Abstract
Increasing crop tolerance to high salinity is an important breeding objective. Some accessions of wild species in the genus Vigna have high salinity tolerance. One accession of V. marina (JP247202) and V. luteola (JP233389) have been previously identified as salt tolerant. However, the mechanisms of tolerance remain elusive and research on the phenotypic responses to salt stress can be a starting point for identifying salt-tolerant mechanisms. This study evaluated plant growth, salt accumulation, PSII effective quantum yield and root system architecture, including total root length, root surface area and root tip number, of three accessions from two Vigna species in response to salt stress. Results showed (1) JP247202 invested in the growth of roots more than shoots under salt stress; (2) JP247202 in salt stress maintained the total root length and surface area as control; (3) JP247202 in salt stress grew more but shorter fine roots, and fewer but longer thick roots; (4) JP247202 in salt stress had a higher amount of water uptake per dry weight; (5) The leaves of JP233389 accumulated high amount of Na without damage, while similar Na accumulation severely damaged the leaves of JP235855, a salt-sensitive accession of V. luteola. In addition, while salt damage started from older leaves in JP235855, it started with younger leaves in JP233389. These findings will facilitate further understanding mechanisms of salt tolerance in V. marina and V. luteola and potentially provide insight into increasing salt tolerance in crop plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil salinity has been one of the limiting factors for agriculture in the last half-century (Kopittke et al. 2019). Salinity causes both osmotic and ion toxic stress on plants, which have impacts on the physiological and biochemical functions of plant cells, inhibiting photosynthesis and growth of both aerial part and root system of plants (Santos et al. 2022). It has been estimated that over 50% of the arable land will be salinized by the year 2050 (Jamil et al. 2011). Increasing salt tolerance is essential to ensure food security.
Some wild plants in the genus Vigna flourish on sandy beaches and exhibit salt tolerance. Vigna marina is “a pantropical plant with sea-drifted seeds” (Miryeganeh et al. 2014; Takayama et al. 2006; Vatanparast et al. 2011). The capability of oceanic dispersal of V. marina and the specific habitat makes it robust against salt stress. In a screening by visual scoring on salt tolerance among cultivated and wild Vigna species, V. marina was superior to other species and 49 accessions out of 50 were resistant (or moderate resistant) in 350 mM NaCl for one week (Yoshida et al. 2020). In the same study, V. luteola exhibited high salt tolerance, with 45 accessions out of 50 being resistant (or moderate resistant) in 200 mM NaCl for one week (Yoshida et al. 2020). In addition to visual scoring, V. marina and V. luteola were assigned as the most tolerant group in terms of relative quantum yield (of the uppermost fully expanded leaves) and shoot biomass (Iseki et al. 2016). V. marina is even more excellent in the following aspects. Yoshida et al. (2020) reported that the plants of V. marina had much higher transpiration rate than V. luteola or other species under salt stress. Moreover, a recent study using autoradiography has shown V. marina remarkably suppressed sodium uptake (Noda et al., 2022). Despite such promising features, their mechanisms for salt tolerance remain unclear.
Besides leaf traits, root system architecture including root length and number of lateral roots change notably in response to salt (Khan et al. 2016; Zou et al. 2022). Research on Arabidopsis has shown that salt stress decreases lateral root length (Julkowska et al. 2014; Zhao et al. 2011; Zolla et al. 2010). In this study, we investigated on the changes in root growth and architecture under salt stress in two species of the genus Vigna.
Materials and methods
Plant materials and growth condition
Three accessions of the genus Vigna were used in this study: JP247202 (Vigna marina) and JP233389 (V. luteola) as salt tolerant accessions, and JP235855 (V. luteola) as salt sensitive accession. Seeds were obtained from the Research Center of Genetic Resources, National Agriculture and Food Research Organization (NARO), Japan (NARO Genebank: https://www.gene.affrc.go.jp/index_en.php). Seeds were sterilized with 70% ethanol and 5% sodium hypochlorite for five minutes each and then rinsed thoroughly with tap water. Sterilized seeds were scratched and germinated on Seramis granules (Effem GmbH, Verden, Germany) for one week. Germinated seedlings were transplanted in hydroponic culture containing 1 × Otsuka house No. 1 and 1 × Otsuka house No. 2) (Otsuka Chemical Co., Osaka, Japan: N, P, K, Ca, and Mg = 18.6, 5.1, 8.6, 8.2 and 3.0 mEq L−1, respectively). Each container contained four-liter culture and held six seedlings (two seedlings for each accession). Plants were grown in a growth chamber (TGC-720-2S, ESPEC MIC Corp.) under LED lamps with light intensity of 180 μmol m−2 s−1 from 6:00 am–20:00 pm (14 h) at 28 °C and in a dark from 20:00 pm to 6:00 am (10 h) at 24 °C.
