Abstract
A variety of insects accumulate high contents of riboflavin (vitamin B2) in their Malpighian tubules (MTs). Although this process is known to be genetically controlled, the mechanism is not known. In the 1940s and the 1950s, several studies showed that riboflavin contents were low in the MTs of some Bombyx mori (silkworm) mutants with translucent larval skin mutations (e.g., w-3, od, oa, and otm) and that genes responsible for these translucent mutations also affected riboflavin accumulation in the MTs. Since the 2000s, it has been shown that the w-3 gene encodes an ABC transporter, whereas genes responsible for od, oa, and otm mutations encode for the biogenesis of lysosome-related organelles. These findings suggest that some genes of ABC transporters and biogenesis of lysosome-related organelles may control the accumulation of riboflavin in MTs. Therefore, we reexamined the effects that translucent mutations have on the accumulation of riboflavin in MTs by using the translucent and wild-type segregants in mutant strains to measure the specific effect that each gene has on riboflavin accumulation (independent of genomic background). We used nine translucent mutations (w-3oe, oa, od, otm, Obs, oy, or, oh, and obt) even though the genes responsible for some of these mutations (Obs, oy, or, oh, and obt) have not yet been isolated. Through observation of larval MTs and measurements of riboflavin content using high-performance liquid chromatography, we found that the oa, od, otm, and or mutations were responsible for low contents of riboflavin in MTs, whereas the Obs and oy mutations did not affect riboflavin accumulation. This indicates that the molecular mechanism for riboflavin accumulation is similar but somewhat different than the mechanism responsible for uric acid accumulation in epidermal cells. We found that the genes responsible for oa, od, and otm mutations were consistent with those already established for uric acid accumulation in larval epidermis. This suggests that these three genes control riboflavin accumulation in MTs through a mechanism similar to that of uric acid accumulation, although we do not yet know why the or mutation also controls riboflavin accumulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A variety of insects accumulate high contents of riboflavin (vitamin B2) in their Malpighian tubules (MTs) (Ishihara 1956; Nickla 1972). The accumulated riboflavin makes MTs exhibit yellow colors (Ishihara 1958a, b). When MTs lack riboflavin, they exhibit white or light-yellow colors (Ishihara 1958c; Van Breugel 1987). The accumulated riboflavin in MTs is thought to contribute to the homeostasis of flavin adenine dinucleotide and flavin mononucleotide (Sang 1956; Nakamura et al. 1992), which play essential roles in a variety of physiological processes. Because riboflavin cannot be synthesized in metazoans (Bacher et al. 2000; Ladenstein et al. 2013; Tuan et al. 2014), it is mainly obtained from their food (Dadd 1985).
In the silkworm Bombyx mori, Koyanagi and Hatamura (1944) first reported that od translucent mutants contained little riboflavin in their MTs. In general, translucent mutations such as od cause a urate granule deficiency in the epidermal cells and subsequently lead to a striking color change in the larval epidermis, from an opaque white to translucent (Tamura and Akai 1990). Kikkawa (1948) reported that the MTs of the wild-type (WT) silkworms stored riboflavin as needle-shaped crystals, whereas MTs of some translucent mutants did not accumulate riboflavin. Thereafter, Aruga et al. (1952a, b) and Eguchi (1955, 1956) showed that the riboflavin contents in MTs and eggs of WT silkworms were greater than those of od translucent mutants. Later, based on his microscopic observations, Ishihara (1958a, b) reported that the needle-shaped granules (crystals) contained high concentrations of riboflavin in their WT MT cells, whereas such granules were absent from MTs of od mutants, suggesting that riboflavin accumulated as cytoplasmic “riboflavin granules” in them.
Ishihara (1958c) compared riboflavin accumulation in MTs among lepidopteran species and speculated that this process must be under genetic control. In addition, he found that several silkworm translucent mutants such as w-3, w-3106, w-3ol (both w-3106 and w-3ol are allelic to w-3), oa, od, and odk had very low contents of riboflavin in their MTs. However, his results also indicated that the quantity of uric acid in epidermal cells did not simply correlate with the accumulation of riboflavin in MTs. For example, the mutants oc, ok, and os contained larger contents of riboflavin than normal silkworms, although they accumulated few urate granules (Ishihara 1958c). Recently, Zhang et al. (2017) reported that another translucent mutant, otm, also possesses riboflavin-deficient MTs. Together, these results suggest that the mechanism for uric acid accumulation in epidermal cells is partly coupled with riboflavin accumulation in MTs. However, due to a lack of molecular information, researchers in the 1940s and the 1950s could not elucidate a mechanism for the accumulation of uric acid and riboflavin in MTs.
