaceum, and P. glaucum) genome, and the map to locate genes on chromosomes of all six millet species was constructed through the PhenoGram (http://visualization.ritchielab.org/phenograms/plot accessed on June 10, 2023 (Wolfe et al. 2013).
Gene structure, conserved motif and conserved protein domain analyses of AMTs
The gene structures (CDS/exon/intron) of all the AMT genes were determined using the Gene Structure Display Server (CSDS) (http://gsds.gao-lab. org/ accessed on June 8, 2023) (Hu et al. 2015). For these analyses, the predicted coding sequence (CDS) of AMT genes and their corresponding genomic DNA sequences were used. The MEME (Multiple Em for Motif Elicitation) online tool (Bailey and Elkan 1994; Bailey et al. 2009) was used to identify the conserved motifs in the promoter regions of AMT genes and AMT amino acid sequences (https://meme-suite.org/meme/tools/meme accessed on June 8, 2023). The conserved domains of AMT proteins were analysed by the NCBI-CD (National Center for Biotechnology Information- Conserved Domain) search tool (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi accessed on June 8, 2023) (Marchler-Bauer and Bryant 2004). The TBtools software was used to integrate the phylogenetic tree, conserved motifs of and domains of millet AMT proteins (Chen et al. 2020).
Phylogenetic tree analysis of AMTs
The full-length amino acid sequences of AMTs from Arabidopsis thaliana, Triticum aestivum, Oryza sativa, Zea mays, Brachypodium distachyon, and Hordium vulgare were downloaded from the Phytozome database (Goodstein et al. 2012). AMT amino acid sequences of two bacteria, viz. Escherichia coli and Nitrosomonas europaea are also downloaded from NCBI (National Center for Biotechnology Information) (https://blast.ncbi.nlm.nih.gov/ accessed on June 6, 2023). To gain a deeper understanding of the taxonomical classification of the poaceae family, the chloroplast maturase K (matK) amino acid sequences were also downloaded. The amino acid sequences of AMTs as well as matK were aligned by MEGA-XI software (Koichiro et al. 2021), and a total of four phylogenetic trees were constructed by the maximum-likelihood method (ML). Bootstrap analysis was calculated for 1000 replicates. The evolutionary tree was visualized on the web-based tool Interactive Tree of Life (iTOL, https://itol.embl.de/ accessed on June 15, 2023) (Letunic and Bork 2021).
Ka/Ks analyses
The synonymous (Ks) and non-synonymous (Ka) substitution rates of the paralogs genes were further investigated by using the Ka_Ks calculator 2.0 (Zhang et al. 2006). A circular ideogram was made by Circos (Krzywinski et al. 2009) using TBTool software (Chen et al. 2020) to facilitate the display of relationships between paralogous pairs by the use of coloured lines. These encode the position, size, and orientation of related genomic elements in the Circos plots.
Synteny analysis of AMT genes
For visualization of protein sequence similarity between these six millet AMT genes, an online visualization tool named Circoletto (http://tools.bat.infspire.org/circoletto/ accessed on June 16, 2023) (Darzentas 2010) was used, which provides fast and informative overview of sequence similarity of search results. These results provide an essential first glimpse of the relationship between protein sequences.
Cis-element analysis of millet AMT promoter regions
The 2 kb upstream genomic DNA sequences of all six millet AMT genes were used for promoter analysis, and the cis-regulatory elements were predicted using the PlantCARE online website (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/ accessed on 18 June 2023) (Lescot et al. 2002). The data were visualised by TBtools (Chen et al. 2020).
Results
Identification of AMT genes in different species
After validation of AMTs by HMM search, a total of 53 AMT protein sequences (S. viridis-7; S. italica-9; E. corocana -12; S. bicolor-5; P. glaucum -8 and P. miliaceum -12) were identified from six millet genomes. The AMTs of these species were termed as per existing rules of nomenclature for gene symbols, such as SvAMTs (derived from S. viridis), SiAMTs (derived from S. italica), EcAMTs (derived from E. corocana), SbAMTs (derived from S. bicolor), PgAMTs (derived from P. glaucum) and PmAMTs (derived from P. glaucum) genes throughout the study.
Physicochemical properties and transmembrane structure analysis of AMT proteins
The physiochemical properties of AMT proteins have been established using parameters such as chromosome location, strand, protein length, molecular weight (MW), isoelectric point (pI), prediction of the hydrophobicity (GRAVY), subcellular location and their family. The lengths of the millet AMT proteins ranged from 304 (SbAMT 2.2b; Sobic.003G344700) to 632 (EcAMT 2.1; ELECO.r07.5BG0417460) amino acids, with molecular weight ranging from 32.07 kD (PmAMT4.1b; GWHPAAEZ055444) to 67.61 kD (EcAMT 2.1; ELECO.r07.5BG0417460) and theoretical pI values ranging from 5.37 (SbAMT 3.3; Sobic.004G173200) to 8.84 (PmAMT1.3b; GWHPAAEZ069937). Subcellular localization prediction showed that all millet AMTs were localized to the cell membrane with few exceptions such as, SbAMT 3.3 (Sobic.004G173200), PmAMT1.3a (GWHPAAEZ021947), PmAMT3.2 (GWHPAAEZ054634), and PmAMT3.3 (GWHPAAEZ070534) are located both in cell membrane as well as vacuole. And the PgAMT1.1 (Pgl_GLEAN_10009225) is located in both cell membrane and mitochondrion whereas PmAMT3.2 (GWHPAAEZ054634) found in cell membrane, vacuole and mitochondrion (Table 1). The grand average of hydropathy (GRAVY) value was calculated for all the millet AMT proteins.
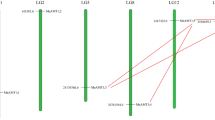
In this study, the predictions of the hydrophobicity of the deduced amino acid sequences indicated that the GRAVY of all millet AMT proteins were above zero, this led to conclusion that these amino acids are polar in nature (Table 1). Further, distribution of all six millet AMTs on chromosomes was analysed. It was observed that AMTs of S. viridis were located on five chromosomes viz. Chr1, Chr3, Chr5, Chr7 and Chr9 (Online Resource: S1), AMTs of S. italica located on five scaffolds with number 1, 3, 5, 7, 9 (Online Resource: S2), AMTs of E. corocana placed on Chr 1A, Chr 1B, Chr 2A, Chr 3A, Chr 3B, Chr 4B and Chr 5B. (Online Resource: S3), AMTs of S. bicolor located on three chromosomes viz, Chr1, Chr3 and Chr4 (Online Resource: S4). PgAMTs were present on three chromosomes (Chr 1, Chr 3 and Chr 6) and on the scaffold 2474 (Fig. 1). Whereas, PmAMTs were located on six chromosomes viz. Chr 1, Chr 3, Chr 4, Chr 5, Chr 6 and Chr 12 (Online Resource: S5). Transmembrane domain analysis of all millet AMTs showed occurrence of conserved transmembrane domains (Fig. 2 and Online Resource: S6-S10). These transmembrane domains regulate membrane localization and transport activity of a protein. Generally, millet AMTs have 11–12 transmembrane domains, whereas, these PgAMT1.1, PmAMT4.1b, SbAMT 2.2, SbAMT 3.3, PmAMT3.2, PgAMT1.2b, PmAMT2.2, PgAMT2.2, PmAMT1.3b and PgAMT1.2a have transmembrane domains varying from 6 to 10) which may be due to the small size of their protein sequences (Table 1).
