The composition and a method of production are proposed for high-porosity materials in low-temperature foaming of liquid glass and ash-slag material from burning of combustible shales used as fill and consumable additives. It is shown that keramzit based on liquid glass must be cooled at the rate 60 K/min, because a porous material with high structural quality factor and with high FeO content is formed at this cooling rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In connection with the constantly rising cost of energy the reduction of energy consumption in the production of efficient building materials and in the operation of buildings is now of great concern. Heat-loss reduction in buildings is accomplished by using new and efficient, including porous (keramzit), heat-insulating materials based on industrial wastes.
The compositions and technological techniques of obtaining porous, grainy, heat-insulating materials (keramzit) based on liquid-glass composition have not been adequately studied, which is an impediment to organizing their production [1]. The porosity of these materials equals, on average, 80 – 90% [2]. These materials are obtained on the basis of foamed liquid glass; they include a wide range of materials, whose primary structure-forming element are the products of thermal or chemical foaming of hydrated alkali silicates [2,3,4].
At present, in connection with the increasing rate of growth of construction, the demand for lightweight porous fillers such as keramzit has grown considerably. Keramzit (ceramic gravel) possesses a nearly spherical shape. The structure of the granules of keramzit is non-uniform. It can be divided into an interior zone (nucleus), which possesses high closed porosity, with gray black color predominating, and a shell with a denser structure, closed porosity, and brown color.
The aim of the present work is to determine the optimal cooling regime for keramzit by means of calculations of the structural quality factor Ksq, the content of FeO, and the phase composition.
Procedure
To study the structure of keramzit in different cooling regimes foamed granules were cooled in air upon extraction from the furnace and in the furnace in a stepped cooling regime.
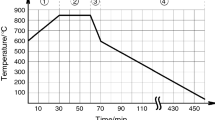
The rate of cooling from the foaming temperature of a sample to the transition of keramzit into a brittle state was equal to 60, 40, 25, and 10 K/min.
The change in the phase composition of the samples was recorded by means of electron microscopy as well as by determining the content of the oxides FeO and Fe2O3.
The di- and trivalent forms of iron were found by the procedure of [4], based on the release of free iodine upon reduction of the trivalent iron by the iodide ion in the presence of potassium iodide. This reaction proceeds in an acid medium without heating in order to avoid volatilization of iodine vapors.
The structural quality factor was calculated in order to determine the influence of the cooling regime on the strength of keramzit:
where R is the strength, MPa, and ρ is the apparent density, kg/m3.
A MIN-8 polarization microscope with different attachments was used to perform quantitative mineralogical analysis.
An ÉMV-100BR electron microscope, the ‘in-transmission’ method, and a platinum-carbon replica were used to obtain information about the phase transformations with keramzit cooled at different rates. X-ray phase analysis with a DRON-6 diffractometer (CoKα radiation) was used to investigate the phase composition.
Experimental Part
Commercial liquid glass, modified by sodium chloride [1, 2, 5], and ash-slag material from burning of combustible shales were used to obtain porous materials (heat-insulating material). Most of the foamed liquid-glass materials are obtained on the basis of sodium liquid glass Na2O·nSiO2 + mH2O, as a rule, with density 1.41 g/cm3 [1, 2].
The ash-slag material from burning of combustible shales were used as the fill and consumable additive. The chemical oxide composition of ash-slag mixture is presented in Table 1, the elemental composition in Table 2, the granulometric (fractional) composition in Table 3, and the technological properties in Table 4.
An x-ray diffraction pattern of the ash-slag material and the microstructure of the ash-slag material from burning of combustible shales are presented in Figs. 1 and 2.
Microstructure of ash-slag mixture from burning of combustible shales: 1 ) glass phase field; 2 ) tabular hematite crystals; 3 ) α-cristobalite crystals of pseudocubic system; 4 ) tabular anorthite crystals; 5 ) pseudomorphoses of glass with short-prismatic mullite; 6 ) fused quartz crystals of prismatic and bi-pyramidal habit; ×15,000.
Aporous fill was obtained by using the optimal composition with the following components (weight content, %): 60 sodium glass; 2 sodium chloride; 38 ash-slag material from burning of combustible shales. An RF patent has been awarded for the presented composition [6].
We are the first to propose a method of obtaining porous fill with relatively low firing temperatures [7].
he (optimal) composition for the production of porous fill was prepared by carefully mixing all components. The obtained mixtures were fed into a forced action mixer in the following order. First, finely ground components and sodium chloride were loaded into a mixer and carefully mixed; next, with the mixer was turned on and sodium glass poured in the form of a fine stream into the ready dry mixture. The mixing was continued until a uniform mass was obtained, in 5 – 7 min. Granules were prepared in a disk granulator.
The main difference of this method consists in the fact that the granules obtained in a disc granulator were heat-treated at 300 – 400°C for 10 – 20 min and then fired at 800 – 900°C for 1 – 3 h. This method made it possible to obtain, at relatively low firing temperatures, a porous fill with high physical and mechanical properties. The firing of montmorillonite clay based keramzit, as a rule, occurs in the temperature interval 1180 – 1200°C. In the present work the keramzit was fired in the temperature interval 900 – 1000°C, using liquid glass. Granules of the obtained keramzit are displayed in Fig. 3.
Electron-microscopic photographs of the keramzit fired at 1000°C (optimal foaming temperature) and cooled at different rates are displayed in Fig. 4.
The phase composition of keramzit cooled at different rates, the structural quality factor Ksq , and the content of iron oxides are presented in Table 5.