In this study, we reexamined the effects of translucent mutations on the accumulation of riboflavin in MTs of silkworms by measuring their riboflavin contents using high-performance liquid chromatography (HPLC). We used translucent and WT segregants of mutant strains to determine the independent effect of each translucent mutant gene (regardless of their genomic backgrounds).
Materials and methods
Silkworm strains
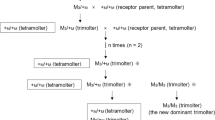
To examine the effects of translucent mutations on riboflavin accumulation in the MTs, we searched SilkwormBase (http://silkworm.nbrp.jp/), Kyushu University, for silkworm strains in which the translucent mutation genotypes segregated as WT (heterozygous) and translucent segregants (homozygous). Nine independent mutations were selected for study (Fig. 1; Fig. S1; Table S1). All silkworm larvae were raised with fresh mulberry leaves under a continuous 12/12 h light/dark cycle at 25 °C.
Genetic background and cross-schemes for the nine mutations used in this study. Detailed information on the respective genes is available at http://silkworm.nbrp.jp/ and Table S1
As shown in Fig. 1, in seven mutant strains, k32, w05, o90, t52, d50, w07, and r50, non-translucent (WT) and translucent larvae (oa, otm, Obs, or, oh, obt, and oy) are segregated, and the genetic backgrounds of both segregants are similar between the respective strains, except for the chromosome carrying the mutant gene. The sex-limited black-egg mutant strain (r01) has a fragment of chromosome 10 containing the + w-3 gene translocated onto the W chromosome in the w-3oe mutant [T(W;10)+w-3 w-3oe]. Because female silkworms are heterogametic (ZW) and males are homogametic (ZZ) (Goldsmith et al. 2005), females exhibit WT egg color and larval translucency phenotypes, whereas males exhibit white egg serosa and translucent larval skin due to the w-3oe mutation. To obtain od segregants with a similar genetic background, we crossed the WT strain p50 (female) with the od mutant strain o06 (male). Because the od gene is located on the Z chromosome, the F1 of p50 × o06 exhibits two different larval skin phenotypes: WT (od/+, male) and translucent (od/W, female).
HPLC analysis of riboflavin contents
We collected the MTs on the day just before spinning of the 5th instar because that is when normal silkworms usually store riboflavin at a high content in their MTs (Nakamura et al. 1992; Zhang et al. 2018). We then performed an HPLC analysis in MTs using the same methods described in Zhang et al. (2018).
Results
Observation of MTs in various mutant strains
We investigated nine mutations (oa, otm, Obs, or, oh, obt, oy, w-3oe, and od), using WT and translucent segregants of the k32, w05, o90, t52, d50, w07, r50, r01, and F1 of p50 × o06 (Fig. 1) strains, respectively, to show their effects on the accumulation of riboflavin in the MTs.
We were able to classify the nine mutations into three groups according to the phenotypic patterns of the MT color (Fig. 2). Group 1 included four mutations: oa, od, otm, and or. The translucent larval segregants (homozygotes) exhibited white MTs, suggesting a lack of riboflavin accumulation, whereas the non-translucent segregants (heterozygotes) exhibited normal yellow MTs. In group 1, larval skin translucency was highly correlated with MT whiteness.
Group 2 included two mutations: Obs and oy. The MTs of larvae with these mutations were yellow regardless of genotype and skin translucency. This suggests that Obs and oy do not affect riboflavin accumulation in the MTs.
Group 3 included three mutations: w-3oe, oh, and obt. In strains with these mutations, MTs were white regardless of genotype and larval skin translucency. In homozygotic segregants, oh/oh, as well as heterozygotic ones, oh/+, MTs appeared white. Similarly, both homozygotic (obt/obt) and heterozygotic (obt/+) segregants had white MTs. One possible explanation is that oh and obt have a dominant effect on MT whitening. Another explanation is that the genetic backgrounds of the d50 and w07 strains contain unknown mutations that affect riboflavin accumulation in MTs. Although T(W;10)+w-3 w-3oe/w-3oe females exhibit non-translucent larval skin, their MTs appeared white. The MTs of the w-3oe/w-3oe males, whose larval skin is translucent, were also white. This indicates that the + w-3 gene on the W chromosome does not complement the MT color phenotype in w-3oe though it is unclear whether the W-linked +w-3 gene is insufficient for riboflavin accumulation, or another hidden mutation in this strain affects the phenotype.