Representative figure chromosomes of Pennisetum glaucum showing distribution of PgAMT genes. The chromosome number is listed below each chromosome while the numbers on the left represent location of the PgAMT genes. (AMTs chromosomal locations for other five millet species are given in Online Resource: S1-S5)
Representative figure of transmembrane structure prediction AMT proteins of Pennisetum glaucum. *Orange line represents outside, purple line indicates on transmembrane and blue line represents inside transmembrane position. (Transmembrane structure prediction of AMTs of other five millet species are given in Online Resource: S6-S10)
Gene structure, conserved motif and conserved protein domain analyses of AMTs
Structural analysis of the AMT proteins of six millet species were carried out expending the conserved domains and motifs based on the evolutionary relationships (Fig. 3). Gene structures of millet AMT proteins were predicted by using their CDS and genomic sequences. The graphical representation derived using GSDS showed that the AMT1 superfamily has less number of introns whereas in AMT2 the presence of introns is very common (Fig. 3). The domain analysis prediction showed that, the ammonium transporter (Ammonium_transp; PFam ID: PF00909, InterPro ID: IPR001905) structural domains are present in all the query proteins. This Ammonium_transp domains have found to relate the cl03012 protein superfamily and are mainly associated with transporting NH4 + across the membrane. In MEME server, the number of motif finder parameter was set to 20, so that upto 20 putative conserved motifs were found from each of the query protein sequences. Throughout the motif analysis it was found that, the subfamilies AMT1 and AMT2 had variable motif compositions. Also, proteins in the same subgroup showed identical motif components. Some of the motifs usually range from 1 to 5 were found present in AMTs of all the species this indicated that there are characteristic motifs of ammonium transporters.
Noticeably, presence of a small motif can cause differences in subgroups and which may give an idea about the evolution of AMTs. In S. viridis AMT proteins, four motifs (motif-3, 6, 7 and 9) were found commonly present in all the AMT proteins of this species (Online Resource: S11). In S. italica AMT proteins, the five motifs (motif-5, 6, 8, 9 and 11) were found common in all AMTs (Online Resource: S12). Whereas in E. corocana, only single motif (motif-1) was found common in both the AMT1 and AMT2 families (Online Resource: S13). Similarly, in P. miliaceum, a single motif (motif-4) was observed common in all the AMTs (Online Resource: S15). Furthermore, in S. bicolor three motifs (motif-1, 2 and 19) are common in all the AMT proteins, (Online Resource: S14). Motif analysis of P. glaucum AMTs showed four common motifs in both the protein subfamily (AMT 1 and AMT 2) (Fig. 4).
Illustration of conserved protein motifs and conserved domain of AMTs in Pennisetum glaucum. An unrooted phylogenetic tree represents AMT1 and AMT2 subfamilies with their respective motifs are represented using different colours and conserved domains are shown by yellow boxes. (Conserved protein motifs and conserved domain analysis of AMTs of other five millet species are given in Online Resource: S11-S15)
Phylogenetic tree analysis of AMTs
A total of 53 millet AMT protein sequences have been identified using sequence search and alignment and used to understand the evolutionary relationships among AMT genes (Fig. 5). The common feature among AMTs of all the millet crops showed that they belong to two subfamilies i.e. AMT1 and AMT2 (AMT2/AMT3/AMT4). The major evolutionary conservation among the transporters exhibited that all millet AMTs stemmed from two major AMT transporter groups necessitates confirmation and further exploration of the evolutionary relationships with the AMTs from other species. The AMT protein sequences of twelve plant species (Setaria viridis, Setaria italica, Eleusine corocana, Sorghum bicolor, Pennisetum glaucum, Panicum miliaceum, Arabidopsis thaliana, Oriza sativa, Zea mays, Hordium vulgare, Triticum aestivum and Brachypodium distachyon) and two bacteria namely, Escherichia coli and Nitrosomonas europaea (ammonia oxidizing bacterium) were used to construct phylogenetic tree (Fig. 6). The phylogenetic tree structure clearly demonstrated the association of AMTs of Poaceae family which include all millets and other cereal species. The AMTs of Poaceae family have some degree of similarity with AMT genes from Arabadopsis thaliana, however, formed a different clade and clearly indicated divergence of the monocot transporters from the dicot ammonium transporters. The exclusion of bacterial group AMTs (E. coli and N. europaea) as an outgroup further, confirmed the AMT relationships and divergences in the phylogenetic grouping.
Phylogenetic tree analysis of AMTs of Setaria viridis (Sv), Setaria italica (Si), Eleusine corocana (Ec), Sorghum bicolour (Sb), Arabidopsis thaliana (At), Oriza sativa (Os), Zea mays (Zm), Hordium vulgare (Hv), Triticum aestivum (Ta) and Brachypodium distachyon (Bd). Two bacterial AMT namely, Escherichia coli (Eco) and Nitrosomona seuropaea (Ne) showing out grouping. *Different colors of circles represent different clusters
Ka/Ks analyses
Nucleotide substitutions in the coding regions may or may not result into amino acid change in the protein. One of the parameters i.e. Ka/Ks ratio which is the measure of the number of nonsynonymous substitutions per nonsynonymous site (Ka) and the number of synonymous substitutions per synonymous site (Ks). This Ka/Ks ration is the measure of selection pressure a gene has experienced during evolution. In the millet AMT family, analysis of selection types of duplicate gene pairs in the SvAMTs, SiAMTs, EcAMTs, SbAMTs, PgAMTs and PmAMTs genes were carried out using the Ka/Ks ratio. Majority of duplicated gene pairs has Ka/Ks ration less than one hence, less nonsynonymous substitutions taken place and the most of millet AMT genes have undergone negative selection. Majority of AMT genes are the resultant products of purifying selection during evolution (Table 2). However, the gene pair SbAMT1.2 and SbAMT4.1 showed that it had undergone neutral selection (Ka/Ks = 1). Schematic representations of the chromosomal distribution and inter chromosomal relationships was studied for all the six millet species by making circos plot of each species separately. These graphical representations showed gene duplication events in circular format. Among the 7 SvAMTs genes, three segmental duplication pairs (SvAMT1.2/SvAMT1.1, SvAMT2.2/SvAMT2.1 and SvAMT3.1/SvAMT3.2 were identified (Online Resource: S16). In S. italica, out of 9 SiAMTs genes only one tandem repeat pair (SiAMT2.3/SiAMT2.2) was found and other 3 pairs (SiAMT4.1/SiAMT2.1, SiAMT3.2/SiAMT3.1 and SiAMT1.1/SiAMT1.2) showed segment duplication (Online Resource: S17). Further, the 12 EcAMTs, 5 segment duplication pairs (EcAMT3.1b/EcAMT3.1a, EcAMT2.2c/EcAMT2.2b, EcAMT2.2d /EcAMT2.2a, EcAMT1.1a/EcAMT1.2a and EcAMT4.2/EcAMT4.1) were identified (Online Resource: S18). In S. bicolor, two segmental duplication pairs were observed out of 5 SbAMTs genes (Online Resource: S19). In P. glaucum, out of 8 genes, one PgAMT pair (PgAMT1.2b/PgAMT1.2a) found as tandem while other two pairs (PgAMT3.2/PgAMT3.3 and PgAMT2.2/PgAMT2.1) showed segmental duplications (Fig. 7). Whereas, in case of P. miliaceum, all the five pairs (PmAMT4.1b/PmAMT4.1a, PmAMT2.1/PmAMT2.3, PmAMT1.3a/PmAMT1.3b, PmAMT1.2b/PmAMT1.2a and PmAMT3.3/PmAMT3.1) among twelve genes showed segmental duplications (Online Resource: S20). The frequency of occurrence of segmental duplication events in the AMTs of these millet species suggest that these duplications plays bigger role in evolution of these genomes. Several research reports mentioned the significant role of tandem and segmental duplication events in gene family expansion and evolution of their genomes (Canon et al. 2004; Panchy et al. 2016; Kuo et al. 2019).