Measurement of dry weight and hydroponic culture consumption
One week after transplanting, we sampled five plants for each accession for measuring the dry weight of day 0. Shoots and roots were separately collected, dried at 50 °C for one week and measured for weight.
The remaining plants were separately transferred to a tin-foil-wrapped 50 ml falcon tubes containing 45 ml of the same hydroponic culture described above with or without 200 mM of NaCl. We used sponges to hold the plants and sealed the tube tops with black tape. We measured the amount of remaining culture by a measuring cylinder every day and renewed the culture after measurement. The amount of culture loss was calculated by subtracting the remaining volume from the starting volume. Thus, we recorded the daily loss of hydroponic culture of each plant for five days.
After five days, we collected five plants (including wilted leaves) for measuring the dry weight of day 5 as described above.
Using the dry weight of day 5 and the total amount of culture consumption by each plant (sum of the amount of culture loss for five days), we calculated the culture consumption per dry weight.
Ion measurement
Methods were the same as documented in Noda et al. (2022). Briefly, after the 3rd leaf fully expanded, plants were transferred to hydroponic culture with 100 mM of NaCl for two days. Roots, lower and upper stems (stem1 and stem2, respectively) and the 1st, 2nd and 3rd leaves (leaf1, leaf2 and leaf3, respectively) were separately sampled from four individual plants and dried at 50 °C for three days. Samples were digested with 69% HNO3 for determination of sodium (Na) and potassium (K) by inductively coupled plasma-mass spectrometry (ICP-MS, NexION 350S, PerkinElmer, Waltham, MA, USA).
Root architectural traits analyses
Salt treatment of 200 mM of NaCl started at one-week after transplanting. On day 5, control and salt-treated roots were cut at the shoot–root junction and stained overnight in neutral red dye solution (0.08 g L−1, Sigma-Aldrich). Eight individual plants were used in this experiment. Stained roots were rinsed with tap water and scanned using an EPSON Perfection V700/V750 scanner. Scanned images were analyzed for total root length, surface area and tip number using WinRHIZO (v. 2008a, Regent Instruments Canada Inc., Arsenault, Pouleur, Messier, & Guay, 1995). Roots were further categorized into three diameter classes (D1: 0–0.15 mm, D2: 0.15–0.3 mm, D3: 0.3 mm) for analysis of total root tip number and average length per root.
Effective quantum yield measurement
Salt treatment of 100 mM, 150 mM and 200 mM of NaCl started at two-weeks after transplanting. Chlorophyll fluorescence was measured using a portable JUNIOR-PAM fluorometer (Heinz Walz GmbH, Pfullingen, Germany) and the Photosystem II (PSII) effective quantum yield (Y(II)) was calculated by the WinControl-3 software (Heinz Walz GmbH, Pfullingen, Germany). The measurement was conducted with a leaf clip with measuring light from blue actinic light of 190 μmol m−2 s−1. The measurement was carried out for each leaflet of the 1st, 2nd and 3rd leaves (leaf1, leaf2 and leaf3, respectively) on four individual plants at time-series of day 0, 1, 2, 3, 5, 7 and 14 days after salt treatment.
Results
Growth evaluation and water uptake
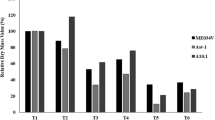
To study the effect of salt stress on plant growth, the dry weight of shoots and roots were measured for plants in control at day 0 and in control and salt at day 5. Compared to day 0, shoot dry weight of all three accessions did not increase at day 5 under salt stress while those in controls significantly increased (Fig. 1a). Root dry weight was significantly increased for JP247202 even under salt stress whereas it did not increase for JP233389 and JP235855 (Fig. 1b).