Riboflavin contents in larval MTs
The riboflavin contents of fresh MTs from each of the nine strains measured using HPLC are shown in Table 1 and Table S2. As with the MT color patterns described above, we were also able to classify the nine mutations into three groups (Table 1 and Table S2), according to the MT riboflavin contents.
In group 1 (oa, od, otm, and or), the MTs in WT (heterozygous) segregants had high riboflavin contents (ca. 800–1,500 µg/g), whereas the riboflavin contents of the translucent (homozygous) segregant MTs were ca. 20-fold lower. In group 1, the translucency of the larval skin was correlated with the riboflavin accumulation deficiency, suggesting that these mutations affect riboflavin accumulation in the MTs via a mechanism similar to that of uric acid accumulation in the epidermis.
In group 2 (Obs and oy), translucent as well as non-translucent (WT) segregants contained riboflavin in their MTs at the same relatively high level (ca. 700–1,400 µg/g). This indicates that Obs and oy have no effect on riboflavin accumulation as expected, based on visual observations.
In group 3 (w-3oe, oh, and obt), the MTs of both non-translucent and translucent segregants had low riboflavin contents (30–90 µg/g) regardless of larval skin translucency. These results indicate that the + oh and + obt genes cannot rescue the MTs from riboflavin deficiency in strains d50 and w07, respectively. Also, the results indicate that the W-translocated +w-3 gene cannot rescue it in w-3oe/w-3oe in strain r01.
Discussion
Previous studies have shown that epidermal translucency is partly coupled with riboflavin accumulation deficiency in the MTs (Koyanagi and Hatamura 1944; Kikkawa 1948; Aruga et al. 1952a, a, b; Eguchi 1955, 1956; Ishihara 1958a, b, c). Therefore, identification of the genes responsible for translucent mutations contributes to our understanding of the riboflavin accumulation mechanism. To date, 11 genes have been reported to be responsible for translucent mutations, and these are categorized according to three major processes resulting in translucency (Zhang et al. 2017): failure of uric acid biosynthesis (oq, xanthine dehydrogenase; oya, molybdenum cofactor (MoCo) synthesis-step 1 enzyme; and og, MoCo sulfurase) (Kômoto 2002; Fujii et al. 2016; Kômoto et al. 2003); failure of uric acid transport (w-3, ATP-binding cassette (ABC) transporter Bmwh3; os, solute carrier family OS; and ok, ABC transporter Bm-ok) (Kômoto et al. 2009; Kiuchi et al. 2011; Wang et al. 2013a); and failure of membrane trafficking and/or intracellular accumulation of uric acid in epidermal cells (ow, od, oa, ov, and otm, subunits of biogenesis of lysosome-related organelle complexes (BLOCs) and the adaptor protein-3 (AP-3) interactive proteins) (Ito et al. 2009; Fujii et al. 2010, 2012; Wang et al. 2013b; Zhang et al. 2017).
In the present study, the nine translucent mutations linked to riboflavin accumulation were categorized into three groups according to patterns of MT riboflavin phenotypes (Fig. 2; Table 1). In the first group, four genes (oa, od, otm, and or) clearly affect riboflavin accumulation, indicating a positive link between uric acid and riboflavin accumulation. The second group (Obs and oy) appears to have no effect on riboflavin accumulation. The third group (w-3oe, oh, and obt) seems to affect riboflavin accumulation in both WT and translucent segregants.
In the first group, the genes for the oa, od, and otm mutations have been identified as BmHPS5, which encodes a subunit necessary for BLOC-2; BmBLOS2, which encodes a subunit of BLOC-1; and Bm-muted, which encodes another subunit of BLOC-1, respectively (Fujii et al. 2010, 2012; Zhang et al. 2017), whereas the gene for or is unknown. The genes for oa, od, and otm are known to play essential roles in uric acid accumulation at the final site, especially in the formation of urate granules in epidermal cells (Fujii et al. 2010, 2012; Zhang et al. 2017). The results of this study indicate that they also probably participate in the final riboflavin accumulation in MT cells. Lastly, although the gene responsible for the or mutation is unknown, its MT phenotype is identical to those of oa, od, and otm, suggesting that the causative or gene also plays a role in riboflavin accumulation in the MTs.