Schematic representations of the chromosomal distribution and inter-chromosomal relationships among AMT genes of Pennisetum glaucum. *Duplication events occurred in AMT gene family of P. glaucum are represented by blue, green, and yellow lines. *Chromosomes are represented in sky blue colors with the chromosomal number indicated inside each chromosome. (Visualization of chromosomal distribution and inter chromosomal relationships of other five millet species are given in the Online Resource: S16-S20)
Synteny analysis of AMT genes
In this study, synteny analysis between six millet AMT proteins (S. viridis, S. italica, E. corocana, S. bicolor, P. glaucum and P. miliaceum) with other species of poaceae family (O. sativa, Z. mays, H. vulgare, T. aestivum and B. distachyon) was performed by circoletto tool to understand the evolutionary history of genomes. In this analysis, amino acid sequences of AMTs of one species were used as query and while the rest of all the AMT sequences used as comparative files. ‘E-value’ and ‘score/max’ ratio parameter was considered to produce the colour bands and the colour of the bands indicate the sequence similarities (blue ≤ 0.25, green ≤ 0.50, orange ≤ 0.75, and red > 0.75). Based on the synteny analyses, maximum high synteny blocks (maximum red coloured bands > 0.75) were identified between AMTs of millets and other poaceae members/species. Considering these maximum red coloured synteny blocks, it can be concluded that, the AMT genes are more conserved in terms of evolutionary and genomic architecture in poaceae family.
Based on best score match parameter in circoletto tool, the best matched AMT sequences of different species showed synteny blocks. The AMTs of the species S. viridis and S. italica showed best synteny association (Online Resource: S21, S22). In case of E. corocana AMTs, 12 best score synteny blocks were identified between E. corocana AMTs and S. viridis, S. italica and S. bicolor AMTs (Online Resource: S23), while in case of S. bicolor 5 synteny blocks with best score was found between S. bicolor and Zea mays, S. viridis, S. italica (Online Resource: S24). In P. glaucum, eight best score synteny blocks were found in between P. glaucum and S. viridis, S. italica, S. bicolor AMTs (Fig. 8). Twelve best score synteny blocks were found to be associated with P. miliaceum AMTs and P. glaucum and S. viridis, S. italica (Online Resource: S25). Genomic dynamicity and evolutionary improvement along mobile elements in the genome of these six studied millet species were determined in these syntenic circles.
Visualization of the sequence similarity of AMT genes between Pennisetum glaucum with other millet AMTs as well as AMTs of different species of poaceae family (Oryza sativa, Zea mays, Hordeum vulgare, Triticum aestivum and B. distachyon). a Representation of synteny of PgAMTs and other AMTs. b Synteny blocks in ‘best score’ matching parameter of circoletto showing best matches between AMTs of P. glaucum and AMTs belongs to same tribe (S. italica, S. viridis and P. miliaceum). (Visualization of synteny analysis of other five millets are given in Online Resource: S21-S25)
Cis-element analysis of millet AMT promoter regions
To study the expression characteristics and potential functions of millet AMT genes, 2000 bp upstream sequences of start codons of the AMT genes of the six studied species were obtained as promoter sequences and used to analyse their cis-acting elements. The comprehensive results showed that millet AMT promoters have numerous cis-elements that respond to endogenous signals related to plant growth and development (viz. zein metabolism, circadian control, endosperm and meristem expression, root-seed-palisade mesophyll cells regulations), growth hormones (mainly auxin, gibberellin, abscisic acid, salicylic acid and methyl jasmonate), and environmental stresses (e.g. light response elements, low temperature stress-related elements, defense and stress, wound, anaerobic induction, anoxic specific induction and drought stress) (Fig. 9, Online Resource: S26-S30, Table. S1). All the millet AMT promoters have cis-elements responsive to light, suggesting an essential role of these AMT genes in plant growth and metabolism. From the data, it was evident that each gene promoter contains response element (s) to different phytohormone (s) with varied numbers ranging from 1 to 20, indicating that these AMT genes are under the regulation of hormone (s) and are involved in the hormone-mediated biological processes. Cis-elements involved in regulation of anaerobic induction are also common in all the millet AMT promoters, suggesting their possible role in plant growth and metabolism in anaerobic conditions. Almost all the promoter sequences have binding site for MYB-transcription factors related to many biological processes, such as plant growth and development, primary and secondary metabolic reactions, different physiological activity and responses to environmental stresses. Cis-elements related to drought- inducibility are also present adequate amount in almost all the promoters.
Representation of promoter cis-element analysis of AMT genes in P. glaucum. a Promoter position information. The different colored markers indicate different predicted cis-acting elements. b Promoter number analysis. The color scale to the right of the heat map represents the number of promoters. (Promoter cis-element analysis of AMT genes of other five millets are given in Online Resource: S26-S30)
Discussion
In plants, the Arabidopsis thaliana AtAMT gene was first recognized as an ammonium transporter (Ninnemann et al. 1994; Sohlenkampet al. 2000). Further analysis in Arabidopsis thaliana, proved that these AMTs also act as ammonium sensors that can sense the signal for cell–cell communication during plant growth and promote root to shoot ammonium translocation (Giehl et al. 2017). Genetic and molecular analysis in rice AMTs also proved that it acts in cell–cell communication and enhance the crown root formation in plants (Luo et al. 2022). In poaceae, several AMT homologues have been reported to play important roles in ammonium transport, such as Triticum aestivum (Li et al. 2017; Jiang et al. 2019), Oriza sativa (Li et al. 2009; Su-mei et al. 2012), Zea mays (Gu et al. 2013; Dechorgnat et al. 2019), Hordium vugare (Han et al. 2016) and Saccharum spontaneum (Wu et al 2021). In some millets, these AMT genes were also identified and are predicted to be associated with plant growth and development via ammonium transport (Maharajan et al. 2022; Ceasar et al. 2023). In Sorghum bicolor, induction of AMTs by arbuscular mycorrhizal fungi was studied which enhances the ammonium transport in plant parts (Koegel et al. 2013). The results suggested that, this AMT gene family has been involved in many biological processes in poaceae family. Millets are highly nutritious cereal crops and realizing their potential as nutraceutical food, much emphasis is given to improvement of these crops. Understanding the genomic loci involved in response, uptake and utilization of the nitrogen, a major nutrient in millet growth and production has utmost significance. There are two transporters involved in nitrogen uptake, the NRTs and AMTs in crop plants. Extensive research on in-silico analysis of NRTs has been carried out in millets. However, information about AMTs in millet crops is scanty. Hence, we performed an in-silico characterization of millet AMT genes that belong to two subfamilies viz. AMT1 and AMT2 (AMT2/AMT3/AMT4). Generally, the approximate length of members AMT gene family are between 400–450 amino acids and the structure can range from 45 to 50 kDa (Ninnemann et al. 1994; Blakey et al. 2002). The present study involved AMTs of six millet species and the length of the amino acids ranged from 304 to 632, and molecular weights ranging from 32.07 to 67.61 kDa are in consensus with earlier research.