Dry weight of shoots (a) and roots (b) at day 0 and day 5 for control and salt-treated (200 mM of NaCl) plants. Orange, green and blue bars represent values for JP247202, JP233389 and JP235855, respectively. Means are calculated using five replications and those not sharing the same alphabet are statistically different (Tukey LSD test, P < 0.05)
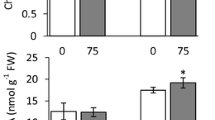
Moreover, we measured the amount of hydroponic culture consumption during the five days of salt treatment, as an indicator of transpiration or water uptake. As shown in Fig. 2, control plants of JP247202 and JP233389 consumed 158 and 157 ml culture per gram dry weight, respectively, while that of JP235855 was 248 ml g−1 dry weight. Under salt stress, however, JP247202 consumed 51 ml culture per gram dry weight, which was significantly higher than JP233389 (34 ml g −1 dry weight) and JP235855 (26 ml g−1 dry weight).
Loss of hydroponic culture per dry weight in control and salt-treated (200 mM of NaCl) plants of three accessions. Orange, green and blue bars represent values for JP247202, JP233389 and JP235855, respectively. Means are calculated using five replications and those not sharing the same alphabet are statistically different (Tukey LSD test, P < 0.05)
Patterns of salt allocation in the plant body
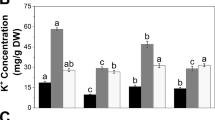
To understand patterns of salt allocation and accumulation in the three accessions, concentration of Na and K was assessed separately in roots, lower and upper stems (stem1 and stem2, respectively) and the 1st, 2nd and 3rd leaves (leaf1, leaf2 and leaf3, respectively). As shown in Fig. 3, Patterns largely differed between three accessions: JP247202 accumulated little Na in the stems and leaves and retained some in the roots; JP233389 allocated Na in the roots and the leaves but little in the stems; JP235855 allocated Na in the whole plant body. As for K, JP247202 accumulated a lot in the whole plant body; JP233389 accumulated less to JP247202; JP235855 accumulated a high concentration of K in the roots but less in the aerial parts. JP247202 had the smallest Na/K ratio in aerial parts among the accessions. JP233389 maintained a low Na/K ratio in the stems but not in the leaves whereas JP235855 had a high Na/K ratio in both stems and leaves.
Concentration of Na, K and Na/K ratio in roots (R), stem1 (S1), stem2 (S2) and leaf1 (L1), leaf2 (L2) and leaf3 (L3) of salt-treated (100 mM) plants on day 2. Orange, green and blue dots represent values for JP247202, JP233389 and JP235855, respectively. Means are calculated using four replications and those not sharing the same alphabet are statistically different (Tukey LSD test, P < 0.05)
Root architectural changes
To understand the effect of salt stress on root architecture, we scanned the roots to evaluate total root length, surface area and tip number (Fig. 4). The results indicated that, compared to the control, JP247202 in salt stress maintained the total root length and surface area, whereas those of the other two were significantly decreased (Fig. 5). On average, the total root length was reduced by 33% for JP233389 and 62% for JP235855. Although a decrease of 21% was observed in JP247202, such a decrease was not significant compared to the control (Fig. 5). A similar trend was found for root surface area (Fig. 5). Root surface area decreased by 25% in JP247202 in salt stress but still not significantly different from control, whereas it decreased by 40% in JP233389 and 66% in JP235855 (Fig. 5). Total root tip number decreased by 16% in JP233389, which was not significant between salt and control, whereas that of the other two were significantly reduced by 31% in JP247202 and 43% in JP235855, respectively (Fig. 5).
Total root length, surface area and tip number of control and salt-treated (200 mM) plants on day 5. Orange, green and blue dots represent values for JP247202, JP233389 and JP235855, respectively. Means are calculated using eight replications. ** stands for the significant difference of P < 0.01 (t-test between control and salt)
Other than global features of root architecture, the scanned image suggested that JP247202 developed more fine roots in salt stress (Fig. 4). Therefore, we categorized roots into three classes by diameter (D1 to D3: representing finer to thicker, see methods for the details) and evaluated total root tip number and average length per root for each category.