In the second group, the Obs and oy mutations had no significant effect on riboflavin accumulation (Table 1). Coincidentally, Ishihara (1958c) reported that os, oc, and ok mutations hardly affected riboflavin accumulation in the MTs. Because the genes for os and ok mutations have been reported to be involved in uric acid transport in larval epidermal cells (Kiuchi et al. 2011; Wang et al. 2013a), the results of this study indicate that os and ok specifically control uric acid accumulation in the epidermis. If the genes responsible for Obs, oy, and oc are identified, it will be possible to explain why they affect only uric acid accumulation.
In the third group, the oh and obt mutations appeared to affect riboflavin accumulation in both heterozygotes and homozygotes. A probable explanation for this is that oh and obt are likely to behave dominantly for riboflavin accumulation but recessively for epidermal translucency (Fig. 1; Table 1). Another possibility is the effects of their genetic backgrounds. Although the d50 strain carries the vit (scanty of vitellin) mutation linked to oh, the vit gene codes for the vitellogenin receptor (Lin et al. 2013) and may not affect riboflavin accumulation in the MTs (Fig. 1). Similarly, the w07 strain carries the q (quail) mutation linked to obt. However, the q gene may not affect the MT phenotype because it encodes a guanylyl cyclase gene, BmGC-I, which controls the pigmentation pattern of larval skin (Yuasa et al. 2016). Nevertheless, there is another possibility that the genetic backgrounds of d50 and w07 carry unknown genes affecting riboflavin accumulation in MTs, respectively. To clarify the effects of oh and obt, further genetic experiments are needed.
Furthermore, the effect of w-3oe should be evaluated thoroughly because we used a special strain, r01, which carries a translocated +w-3 gene on the W chromosome (Fig. 1) with a w-3oe/w-3oe background. One possible reason why the +w-3 gene cannot rescue the riboflavin accumulation is that the translocated +w-3 gene does not encode enough gene products to remedy the riboflavin deficiency even though it is sufficient to remedy epidermal translucency and ommochrome deficiency in the serosa in w-3oe/w-3oe. The gene for w-3 has been identified as Bmwh3 (Kômoto et al. 2009), which encodes a membrane-spanning ABC transporter involved in uric acid and ommochrome precursor transport (Tatematsu et al. 2011; Wang et al. 2013a). To date, three w-3 alleles (w-3, w-3106, w-3ol) have been reported to affect riboflavin accumulation (Ishihara 1958c; Abraham et al. 2000; Kobayashi et al. 2010) in a genetically recessive manner. These observations suggest that Bmwh3 controls riboflavin transport in the MT cells via a mechanism similar to that of uric acid and ommochrome precursor transport. Nevertheless, we cannot exclude another possibility that the genetic background of r01 carries a hidden gene affecting riboflavin accumulation in MTs.
Based on the results of both the present study and Ishihara’s (1958c) study, we conclude that odk, w-3, oa, od, otm, or, oh, and obt mutations are involved in the accumulation of both uric acid and riboflavin in the silkworm, whereas os, ok, og, oc, Obs, and oy mutations function only in uric acid accumulation and do not affect riboflavin accumulation in the MTs (Table 2). This finding supports our hypothesis that the molecular mechanism of riboflavin accumulation bears some similarity to that of uric acid accumulation. Currently, the genes responsible for the oc, odk, Obs, oy, or, oh, and obt mutations are unknown. Uncovering the genes responsible for these mutations can be achieved by positional cloning. Further molecular biological studies of odk, or, oh, and obt mutations will provide a better understanding of the common mechanism in riboflavin accumulation and uric acid accumulation.