Structural analysis AMT genes of millet revealed that the two subfamilies AMT1 and AMT2 exhibit divergent exon–intron patterns (Fig. 3). The structure of AMT genes of millet are highly conserved, among all the studied millets. AMT1 of P. glaucum (PgAMT1.2b, PgAMT1.2a, PgAMT1.1) and P. miliaceum (PmAMT1.2b, PmAMT1.3a) have introns in it, others are intronless. Similar research in MdAMT1 of Apple and GmAMT1 of Soybean reported absence of introns in AMT1 sub family (Huang et al. 2022; Yang et al. 2023). In Populus, Lotus japonicus, chilli pepper, most AMT1 genes have no introns in it, with the exception of LjAMT1.1, PtAMT1.7 and CaAMT1.1 that have one intron (Wu et al. 2015; Wang et al. 2022; Fang et al 2023). Millet AMT2 genes contain introns (ranges from 1 to 3), exons, and UTRs. The lengths of the UTRs, exons and introns vary among these AMT2 genes. Introns are usually involved in the regulation of gene expression and/or RNA stability (Shaul 2017). Mutations in critical regions in gene structure, including upstream region and coding sequence site may alter the expression patterns of members of gene family under evolution events (Heidari et al. 2022; Yaghobi and Heidari 2023). The lack of introns in the AMT1 subfamily genes suggests that the expression of these genes is essentially regulated at the transcriptional level. Large variations in the length and number of introns in different AMT2 subfamily genes indicate that these genes may undergo more complicated regulation, such as mRNA transport, alternative splicing, or chromatin assembly, which have been reported previously (Zhao et al. 2014; Jo and Choi 2015).
The phylogenetic analysis for ammonium transporters genes of six millets (Fig. 5) revealed that S. viridis, S. italica, P. glaucum and P. miliaceum shares a close relationship after alignment of retrieved proteins sequences of all the AMTs. This could be due to taxonomic commonality for instance, these four species (S. viridis, S. italica, P. glaucum and P. miliaceum) belong to the same tribe Paniceae (Li and Bruntnell 2011). Further, combined phylogenetic analysis using AMT proteins of all cereals (millets, rice, wheat, maize, barley and brachypodium), arabidopsis and bacterial AMTs (E. coli and N. europaea) evidently identified close association among six millets species for two ammonium transporter subfamilies. The maize transporter (ZmAMTs) were found closely related with millet AMTs as Zea mays (maize) is a member of Andropogoneae, which is a sister tribe to millet family, the Paniceae (Li and Brutnell 2011). The phylogenetic analysis of all millet AMTs and the combined phylogenetics involving AMTs of rice, wheat, maize, arabadopsis AMTs and bacterial AMTs (E. coli and N. europaea) clustered into conspicuous two subgroups of AMTs and similar findings of AMTs grouping has been reported in several investigations carried on other crops such as, soybean (Yang et al. 2023), poplus (Wu et al. 2015) and cassava (Xia et al. 2023).
In synteny analysis, the high score synteny blocks (red > 0.75) reinforce the idea that, AMT genes of poaceae are conserved in this family (Fig. 8, Online Resource: S21-S25). Five studied millets (S. viridis, S. italica, P. glaucum, P. miliaceum and S. bicolor) belongs to the subfamily panicoideae showed the best score synteny blocks frequently, imparting knowledge about the conservation of AMT genes in this subfamily. Again, AMTs of S. viridis, S. italica, P. glaucum and P. miliaceum exhibit maximum best scores synteny blocks as they belong to the same tribe paniceae. Furthermore, best score synteny blocks were found between S. bicolor and Z. mays, which again supporting the concept that AMTs are also conserved in tribes, as those two belongs to the identical tribe andropogoneae. In best match synteny analysis of E. corocana synteny blocks were also appeared between E. corocana and other millet AMTs, but the frequency is low. E. corocana belongs to chloridoideae subfamily, which is a close relative of subfamily panicoideae, and this may suggest that, there are resemblance of AMT genes between two closely related sister subfamilies. No best score synteny blocks were found between millet AMTs and other members of poaceae viz. O. sativa (subfamily: oryzoideae), B. distachyon, T. aestivum and H. vulgare (subfamily: pooideae) considered for this study, as they shared distant relationship from panicoideae subfamily. The phylogenetic tree generated by chloroplast matK genes of all the species of poaceae family in this study gives a depiction of taxonomic classification of poaceae family (Fig. 10), (Sorenget al. 2015, 2017, 2022). Interestingly, the phylogenetic tree constructed using AMT1.1 gene of all the previously studied Poaceae family crops reflected precisely the same pattern as proposed in their taxonomic classification (Fig. 10). This suggests that, in the course of evolution, AMT genes were also evolved by means of gene flow, natural selection, mutation or genetic drift.
A promoter is a region of DNA upstream of a gene where relevant proteins viz. RNA polymerase and transcription factors have to bind and initiate transcription of that gene (Hernandez-Garcia and Finer 2014). The level of transcriptional activation in eukaryotes is coordinated by upstream cis-acting elements in the regulation of gene expression, which are key links in plant environmental responses. Plant gene promoters contain a variety of important cis-acting elements that are involved in regulating the expression of corresponding downstream genes at the transcriptional level, thereby enabling plants to resist environmental stresses (Li et al. 2020). Cis-acting regulatory element analysis of millet AMTs promoter regions revealed a great abundance of light responsive elements, which implies that AMT gene expression is closely associated with photosynthesis and might be diurnally regulated. In research with Arabidopsis AMTs, AtAMT1.3 exhibited a typical diurnal pattern of change in expression; absorption of ammonium increased significantly towards the end of the day's light, and decreased as light intensity decreased (Gazzarrini et al. 1999). Additionally, two tomato AMTs (LeAMT1.2 and LeAMT1.3) also demonstrated rhythmic regulation (Von Wirén et al. 2000). Further, all the AMT genes share cis-elements responsive to anaerobic conditions. This has functional application in rice where AMTs has been widely studied for their role to uptake and utilize ammonium form of nitrogen in anaerobic conditions (Konishi and Feng. 2021). Cis-element analysis of AMT genes in majority of millet species showed involvement of at least one cis element in host defense response to the various biotic stresses in this study. It has been revealed in wheat and rice that ammonium transporters 1.1, 1.3, and 2.3 are associated with defense response to pathogens (Wu et al. 2022; Li et al. 2017; Jiang et al. 2019). Similarly evidences in support of role of AMTs in plant–microbe symbiosis e.g. LjAMT2.1 and LjAMT2.2 of Lotus japonicus and MtAMT2.3 of Medicago truncatula could be involved in ammonium transport from the host plants to nitrogen-fixing rhizobia and arbuscular mycorrhizae (Simon-Rosin et al. 2003; Guether et al. 2009; Breuillin-Sessoms et al. 2015). In addition, these AMT genes are under the control of different phytohormone (s) during the development and their response varies under diverse environmental conditions, thereby co-ordinately regulating ammonium uptake and metabolism.