As a result, JP247202 increased the number of D1 roots to more than double in salt stress while decreased the average length per root. In contrast, it dramatically decreased the number of D2 and D3 roots while increased the average length (Fig. 6). Thus, JP247202 produced more but shorter D1 roots, and fewer but longer D2 and D3 roots.
Total root tip number and average length per root of D1, D2 and D3 roots of control and salt-treated (200 mM) plants. D1, D2 and D3 represent roots with diameters of 0–0.15 mm, 0.15–0.3 mm and 0.3 mm, respectively. Orange, green and blue dots represent values for JP247202, JP233389 and JP235855, respectively. Means are calculated using eight replications. * and ** stands for the significant difference of P < 0.05 and P < 0.01, respectively (t-test between control and salt)
JP233389 showed a similar trend as JP247202 in the change of tip number and average length for all three root classes, though not significant in the tip number and average length for D1 and D2 roots (Fig. 6).
In contrast, JP235855 decreased in tip number and average length for all three root classes (Fig. 6).
Spatio-temporal patterns of PSII Effective quantum yield
In addition to the evaluation of root growth, the PSII effective quantum yield (Y(II)) was estimated by measuring the fluorescence of chlorophyll in the 1st, 2nd and 3rd leaves (leaf1, leaf2 and leaf3, respectively) in 100, 150 and 200 mM of NaCl at seven time points from 0 and 14 days after salt treatment.
Not surprisingly, Y(II) of JP247202 was stable across time regardless of salt concentrations and position of leaves, except for a slight decrease at day 14 at 200 mM of NaCl (Fig. 7).
The pattern of Y(II) in JP233389 was similar to that of JP247202 for leaf1 and leaf2, where no decrease was observed. However, Y(II) of leaf3 decreased to zero by day 14 under 200 mM of NaCl (Fig. 7).
As for JP235855, Y(II) decreased in all the leaves under any conditions of salt stress (Fig. 7). In 100 mM, leaf1 and leaf2 lost fluorescence on day 14 while leaf3 still maintained its Y(II). At 150 mM, Y(II) dropped to zero on day 7 in all the three leaves. At 200 mM, Y(II) in leaf1 dramatically decreased at day 2. It completely lost fluorescence on day 3, and this happened to leaf2 and leaf3 on day 5.
In summary, JP247202 kept Y(II) even in 200 mM NaCl for at least two weeks. JP233389 also kept Y(II) high but the leaf3 had completely lost Y(II) at day 14 in 200 mM NaCl. In contrast, JP235855 lost Y(II) over time under all tested conditions and the lower leaves were impaired earlier than the upper leaves.
Discussion
This study provided evidence of the difference in root growth of three accessions in the genus Vigna in response to salt stress. JP247202, an accession of the most salt-tolerant species V. marina across the genus, invested in the dry matter production of roots more than shoots under salt stress (Fig. 1). JP247202 maintained the total root length and surface area under salt stress (Fig. 5), despite the root dry weight was reduced (Fig. 1). This contrasts with JP233389 and JP235855, the tolerant and sensitive accessions of V. luteola, respectively, and those reported in other research (Ijaz et al. 2019; Mu et al. 2022; Robin et al. 2016), where salt stress dramatically inhibits root growth (Fig. 5). In addition, JP247202 was able to maintain higher transpiration than the other two accessions under salt stress. Although the amount of culture loss was the sum of transpiration from leaves and evaporation from culture surface, we assume the difference between the accessions mainly came from transpiration as evaporation differed little because all plants were grown in the same growth chamber. Given root length and surface areas are correlated with capacity of water uptake (Tachibana et al., 1983), we consider the maintenance of root growth in JP247202 could be an adaptive response to low osmotic pressure under salt stress.
Another interesting observation was the dramatic change in root architecture of JP247202 in response to salt stress (Fig. 6). JP247202 under salt stress grew more but shorter D1 roots, and fewer but longer D2 and D3 roots compared to control (Fig. 6). Another tolerant accession JP233389 showed the similar trend as JP247202 in all parameters but the change was less dramatic, whereas the sensitive accession JP235855 showed the opposite trend in the tip number of D1 roots and average length of D2 and D3 roots (Fig. 6). The correlation between these changes in root architecture and water uptake (Fig. 2) suggests such root architectures of JP247202 and JP233389 were important for water uptake against low osmotic pressure under salt stress. A study by Zarebanadkouki et al. (2013) supports this argument regarding the importance of increasing short roots for water uptake, as radial flux of water uptake is higher in shorter roots than in longer roots.