References
Abraham EG, Sezutsu H, Kanda T et al (2000) Identification and characterisation of a silkworm ABC transporter gene homologous to Drosophila white. Mol Gen Genet 264:11–19
Aruga H, Eguchi M, Terao K (1952a) On riboflavin in the blood and Malpighian tubes in the larva of Bombyx mori. In: 4th Meeting of Kanto Branch of Japanese Society of Sericultural Science (in Japanese)
Aruga H, Yoshitake N, Eguchi M (1952b) Studies on the mechanism of the expressions of the translucent and lemon genes in the silkworm. (I) On the expressions of the translucent and lemon genes in the silkworm egg. J Seric Sci Jpn 21:255–263 (in Japanese with English summary)
Bacher A, Eberhardt S, Fischer M et al (2000) Biosynthesis of vitamin B2 (riboflavin). Annu Rev Nutr 20:153–167
Dadd RH (1985) Nutrition: organisms. In: Kerkut GA, Gilbert LI (eds) Comparative insect physiology, biochemistry and pharmacology, vol 4. Pergamon, Oxford, pp 313–390
Eguchi M (1955) Comparison of vitamin B2 contents in the blood and eggs between normal and od-translucent silkworms (Bombyx mori L.). J Seric Sci Jpn 24:350–352 (in Japanese with English summary)
Eguchi M (1956) Changes of the riboflavin content in several organs and tissues of od and normal silkworms, Bombyx mori during metamorphosis. Jpn J Genet 31:279–283
Fujii T, Daimon T, Uchino K et al (2010) Transgenic analysis of the BmBLOS2 gene that governs the translucency of the larval integument of the silkworm, Bombyx mori. Insect Mol Biol 19:659–667
Fujii T, Banno Y, Abe H et al (2012) A homolog of the human Hermansky-Pudluck Syndrome-5 (HPS5) gene is responsible for the oa larval translucent mutants in the silkworm, Bombyx mori. Genetica 140:463–468
Fujii T, Yamamoto K, Banno Y (2016) Molybdenum cofactor deficiency causes translucent integument, male-biased lethality, and flaccid paralysis in the silkworm Bombyx mori. Insect Biochem Mol Biol 73:20–26
Goldsmith MR, Shimada T, Abe H (2005) The genetics and genomics of the silkworm, Bombyx mori. Annu Rev Entomol 50:71–100
Ishihara R (1956) Studies on the Malpighian tubules of the silkworm, Bombyx mori L. (I) The accumulation of riboflavin in the Malpighian tubules and the riboflavin content of feces in the silkworm. J Seric Sci Jpn 25:135–140 (in Japanese with English summary)
Ishihara R (1958a) Studies on the Malpighian tubules of the silkworm, Bombyx mori L. (IV) Reciprocal transplantation of the Malpighian tubules between od-oily larva and normal larva. J Seric Sci Jpn 27:193–198 (in Japanese with English summary)
Ishihara R (1958b) Studies on the Malpighian tubules of the silkworm, Bombyx mori L. (V) Supplementary studies on the accumulation of riboflavin in the Malpighian tubules. J Seric Sci Jpn 27:374–381 (in Japanese with English summary)
Ishihara R (1958c) Studies on the Malpighian tubules of the silkworm, Bombyx mori L. (VI) Riboflavin content in the Malpighian tubules of several oily mutants. J Seric Sci Jpn 27:382–387 (in Japanese with English summary)
Ito K, Katsuma S, Yamamoto K et al (2009) A 25 bp-long insertional mutation in the BmVarp gene causes the waxy translucent skin of the silkworm, Bombyx mori. Insect Biochem Mol Biol 39:287–293
Kikkawa H (1948) On the tryptophan-pigment in the silkworm, Bombyx mori. Seibutsugaku no Shinpo. Tokyo Kyoritsu Press 3:36–70 (in Japanese)
Kiuchi T, Banno Y, Katsuma S et al (2011) Mutations in an amino acid transporter gene are responsible for sex-linked translucent larval skin of the silkworm, Bombyx mori. Insect Biochem Mol Biol 41:680–687
Kobayashi I, Uchino K, Iizuka T et al (2010) Rescue of the Aojuku white-egg translucent (w-3 ol) Bombyx mori mutant by transgenic expression of the wild-type Bmwh3 gene. J Insect Biotecnol Sericol 79:111–116
Kômoto N (2002) A deleted portion of one of the two xanthine dehydrogenase genes causes translucent larval skin in the oq mutant of the silkworm (Bombyx mori). Insect Biochem Mol Biol 32:591–597