Conclusion
The ammonium transporter gene (AMT) family plays a key role in the acquisition and transport of NH4 + forms of nitrogen in plants. This study identified a total of 53 AMT genes in the genomic sequences of the six millet species and classified them into two subfamilies, AMT1 and AMT2 (AMT2/AMT3/AMT4), based on phylogenetic analysis. The expansion of millet AMTs is the outcome of segmental and tandem duplication events in evolution. Syntenic conservation was observed in the structure and function of ammonium transporters in members of Poaceae. Promoter analysis of millet AMTs showed the presence of cis-elements regulating light response, anaerobic induction, growth hormones, drought stress, biotic stress, and several endogenous signals related to plant growth and development. This study provides in-depth information about the ammonium transporter gene family in millets, which would assist in improving nitrogen use efficiency through genomic manipulation of the expression patterns of these transporters.
Data availability
All data generated or analysed during this study are available from the corresponding author upon request.
References
Adeola O, Orban JI (1995) Chemical composition and nutrient digestibility of pearl millet (Pennisetum glaucum) fed to growing pigs. J Cereal Sci 22:177–184. https://doi.org/10.1016/0733-5210(95)90048-9
Ahmad Z, Nadeem F, Wang R, Diao X, Han Y, Wang X, Li X (2018) A Larger root system is coupled with contrasting expression patterns of phosphate and nitrate transporters in foxtail millet [Setaria italica (L.) Beauv.] under phosphate limitation. Front Plant Sci 9:1367. https://doi.org/10.3389/fpls.2018.01367
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Amadou I, Gounga ME, Guo-Wei L (2013) Millets: Nutritional composition, some health benefits and processing—A review. Emir. J Food Agric 25(7):501–508. https://doi.org/10.9755/ejfa.v25i7
Anitha S, Govindaraj M, Kane-Potaka J (2020) Balanced amino acid and higher micronutrients in millets complements legumes for improved human dietary nutrition. Cereal Chem 97:74–84. https://doi.org/10.1002/cche.10227
Avin-Wittenberg T, Baluška F, Bozhkov PV et al (2018) Autophagyrelated approaches for improving nutrient use efciency and crop yield protection. J Exp Bot 69:1335–1353. https://doi.org/10.1093/jxb/ery069
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. https://doi.org/10.1093/nar/gkp335
Bailey TL, Elkan C (2004) CD-Search: Protein domain annotations on the fly. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In: Proceedings of the 2nd international conference on intelligent systems for molecular biology, Stanford, CA, USA. Nucleic Acids Res. International Conference on Intelligent Systems for Molecular Biology 2:28–36 A Marchler-Bauer, Bryant SH, eds. 32) [Web server issue]: W327–W331.
Baligar VC, Fageria NK, He ZL (2001) Nutrient use efciency in plants. Commun Soil Sci Plant Anal. https://doi.org/10.1081/CSS-100104098
Bariya H, Ahish P (2014) Nutrient use efciency in plants: concepts and approaches. Plant Ecophysiol. https://doi.org/10.1007/978-3-319-10635-9
Blakey D, Leech A, Thomas GH, Coutts G, Findlay K, Merrick M (2002) Purification of the escherichia coli ammonium transporter AmtB reveals a trimeric stoichiometry. Biochem J 364(Pt 2):527–535. https://doi.org/10.1042/BJ20011761
Bloom AJ, Sukrapanna SS, Warner RL (1992) Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol 99:1294–1301. https://doi.org/10.1104/pp.99.4.1294
Boschiero BN, Mariano E, Azevedo RA, Trivelinb PCO (2019) Influence of nitrate—ammonium ratio on the growth, nutrition, and metabolism of sugarcane. Plant Physiol Bioch 139:246–255. https://doi.org/10.1016/j.plaphy.2019.03.024
Breuillin-Sessoms F, Floss DS, Gomez SK, Pumplin N, Ding Y, Levesque-Tremblay V, Noar RD, Daniels DA, Bravo A, Eaglesham JB, Benedito VA, Udvardi MK, Harrison MJ (2015) Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 27:1352–1366. https://doi.org/10.1105/tpc.114.131144
Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol 4:10. https://doi.org/10.1186/1471-2229-4-10
Castro-Rodríguez V, Assaf-Casals I, Pérez-Tienda J, Fan X, Avila C, Miller A, Cánovas FM (2016) Deciphering the molecular basis of ammonium uptake and transport in maritime pine. Plant Cell Environ 39:1669–1682. https://doi.org/10.1111/pce.12692
Ceasar SA (2023) Foxtail millet (Setaria italica) as a model system to study and improve the nutrient transport in cereals. Plant Growth Regul 99:3–10. https://doi.org/10.1007/s10725-022-00878-x
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–1202. https://doi.org/10.1016/j.molp.2020.06.009
Chou KC, Shen HB (2010) Plant-mPLoc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 5(6):e11335. https://doi.org/10.1371/journal.pone.0011335
CNCB-NGDC Members and Partners (2023) Database resources of the national genomics data center, China national center for bioinformation in 2023. Nucleic Acids Res 51(D1):D18–D28. https://doi.org/10.1093/nar/gkac1073
Couturier J, Montanini B, Martin F, Brun A, Blaudez D, Chalot M (2007) The expanded family of ammonium transporters in the perennial poplar plant. New Phytol 174:137–150. https://doi.org/10.1111/j.1469-8137.2007.01992.x
Darzentas N (2010) Circoletto: visualizing sequence similarity with Circos. Bioinformatics 26:2620–2621. https://doi.org/10.1093/bioinformatics/btq484
Dechorgnat J, Francis KL, Dhugga KS, Rafalski JA, Tyerman SD, Kaiser BN (2019) Tissue and nitrogen-linked expression profiles of ammonium and nitrate transporters in maize. BMC Plant Biol 19:206. https://doi.org/10.1186/s12870-019-1768-0
Duan J, Tian H, Gao Y (2016) Expression of nitrogen transporter genes in roots of winter wheat (Triticum aestivum L.) in response to soil drought with contrasting nitrogen supplies. Crop Pasture Sci 67:128–136. https://doi.org/10.1071/CP15152
Dube T, Mlilo C, Moyo P, Ncube C, Phiri K (2018) Will adaptation carry the future? Questioning the long-term capacity of smallholder farmers’ adaptation strategies against climate change in Gwanda District, Zimbabwe. J Hum Ecol 61:20–30. https://doi.org/10.1080/09709274.2018.1452866
Fang L, Wang M, Chen X, Zhao J, Wang J, Liu J (2023) Analysis of the AMT gene family in chili pepper and the effects of arbuscular mycorrhizal colonization on the expression patterns of CaAMT2 genes. BMC Genomics 24(1):158. https://doi.org/10.1186/s12864-023-09226-3
FAO (Food and Agriculture Organization) (2017) World food situation. http://www.fao.org/worldfoodsituation/csdb/en/ Accessed June 1, 2023.