However, we note that root growth or architecture are not the only determinant of water uptake. For example, JP233389 had even more D1 roots than JP247202, but its water uptake was not as high as that of JP247202. Namely, JP247202 achieved the highest water uptake even with fewer D1 roots and similar total root length and surface area compared to others (Figs. 2, 5, 6). These facts suggest that JP247202 has some features other than root architecture contributing to its water uptake under salt stress. The explanation could be a physiological aspect of the root including adjustment of osmotic pressure, to which the higher K content of JP247202 might contribute (Fig. 3).
While JP247202 had a higher amount of water uptake (Fig. 2), it was excellent in suppressing Na accumulation (Fig. 3) (Noda et al. 2022). This is surprising, considering the higher water uptake is, the higher risk of Na uptake is. JP247202 might avoid this risk, at least to some extent, by allocating a high amount of K in the plant (Fig. 3). The higher K concentration in a cell lowers the chance of Na entrance into a cell across the plasma membrane (Schachtman and Liu 1999). This feature highly contrasts with JP235855, where it failed in transporting K, though stocked a lot in the root, to the stem or the leaves. However, as high K does not always mean low Na (see leaf2 in JP233389 in Fig. 3), JP247202 could also have other mechanisms such as apoplastic barriers and active exclusion of Na, which should be examined in future research.
Being salt tolerant as well, JP233389 had both similarities and differences with JP247202. For root characteristics, JP233389 exhibited a similar pattern to JP247202 in that it increased tip number for D1 roots, but not D2 and D3 roots (Fig. 6). JP233389 was as good as JP247202 in retaining Y(II) except leaf3 at day14 under 200 mM NaCl (Fig. 7). However, it accumulated more Na in leaves than JP247202 (Fig. 3). While JP247202 in salt stress invested in the growth of roots more than shoots and maintained the total root length and surface area, JP233389 did not (Figs. 1, 5). Thus, JP233389 shares some mechanisms of salt tolerance with JP247202 but has also evolved unique features. One such unique feature of JP233389 could be Na compartmentalization to vacuoles.
Moreover, JP233389 showed salt damage in leaf3 (200 mM) while leaf1 and leaf2 maintained Y(II) (Fig. 7). This contrasts with JP235855 where upper leaves tended to maintain Y(II) for longer period (Fig. 7). These results confirmed our previous study that JP233389 allocates sodium to the upper leaves while other species (including tolerant and sensitive) allocate sodium to the lower leaves (Noda et al., 2022). We have considered this system of JP233389 could be reasonable as long as it keeps growing and generating new leaves (Noda et al., 2022), as it does not have to sacrifice any leaves. However, we should also examine whether the system leads to disastrous outcome for JP233389 or there is another mechanism to make it survive when salt stress is above a certain threshold.
By the series of observations, this study revealed aspects of phenotypic responses to salt stress in accessions of V. marina and V. luteola but several outstanding questions remain.
-
1.
What is the mechanism of D1 roots contributing to maintenance of water uptake?
-
2.
How does JP247202 suppress Na accumulation in the plant body?
-
3.
What is the mechanism of salt allocation in the leaves of JP233389?
-
4.
How does JP233389 survive (or not) if salt stress is over 200 mM?