Kômoto N, Sezutsu H, Yukuhiro K et al (2003) Mutations of the silkworm molybdenum cofactor sulfurase gene, og, cause translucent larval skin. Insect Biochem Mol Biol 33:417–427
Kômoto N, Quan GX, Sezutsu H et al (2009) A single-base deletion in an ABC transporter gene causes white eyes, white eggs, and translucent larval skin in the silkworm w-3 oe mutant. Insect Biochem Mol Biol 39:152–156
Koyanagi T, Hatamura M (1944) Relationship between the od translucency and flavin accumulation in the silkworm, Bombyx mori (Preceding report). Medicine Biology (Tokyo) 5:605–608 (in Japanese)
Ladenstein R, Fischer M, Bacher A (2013) The lumazine synthase/riboflavin synthase complex: shapes and functions of a highly variable enzyme system. FEBS J 280:2537–2563
Lin Y, Meng Y, Wang YX et al (2013) Vitellogenin receptor mutation leads to the oogenesis mutant phenotype “scanty vitellin” of the silkworm, Bombyx mori. J Biol Chem 288:13345–13355
Nakamura M, Yanagawa H, Kuarta K (1992) Homeostasis of vitamin B2 and role of the Malpighian tubes in the silkworm, Bombyx mori. J Seric Sci Jpn 61:52–58 (in Japanese with English summary)
Nickla H (1972) Interaction between pteridine synthesis and riboflavin accumulation in Drosophila melanogaster. Can J Genet Cytol 14:105–111
Sang JH (1956) The quantitative nutritional requirements of Drosophila melanogaster. J Exp Biol 33:45–72
Tamura T, Akai H (1990) Comparative ultrastructure of larval hypodermal cell in normal and oily Bombyx mutants. Cytologia 55:519–530
Tatematsu K, Yamamoto K, Uchino K et al (2011) Positional cloning of silkworm white egg 2 (w-2) locus shows functional conservation and diversification of ABC transporters for pigmentation in insects. Genes Cells 16:331–342
Tuan PA, Zhao S, Kim JK et al (2014) Riboflavin accumulation and molecular characterization of cDNAs encoding bifunctional GTP cyclohydrolase II/3,4-dihydroxy-2-butanone 4-phosphate synthase, lumazine synthase, and riboflavin synthase in different organs of Lycium chinense plant. Molecules 19:17141–17153
Van Breugel FM (1987) Differential riboflavin deposition in white and variegated white mutants of Drosophila hydei. Dev Genet 8:45–58
Wang L, Kiuchi T, Fujii T et al (2013a) Mutation of a novel ABC transporter gene is responsible for the failure to incorporate uric acid in the epidermis of ok mutants of the silkworm, Bombyx mori. Insect Biochem Mol Biol 43:562–571
Wang L, Kiuchi T, Fujii T et al (2013b) Reduced expression of the dysbindin-like gene in the Bombyx mori ov mutant exhibiting mottled translucency of the larval skin. Genome 56:101–108
Yuasa M, Kiuchi T, Banno Y et al (2016) Identification of the silkworm quail gene reveals a crucial role of a receptor guanylyl cyclase in larval pigmentation. Insect Biochem Mol Biol 68:33–40
Zhang H, Kiuchi T, Wang L et al (2017) Bm-muted, orthologous to mouse muted and encoding a subunit of the BLOC-1 complex, is responsible for the otm translucent mutation of the silkworm Bombyx mori. Gene 629:92–100
Zhang H, Kiuchi T, Hirayama C et al (2018) Bombyx ortholog of the Drosophila eye color gene brown controls riboflavin transport in Malpighian tubules. Insect Biochem Mol Biol 92:65–72
Acknowledgements
This study was in part supported by KAKENHI (15H02482, 24658048, and 22128004). The silkworm strains and related information were provided by National BioResource Project, Japan. We thank the anonymous reviewers for their criticism of the manuscript. We thank the Institute for Sustainable Agro-ecosystem Services, University of Tokyo, for facilitating the mulberry cultivation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Kiuchi, T., Hirayama, C. et al. A reexamination on the deficiency of riboflavin accumulation in Malpighian tubules in larval translucent mutants of the silkworm, Bombyx mori. Genetica 146, 425–431 (2018). https://doi.org/10.1007/s10709-018-0034-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-018-0034-y