Ferreira LM, De Souza VM, Tavares OCH, Zonta E, Santa-Catarina C, De Souza SR, Fernandes MS, Santos LA (2015) OsAMT1.3 expression alters rice ammonium uptake kinetics and root morphology. Plant Biotechnol Rep 9:221–229. https://doi.org/10.1007/s11816-015-0359-2
Filiz E, Akbudak MA (2020) Ammonium transporter 1 (AMT1) gene family in tomato (Solanum Lycopersicum L.): Bioinformatics, physiological and expression analyses under drought and salt stresses. Genomics 112:3773–3782. https://doi.org/10.1016/j.ygeno.2020.04.009
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A (2003) Expasy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788. https://doi.org/10.1093/nar/gkg563
Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB, von Wirén N (1999) Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 11:937–948. https://doi.org/10.1093/nar/gkg563
Giehl RFH, Laginha AM, Duan F, Rentsch D, Yuan L, von Wirén N (2017) A critical role of AMT2;1 in root-to-shoot translocation of ammonium in Arabidopsis. Mol Plant 10:1449–1460. https://doi.org/10.1016/j.molp.2017.10.001
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:D1178–D1186. https://doi.org/10.1093/nar/gkr944
Goron TL, Raizada MN (2015) Genetic diversity and genomic resources available for the small millet crops to accelerate a New Green Revolution. Front Plant Sci 6:157. https://doi.org/10.3389/fpls.2015.00157
Gu R, Duan F, An X, Zhang F, Vonwirén N, Yuan L (2013) Characterization of AMT-mediated high-affinity ammonium uptake in roots of maize (Zea mays L.). Plant Cell Physiol 54:1515–1524. https://doi.org/10.1093/pcp/pct099
Guether M, Neuhäuser B, Balestrini R, Dynowski M, Ludewig U, Bonfante P (2009) A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol 150:73–83. https://doi.org/10.1104/pp.109.136390
Han M, Wong J, Su T, Beatty PH, Good AG (2016) Identification of nitrogen use efficiency genes in barley: Searching for QTLs controlling complex physiological traits. Front Plant Sci 7:1587. https://doi.org/10.3389/fpls.2016.01587
Hao DL, Zhou JY, Yang SY, Qi W, Yang KJ, Su YH (2020) Function and regulation of ammonium transporters in plants. Int J Mol Sci 21:3557. https://doi.org/10.3390/ijms21103557
Hassan ZM, Sebola NA, Mabelebele M (2021) The nutritional use of millet grain for food and feed: A review. Agric Food Secur 10:16. https://doi.org/10.1186/s40066-020-00282-6
Hawkesford MJ (2012) Improving nutrient use effciency in crops. Wiley, Hoboken. https://doi.org/10.1002/9780470015902.a0023734
Heidari P, Puresmaeli F, Mora-Poblete F (2022) Genome-wide identification and molecular evolution of the magnesium transporter (MGT) gene family in Citrullus lanatus and Cucumis sativus. Agronomy 12:2253. https://doi.org/10.3390/agronomy12102253
Hernandez-Garcia CM, Finer JJ (2014) Identification and validation of promoters and cis-acting regulatory elements. Plant Sci 217–218:109–119. https://doi.org/10.1016/j.plantsci.2013.12.007
Hittalmani S, Mahesh HB, Shirke MD, Biradar H, Uday G, Aruna YR, Lohithaswa HC, Mohanrao A (2017) Genome and transcriptome sequence of Finger millet (Eleusine coracana (L.) Gaertn) provides insights into drought tolerance and nutraceutical properties. BMC Genomics 18:465. https://doi.org/10.1186/s12864-017-3850-z
Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G (2015) GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31:1296–1297. https://doi.org/10.1093/bioinformatics/btu817
Huang L, Zhang H, Zhang H, Deng XW, Wei N (2015) HY5 regulates nitrite reductase 1 (NIR1) and ammonium transporter1;2 (AMT1;2) in Arabidopsis seedlings. Plant Sci 238:330–339. https://doi.org/10.1016/j.plantsci.2015.05.004
Huang L, Li J, Zhang B, Hao Y, Ma F (2022) Genome-wide identification and expression analysis of AMT gene family in apple (Malus domestica Borkh.). Horticulturae 8:457. https://doi.org/10.3390/horticulturae8050457
Hurst LD (2002) The Ka/Ks ratio: diagnosing the form of sequence evolution. Trends Genet 18:486. https://doi.org/10.1016/s0168-9525(02)02722-1
Jiang J, Zhao J, Duan W, Tian S, Wang X, Zhuang H, Fu J, Kang Z (2019) TaAMT2;3a, a wheat AMT2-type ammonium transporter, facilitates the infection of stripe rust fungus on wheat. BMC Plant Biol 19:239. https://doi.org/10.1186/s12870-019-1841-8
Jo BS, Choi SS (2015) Introns: The functional benefits of introns in genomes. Genomics Inform 13:112–118. https://doi.org/10.5808/GI.2015.13.4.112
Kiba T, Krapp A (2016) Plant nitrogen acquisition under low availability: regulation of uptake and root architecture. Plant Cell Physiol 57:707–714. https://doi.org/10.1093/pcp/pcw052
Kobae Y, Tamura Y, Takai S, Banba M, Hata S (2010) Localized expression of arbuscular mycorrhiza-inducible ammonium transporters in soybean. Plant Cell Physiol 51:1411–1415. https://doi.org/10.1093/pcp/pcq099
Koegel S, Ait Lahmidi N, Arnould C, Chatagnier O, Walder F, Ineichen K, Boller T, Wipf D, Wiemken A, Courty PE (2013) The family of ammonium transporters (AMT) in Sorghum bicolor: Two AMT members are induced locally, but not systemically in roots colonized by arbuscular mycorrhizal fungi. New Phytol 198:853–865. https://doi.org/10.1111/nph.12199
Konishi N, Ma JF (2021) Three polarly localized ammonium transporter 1 members are cooperatively responsible for ammonium uptake in rice under low ammonium condition. New Phytol 232:1778–1792. https://doi.org/10.1111/nph.17679
Krogh A, Larsson B, Von Heijne G, Sonnhammer EL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. https://doi.org/10.1006/jmbi.2000.4315
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: An information aesthetic for comparative genomics. Genome Res 19:1639–1645. https://doi.org/10.1101/gr.092759.109
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Kuo YT, Chao YT, Chen WC, Shih MC, Chang SB (2019) Segmental and tandem chromosome duplications led to divergent evolution of the chalcone synthase gene family in Phalaenopsis orchids. Ann Bot 123:69–77. https://doi.org/10.1093/aob/mcy136
Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M, Karthikeyan AS, Lee CH, Nelson WD, Ploetz L, Singh S, Wensel A, Huala E (2012) The Arabidopsis information resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res 40:D1202–D1210. https://doi.org/10.1093/nar/gkr1090
Lanquar V, Loqué D, Hörmann F, Yuan L, Bohner A, Engelsberger WR, Lalonde S, Schulze WX, von Wirén N, Frommer WB (2009) Feedback inhibition of ammonium uptake by a phospho-dependent allosteric mechanism in Arabidopsis. Plant Cell 21:3610–3622. https://doi.org/10.1105/tpc.109.068593
Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, Mcwilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327. https://doi.org/10.1093/nar/30.1.325