The amorphous silica formed in keramzit during mulletization dissolves in the melt. The amount of glass phase and keramzit cooled in air is equal to 69.8% and the structural quality factor 11.2 (see Table 5).
In the case of keramzit cooled at the rate 60 K/min extensive fields of a glass phase, fine tabular magnetite and hematite crystals, large isolated crystals of α-cristobalite, and fragments of quartz and anorthite crystals can be seen under a microscope (see Fig. 4a ) [8, 9].
The glass-phase content in samples cooled at 60 K/min increases, and the total content of iron oxides decreases (see able 5), evidently, as a result of a portion of the iron oxide transitioning into the glass. In the case of rapid reduction of the firing temperature (60 K/min), apparently, a reductive medium predominates inside the sample, so that the FeO content in the sample increases.
Cooling rate reduction to 40 K/min preserves the significant glass-phase fields (see Fig. 4b), and the magnetite content decreases (see Table 5), while at the same time isolated tabular and plate-shaped hematite crystals are observed.
FUSED crystals of quartz, α-cristobalite, individual crystals of short-prism mullite, and a glass-phase field can be seen under a microscope. The contents of the glass phase decreases and the total iron oxide content increases, but the FeO amount decreases. A reduction of the glass-phase content results in reduction of Ksq (see Table 5).
G. V. Kukolev asserts [10] that in the process of amount the material the liquid phase not only pulls together crystal grains but also promotes the recrystallization of the solid phase. If the wetting capacity of the melt is good, liquid can penetrate into capillaries and act as a cementing binder by means of thin films formed on the interphase contacts. If the amounts of melt are large and the viscosity of the liquid high, gases become trapped as a result of pinning of the pores, which affects the properties of the ceramic and increases the structural quality factor Ksq.
Thus, the liquid phase densifies and strengthens the ceramic, and it influences the effectiveness of the structure of sintered materials and the magnitude of the thermal effects and loosens the lattice of different crystalline compounds or it dissolves them and actively participates in the important process of phase formation of the ceramic.
Upon lowering the cooling rate to 25 K/min fused quartz crystals with prismatic habit and individual crystals of cristobalite can be seen under the microscope, but the amount of glass phase decreases (see Fig. 4c).
The reduction of the cooling rate of keramzit to 10 K/min results in reduction of the amount of glass phase, reduction of the structural quality factor, and increase of the Fetotal content. Under the microscope one can see the glass phase field, large individual quartz crystals, fused quartz and anorthite crystals, large sharply delineated plate-like hematite crystals, large individual crystals of α-cristobalite, and short-prism mullite crystals.
Conclusions
It was shown that high-quality porous fill can be obtained on the basis of liquid glass and industrial waste. The compositions and a method of producing high-porosity materials with low-temperature foaming of liquid glass and ash-slag material from burning of combustible shales, which is used as fill and a consumable additive, were proposed. It was shown that keramzit based on liquid glass must be cooled at 60 K/min because a porous material with closed pores with a high structural quality factor and the highest FeO content is formed at this cooling rate.
References
A. I. Kudyakov, N. A. Svergunova, and M. Yu. Ivanov, Granular Insulating Material Based on a Modified Liquid Glass Composition [in Russian], Izd. TGASU, Tomsk (2010).
E. S. Abdrakhimova and V. Z. Abdrakhimov, “Highly porous thermal insulating material based on liquid glass,” Fiz. Khim. Stekla, 43(2), 222 – 230 (2017).
V. Z. Abdrakhimov, “Study of the phase composition of thermal-insulation materials based on solid salt slags and liquid glass,” Izv. Vysh. Ucheb. Zaved., Stoitel’stvo, No. 11 – 12, 33 – 39 (2008).
V. F. Gillebrand and G. A. Lendel, A Practical Guide to Inorganic Analysis [in Russian], Khimiya, Moscow (1965).
E. S. Abdrakhimova, V. Z. Abdrakhimov, and A. K. Kairakbaev, The Use of Wastes from the Fuel and Energy Complex in the Production of Thermal-Insulation Materials Based on Liquid Glass Compositions [in Russian], Kazakhsk.-Rus. Mezhdunar. Universitet, Aktobe (2016).
V. Z. Abdrakhimov, “Composition for the production of waterresistant porous filler, RF Pat. 2478084, C2 SB 14B 14/24,” Byull. Izobr. Polezn. Modeli, No. 9 (2013), publ. March 27, 2013.
V. Z. Abdrakhimov, V. K. Semenychev, V. A. Kulikov, and E. S. Abdrakhimova, “Method of producing porous aggregate, Pat. 2426710, C1 C04B 38/06, 33/132,” Byull. Izobr. Polezn. Modeli, No. 23 (2011), publ. August 20, 2011.
V. F. Pavlov, Physical and Chemical Principles of Firing Building Ceramic Articles [in Russian], Stroiizdat, Moscow (1977).
E. S. Abdrakhimova and V. Z. Abdrakhimov, “Structural transformations of iron compounds in clayey materials according to Mössbauer spectroscopy data,” Zh. Fiz. Khim., 80(7), 1 – 8 (2011).
G. V. Kukolev, Silicon Chemistry and Physical Chemistry of Silicates [in Russian], Vyssh. Shkola, Moscow (1966).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 9, pp. 40 – 44, September, 2018.
Rights and permissions
About this article
Cite this article
Abdrakhimova, E.S. Optimal Cooling Rate of Porous Fill Based on Liquid-Glass Compositions. Glass Ceram 75, 366–369 (2019). https://doi.org/10.1007/s10717-019-00086-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-019-00086-6