References
dos Santos TB, Ribas AF, de Souza SGH, Budzinski IGF, Domingues DS (2022). Physiological responses to drought, salinity, and heat stress in plants: a review. Stresses 2(1): 113–135
Ijaz B, Sudiro C, Jabir R, Schiavo FL, Hyder MZ, Yasmin T (2019) Adaptive behaviour of roots under salt stress correlates with morpho-physiological changes and salinity tolerance in rice. Int J Agric Biol 21(3):667–674
Iseki K, Takahashi Y, Muto C, Naito K, Tomooka N (2016) Diversity and evolution of salt tolerance in the genus Vigna. PLoS ONE 11(10):e0164711
Jamil A, Riaz S, Ashraf M, Foolad MR (2011) Gene expression profiling of plants under salt stress. Crit Rev Plant Sci 30(5):435–458
Julkowska MM, Hoefsloot HCJ, Mol S, Feron R, de Boer GJ, Haring MA, Testerink C (2014) Capturing arabidopsis root architecture dynamics with root-fit reveals diversity in responses to salinity. Plant Physiol 166:1387–1402
Khan MA, Gemenet DC, Villordon A (2016) Root system architecture and abiotic stress tolerance: current knowledge in root and tuber crops. Front Plant Sci 7:1584
Kopittke PM, Menzies NW, Wang P, McKenna BA, Lombi E (2019) Soil and the intensification of agriculture for global food security. Environ Int 132:105078
Miryeganeh M, Takayama K, Tateishi Y, Kajita T (2014) Long-distance dispersal by sea-drifted seeds has maintained the global distribution of Ipomoea pes-caprae subsp. brasiliensis (Convolvulaceae). PLoS One 9(4):e91836
Mu DW, Feng NJ, Zheng DF, Zhou H, Liu L, Chen GJ, Mu B (2022) Physiological mechanism of exogenous brassinolide alleviating salt stress injury in rice seedlings. Sci Rep 12(1):20439
Noda Y, Sugita R, Hirose A, Kawachi N, Tanoi K, Furukawa J, Naito K (2022). Diversity of Na+ allocation in salt-tolerant species of the genus Vigna. Breed Sci 72(4): 326–331
Robin AHK, Matthew C, Uddin MJ, Bayazid KN (2016) Salinity-induced reduction in root surface area and changes in major root and shoot traits at the phytomer level in wheat. J Exp Bot 67(12):3719–3729
Schachtman D, Liu W (1999) Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci 4(7):281–287
Tachibana Y, Ohta Y (1983) Root surface area, as a parameter in relation to water and nutrient uptake by cucumber plant. J Soil Sci Plant Nutr 29(3):387–392
Takayama K, Kajita T, Murata J, Tateishi Y (2006) Phylogeography and genetic structure of Hibiscus tiliaceus-Speciation of a pantropical plant with sea-drifted seeds. Mol Ecol 15:2871–2881
Vatanparast M, Takayama K, Sousa MS, Tateishi Y, Kajita T (2011) Origin of Hawaiian endemic species of Canavalia (Fabaceae) from sea-dispersed species revealed by chloroplast and nuclear DNA sequences. J Jpn Botany 86:15–25
Yoshida J, Tomooka N, Yee Khaing T, Shantha PS, Naito H, Matsuda Y, Ehara H (2020) Unique responses of three highly salt-tolerant wild Vigna species against salt stress. Plant Prod Sci 23(1):114–128
Zarebanadkouki M, Kim YX, Carminati A (2013) Where do roots take up water? Neutron radiography of water flow into the roots of transpiring plants growing in soil. New Phytol 199(4):1034–1044
Zhao Y, Wang T, Zhang W, Li X (2011) SOS3 mediates lateral root development under low salt stress through regulation of auxin redistribution and maxima in Arabidopsis. New Phytol 189:1122–1134
Zolla G, Heimer YM, Barak S (2010) Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J Exp Bot 61:211–224
Zou Y, Zhang Y, Testerink C (2022) Root dynamic growth strategies in response to salinity. Plant Cell Environ 45(3):695–704
Acknowledgements
This work was financially supported by Moonshot Research & Development Program for Agriculture, Forestry and Fisheries by Cabinet Office, Government of Japan (20350204). We appreciate Dr. Matthias Wissuwa and Dr. Juan Pariasca Tanaka for providing a facility for root scans and analyses. We appreciate Dr. Jimmy Burridge for his kind help in correcting and polishing English.
Funding
This work was financially supported by Moonshot Research & Development Program for Agriculture, Forestry and Fisheries by Cabinet Office, Government of Japan (JPJ009273).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, F., Iki, Y., Tanoi, K. et al. Phenotypic responses in the root of salt-tolerant accessions of Vigna marina and Vigna luteola under salt stress. Genet Resour Crop Evol 71, 2631–2640 (2024). https://doi.org/10.1007/s10722-023-01794-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-023-01794-3