Letunic I, Bork P (2021) Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49(W1):W293–W296. https://doi.org/10.1093/nar/gkab301
Letunic I, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40(Database issue):D302-5. https://doi.org/10.1093/nar/gkr931
Li P, Brutnell TP (2011) Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. J Exp Bot 62:3031–3037. https://doi.org/10.1093/jxb/err096
Li BZ, Merrick M, Li SM, Li HY, Zhu SW, Shi WM, Su YH (2009) Molecular basis and regulation of ammonium transporter in rice. Rice Sci 16:314–322. https://doi.org/10.1016/S1672-6308(08)60096-7
Li S, Li B, Shi W (2012) Expression patterns of nine ammonium transporters in rice in response to N status. Pedosphere 22(6):860–869. https://doi.org/10.1016/S1002-0160(12)60072-1
Li C, Tang Z, Wei J, Qu H, Xie Y, Xu G (2016) The OsAMT1.1 gene functions in ammonium uptake and ammonium-potassium homeostasis over low and high ammonium concentration ranges. J Genet Genomics 43:639–649. https://doi.org/10.1016/j.jgg.2016.11.001
Li R, Zhu F, Duan D (2020) Function analysis and stress-mediated cis-element identification in the promoter region of VqMYB15. Plant Signal Behav 15(7):1773664. https://doi.org/10.1080/15592324.2020.1773664
Li T, Liao K, Xu X, Gao Y, Wang Z, Zhu X, Jia B, Xuan Y (2017) Wheat ammonium transporter (AMT) gene family: diversity and possible role in host-pathogen interaction with stem rust. In: Front Plant Sci 8:1637. https://doi.org/10.3389/fpls.2017.01637
Loqué D, von Wirén N (2004) Regulatory levels for the transport of ammonium in plant roots. J Exp Bot 55(401):1293–1305. https://doi.org/10.1093/jxb/erh147
Loqué D, Yuan L, Kojima S, Gojon A, Wirth J, Gazzarrini S, Ishiyama K, Takahashi H, von Wirén N (2006) Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J 48(4):522–534. https://doi.org/10.1111/j.1365-313X.2006.02887
Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Song MGH, JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A, (2020) CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res 48(D1):D265–D268. https://doi.org/10.1093/nar/gkz991
Ludewig U, Wilken S, Wu B, Jost WH, Obrdlik P, El Bakkoury ME, Marini AM, André B, Hamacher T, Boles E, von Wirén N, Frommer WB (2003) Homo- and hetero-oligomerization of ammonium transporter-1 NH4 uniporters. J Biol Chem 278:45603–45610. https://doi.org/10.1074/jbc.M307424200
Luo L, Zhu M, Jia L, Xie Y, Wang Z, Xuan W (2022) Ammonium transporters cooperatively regulate rice crown root formation responding to ammonium nitrogen. J Exp Bot 73:3671–3685. https://doi.org/10.1093/jxb/erac059
Maharajan T, Ceasar SA, Ajeesh Krishna TPA (2022) Finger Millet (Eleusine coracana (L.) Gaertn): nutritional importance and nutrient transporters. Crit Rev Plant Sci 41:1–31. https://doi.org/10.1080/07352689.2022.2037834
Marchler-Bauer A, Bryant SH (2004) CD-search: protein domain annotations on the fly. Nucleic Acids Res 32:W327–W331. https://doi.org/10.1093/nar/gkh454
Marcos de Leão R, Hülse G, de Souza S, Benedito Dos Santos T (2020) An in silico data mining of the ammonium transporter gene family in Ananas comosus L. Colloq Agrariae 16:10–24. https://doi.org/10.5747/ca.2020.v16.n6.a403
Marini AM, Soussi-Boudekou S, Vissers SD, André B (1997) A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol 17:4282–4293. https://doi.org/10.1128/MCB.17.8.4282
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105:1141–1157. https://doi.org/10.1093/aob/mcq028
Mcdonald TR, Ward JM (2016) Evolution of electrogenic ammonium transporters (AMTs) Front Plant Sci 7:352. https://doi.org/10.3389/fpls.2016.00352
Naeem M, Ansari AA, Gill SS (2017) Essential plant nutrients: uptake, use efciency, and management. Springer, Cham
Nieves-Cordones M, Rubio F, Santa-María GE (2020) Editorial: nutrient use-efciency in plants: an integrative approach. Front Plant Sci 11:623976. https://doi.org/10.3389/fpls.2020.623976
Ninnemann O, Jauniaux JC, Frommer WB (1994) Identification of a high affinity NH4+ transporter from plants. EMBO J 13:3464–3471. https://doi.org/10.1002/j.1460-2075.1994.tb06652.x
Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, Thibaud-Nissen F, Malek RL, Lee Y, Zheng L, Orvis J, Haas B, Wortman J, Buell CR (2007) The TIGR rice genome annotation resource: improvements and new features. Nucleic Acids Res 35:D883–D887. https://doi.org/10.1093/nar/gkl976
Panchy N, Lehti-Shiu M, Shiu SH (2016) Evolution of gene duplication in plants. Plant Physiol 171:2294–2316. https://doi.org/10.1104/pp.16.00523
Patel K, Gartaula H, Johnson D, Karthikeyan M (2015) The interplay between household food security and wellbeing among small-scale farmers in the context of rapid agrarian change in India. Agric Food Secur 4(1):16. https://doi.org/10.1186/s40066-015-0036-2
Ravindran G (1991) Studies on millets: proximate composition, mineral composition, and phytate and oxalate contents. Food Chem 39:99–107. https://doi.org/10.1016/0308-8146(91)90088-6
Santos TB, Lima JE, Felicio MS, Soares JDM, Domingues DS (2017) Genome-wide identification, classification and transcriptional analysis of nitrate and ammonium transporters in Coffea. Genet Mol Biol 40:346–359. https://doi.org/10.1590/1678-4685-GMB-2016-0041
Sharma KK, Ortiz R (2000) Program for the application of genetic transformation for crop improvement in the semi-arid tropics. In Vitro Cell Dev Biol -Plant 36:83–92. https://doi.org/10.1007/s11627-000-0019-1
Shaul O (2017) How introns enhance gene expression. Int J Biochem Cell Biol 91(1):145–155. https://doi.org/10.1016/j.biocel.2017.06.016
Shelden MC, Dong B, de Bruxelles GL, Trevaskis B, Whelan J, Ryan PR, Howitt SM, Udvardi MK (2001) Arabidopsis ammonium transporters, AtAMT1;1 and AtAMT1;2, have different biochemical properties and functional roles. Plant Soil 231:151–160. https://doi.org/10.1023/A:1010303813181
Shobana S, Sreerama YN, Malleshi NG (2009) Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem 115:1268–1273. https://doi.org/10.1016/j.foodchem.2009.01.042
Shweta M (2015) Pearl millet nutritional value and medicinal uses. IJARIIE 1:414–418. https://doi.org/10.1017/s0003598x00061378
Simon-Rosin U, Wood C, Udvardi MK (2003) Molecular and cellular characterisation of LjAMT2;1, an ammonium transporter from the model legume Lotus japonicus. Plant Mol Biol 51:99–108. https://doi.org/10.1023/a:1020710222298
Sohlenkamp C, Shelden M, Howitt S, Udvardi M (2000) Characterization of arabidopsis AtAMT2, a novel ammonium transporter in plants. FEBS Lett 467:273–278. https://doi.org/10.1016/s0014-5793(00)01153-4
Sonoda Y, Ikeda A, Saiki S, von Wirén N, Yamaya T, Yamaguchi J (2003) Distinct expression and function of three ammonium transporter genes (OsAMT1;1–1;3) in rice. Plant Cell Physiol 44(7):726–734. https://doi.org/10.1093/pcp/pcg083
Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Zuloaga FO, Judziewicz EJ, Filgueiras TS, Davis JI, Morrone O (2015) A worldwide phylogenetic classification of the Poaceae (Gramineae). J Syst Evol 53:117–137. https://doi.org/10.1111/jse.12262
Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Teisher JK, Clark LG, Barberá P, Gillespie LJ, Zuloaga FO (2017) A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications. J Syst Evol 55:259–290
Soreng RJ, Peterson PM, Zuloaga FO, Romaschenko K, Clark LG, Teisher JK, Gillespie LJ, Barberá P, Welker CAD, Kellogg EA, Li D-Z, Davidse G (2022) A worldwide phylogenetic classification of the Poaceae (Gramineae) III: an update. J Syst Evol 60:476–521. https://doi.org/10.1111/jse.12847
Su-Mei LI, Bao-Zhen LI, Wei-Ming SHI (2012) Expression patterns of nine ammonium transporters in rice in response to N status. Pedosphere 22(6):860-869. https://doi.org/10.1016/S1002-0160(12)60072-1
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. https://doi.org/10.1093/molbev/msab120
Tang M, Li Y, Chen Y, Han L, Zhang H, Song Z (2020) Characterization and expression of ammonium transporter in peach (Prunus persica) and regulation analysis in response to external ammonium supply. Phyton 89:925–941. https://doi.org/10.32604/phyton.2020. 011184
Taylor JRN (2004) In Wrigley C, Corke H, Walker CE (Eds.) (2013) Millet. In encyclopaedia in grain science, 2. Millets: Nutritional composition, some health benefits and processing – A review. Emirates J Food Agric Elsevier I Amadoubr, Le M, eds. 25:501–508, (253–261) https://doi.org/10.9755/ejfa.v25i7.12045
Tegeder M, Masclaux-Daubresse C (2018) Source and sink mechanisms of nitrogen transport and use. New Phytol 217:35–53. https://doi.org/10.1111/nph.14876
von Wirén N, Lauter FR, Ninnemann O, Gillissen B, Walch-Liu P, Engels C, Jost W, Frommer WB (2000) Differential regulation of three functional ammonium transporter genes by nitrogen in root hairs and by light in leaves of tomato. Plant J 21:167–175. https://doi.org/10.1046/j.1365-313x.2000.00665.x
Voorrips RE (2002) MapChart: Software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78. https://doi.org/10.1093/jhered/93.1.77
Wang Y, Zhou W, Wu J, Xie K, Li X (2022) 2 promotes ammonium nitrogen transport during arbuscular mycorrhizal fungi symbiosis in Lotus japonicus. Int J Mol Sci 23(17): LjAMT2. https://doi.org/10.3390/ijms23179522
Williams LE, Miller AJ (2001) Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu Rev Plant Physiol Plant Mol Biol 52:659–688. https://doi.org/10.1146/annurev.arplant.52.1.659
Wolfe D, Dudek S, Ritchie MD, Pendergrass SA (2013) Visualizing genomic information across chromosomes with phenogram. BioData Min 6(1):18. https://doi.org/10.1186/1756-0381-6-18
Wu X, Yang H, Qu C, Xu Z, Li W, Hao B, Yang C, Sun G, Liu G (2015) Sequence and expression analysis of the AMT gene family in poplar. Front Plant Sci 6:337. https://doi.org/10.3389/fpls.2015.00337
Wu Z, Gao X, Zhang N, Feng X, Huang Y, Zeng Q, Wu J, Zhang J, Qi Y (2021) Genome-wide identification and transcriptional analysis of ammonium transporters in Saccharum. Genomics 113:1671–1680. https://doi.org/10.1016/j.ygeno.2021.04.001
Wu XX, Yuan P, Chen H, Kumar V, Kang SM, Jia B, Xuan YH (2022) Ammonium transporter 1 increases rice resistance to sheath blight by promoting nitrogen assimilation and ethylene signalling. Plant Biotechnol J 20:1085–1097. https://doi.org/10.1111/pbi.13789
Xia J, Wang Y, Zhang T, Pan C, Ji Y, Zhou Y, Jiang X (2023) Genome-wide identification, expression profiling, and functional analysis of ammonium transporter 2 (AMT2) gene family in cassava (Manihot esculenta crantz). Front Genet 14:1145735. https://doi.org/10.3389/fgene.2023.1145735
Xu J, Peng S, Yang S, Wang W (2012) Ammonia volatilization losses from a rice paddy with different irrigation and nitrogen managements. Agric Water Manag 104:184–192. https://doi.org/10.1016/j.agwat.2011.12.013
Yaghobi M, Heidari P (2023) Genome-wide analysis of aquaporin gene family in Triticum turgidum and its expression profile in response to salt stress. Genes 14(1):202. https://doi.org/10.3390/genes14010202
Yang W, Dong X, Yuan Z, Zhang Y, Li X, Wang Y (2023) Genome-wide identification and expression analysis of the ammonium transporter family genes in soybean. Int J Mol Sci 24(4):3991. https://doi.org/10.3390/ijms24043991
Yuan L, Loqué D, Kojima S, Rauch S, Ishiyama K, Inoue E, Takahashi H, von Wirén N (2007) The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 19:2636–2652. https://doi.org/10.1105/tpc.107.052134
Yuan L, Graff L, Loqué D, Kojima S, Tsuchiya YN, Takahashi H, von Wirén N (2009) AtAMT1;4, a pollen-specific high-affinity ammonium transporter of the plasma membrane in Arabidopsis. Plant Cell Physiol 573(50):13–25. https://doi.org/10.1093/pcp/pcn186
Yuan L, Gu R, Xuan Y, Smith-Valle E, Loqué D, Frommer WB, von Wirén N (2013) Allosteric regulation of transport activity by heterotrimerization of Arabidopsis ammonium transporter complexes in vivo. Plant Cell 25:974–984. https://doi.org/10.1105/tpc.112.108027
Zhang Z, Li J, Zhao XQ, Wang J, Wong GKS, Yu J (2006) KaKs_Calculator: Calculating Ka and Ks through model selection and model averaging. Genom Proteom Bioinform 4:259–263. https://doi.org/10.1016/S1672-0229(07)60007-2
Zhao Y, Sun J, Xu P, Zhang R, Li L (2014) Intron-mediated alternative splicing of WOOD-ASSOCIATED NAC TRANSCRIPTION FACTOR1B regulates cell wall thickening during fiber development in Populus species. Plant Physiol 164:765–776. https://doi.org/10.1104/pp.113.231134
Acknowledgements
*Financial support to Dr. Tanushree Sarkar working as a Research Associate from Bhabha Atomic Research Centre, India is gratefully acknowledged.
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Contributions
Both the authors equally contributed to the study conception, design, data analysis, manuscript writing and correction and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflict of interest.
Human and animal rights
This research does not involve any human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sarkar, T., Bakshi, S. Ammonium transporter genes in millets: insights into structure, function, evolutionary conservation, divergence, and phylogenetic analysis. Genet Resour Crop Evol (2024). https://doi.org/10.1007/s10722-024-02092-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10722-024-02092-2