Abstract
Silkworm moltinism is closely related to many important economic characters such as cocoon yield and cocoon filament size. Farnesoic acid methyltransferase (FAMeT) is the rate-limiting enzyme for the conversion of farnesoic acid to methyl farnesoate which acts as juvenile hormone in crustaceans. Silkworm has seven FAMeT genes in its genome but the precise role of BmFAMeT is still unclear. Our results showed that BmFAMeT6 expression had a clear difference between two different moltinism strains, the M3/M3 (dominant trimolter) and the +M/+M (tetramolter). Declining BmFAMeT6 expression in the M3/M3 could increase the number of larval molts/instars and convert trimolter to tetramolter, and injecting dsBmFAMeT6 could significantly improve the tetramolter rate in several other dominant trimolter strains. These data demonstrated that BmFAMeT6 is relevant to the moltinism of the dominant trimolter. Interestingly, BmFAMeT6 expression was high at the early larval stage and low at the final instar. BmFAMeT6 protein signal was stronger on the first day than on the second day within each instar and was very weak at the last larval instar. BmFAMeT6-specific fluorescence was localized on the corpus allatum. It suggests a correlation between BmFAMeT6 expression and juvenile hormone titers. We speculated that decreasing BmFAMeT6 could affect the moltinism in the dominant trimolter, which may be interrelated with the close relationship between BmFAMeT6 and juvenile hormone titers in silkworm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Farnesoic acid O-methyltransferase (FAMeT) catalyzes the methylation of farnesoic acid (FA) to methyl farnesoate (MF) with the cofactor S-adenosyl-methionine, which is the final rate-limiting step for the biosynthesis of methyl farnesoate (as juvenile hormone) in crustaceans (Laufer et al. 1987; Holford et al. 2004; Nagaraju 2007). The FAMeT gene was first identified in the shrimp Metapenaeus ensis De Haan, 1844 (Decapoda) (Gunawardene et al. 2001), subsequently has been isolated from several other crustaceans (Holford et al. 2004; Hui et al. 2008; Duan et al. 2014; Buaklin et al. 2015; Qian and Liu 2019).
In the fruit fly Drosophila melanogaster Meigen, 1830 (Diptera) a protein with high homology to crustaceans farnesoic acid O-methyltransferase is encoded by CG10527 but has no activity on MF biosynthesis and is not essential for survival and fertility (Burtenshaw et al. 2008; Zhang et al. 2010; Wen et al. 2015;). Knockdown experiments have not revealed involvement of FAMeT in juvenile hormone (JH) biosynthesis and in the change of oocyte lengths in the desert locust Schistocerca gregaria Forskål, 1775 (Orthoptera), which is in accord with the research findings on Drosophila CG10527 (Marchal et al. 2011). However, the FAMeT enzyme reveals a high preference for FA in the Asian citrus psyllid Diaphorina citri Kuwayama, 1908 (Hemiptera) and its expression is consistent with JHs titers in the Mediterranean fruit fly Ceratitis capitata (Wiedemann 1824) (Diptera) (Vannini et al. 2010; Marchal et al. 2011; Van Ekert et al. 2015). Further, FAMeT transcripts have a high level in the abdomen and a positive change during the vitellogenesis in female adults, but only have a low level in the ovary in the brown planthopper Nilaparvata lugens Stal, 1854 (Homoptera) (Liu et al. 2010). In the stingless bee, two FAMeT isoforms resulted from alternative splicing exhibit marked differences between workers and queens in representative stages of Melipona scutellaris Linnaeus, 1758 (Hymenoptera) (Vieira et al. 2008). These reports indicate that the function of FAMeT is different among insect species.
Bombyx mori Linnaeus, 1758 (silkworm), a good model of Lepidoptera, has seven genes (BmFAMeT1 to BmFAMeT7) encoding FAMeT homologues in its genome, but only three (BmFAMeT5, BmFAMeT6 and BmFAMeT7) of them have been analyzed by RT-PCR. And the result displayed that the three genes were shown to continuously express from late embryo to adult (Meng et al. 2013). In this study, we produced the near-isogenic lines and proposed that BmFAMeT6 is most likely related to the moltinism of dominant trimolters by several analysis including RNAi, Western blotting, and immunofluorescence.
Materials and methods
Silkworm strains
Silkworm ZhongXian (the +M/+M, tetramolter), the M3/M3 (dominant trimolter), S4 (dominant trimolter), S6 (dominant trimolter), S11 (dominant trimolter) and HeiShan (dominant trimolter) strains were maintained at the Southwest University in China and were reared under standard conditions 24–26 °C and 70–85% RH with a photoperiod of 12:12 LD.
RNA extraction, cDNA synthesis and cloning
Total RNA was purified using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Then ezDNase was added to the RNA sample incubating for 2 min at 37 °C for the fast removal of genomic DNA from RNA preparations. cDNA was synthesized using oligo (dT) primers and Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega). The target fragment on the full-length mRNA sequence of BmFAMeT6 was obtained using specific primers (Online resource 1: Table S1) and then was cloned into pMD19-T vector and verified by sequencing.
Recombinant protein expression, purification and production of the antibody
The ORF sequence of BmFAMeT6 has cloned into pET32 a (+) vector by EcoR I and XhoI sites (Online resource 1: Table S1), and then the BmFAMeT6-pET32 a (+) plasmid was transformed into E. coli strain BL21 cells. The recombinant protein containing the His-tag was induced to express by IPTG and subsequently purified by affinity chromatography using a Ni2+ column. The polyclonal anti-BmFAMeT6 from the male adult rabbit was produced by JinSiRui Biotechnology Company (China) (Online resource 1: Fig. S1).
RNAi
Primers for the dsRNA synthesis were listed in Online resource 1: Table S2 and the dsRNA was synthesized by the T7 RiboMAX™ Express RNAi System (Promega). The integrity of dsRNA was confirmed by non-denaturing agarose gel electrophoresis (Online resource 1: Fig. S2). The dsRNA was diluted to the concentration at 6 μg/μL using RNase-free water for injection.
Experimental larvae were chosen to base on similar weight within the strain in the same instar. After surface-sterilization with 70% alcohol, larvae were cold anesthetized and injected liquid using a homemade capillary syringe through the third or fourth spiracle into hemocoel to avoid bleeding as much as possible. Each larva was injected twice, and for the first injection 2 μL solutions were used on the left side at 0 h, and for the second injection 3 μL solutions were used on the right side at 12 h after entering the second molting process. The control larvae were injected with RNase-free water (ddH2O) or dsRNA of EGFP (dsEGFP) under the same conditions, and the injected individuals were kept at 25 °C.
Semi-quantitative RT-PCR
The primers listed in Online resource 1: Table S3, according to their CDS sequence, were designed for the expression analysis of BmFAMeT6 (KC888750), BmACAT (AB274988, encoding acetoacetyl CoA thiolase), BmHMGR (AB274990, encoding 3-hydroxy-3-methylglutaryl-CoA reductase), BmHMGS (AB274989, encoding 3-hydroxy-3-methylglutaryl-CoA synthase), BmMevK (AB274991, encoding mevalonate kinase), BmMevPK (AB274992, encoding Phosphomevalonate kinase), BmMevPPD (AB274993, encoding diphosphomevalonate decarboxylase), BmIPPI (DQ311366, encoding isopentenyl-diphosphate delta isomerase), BmFPPS (AB274995, encoding farnesyl diphosphate synthase), BmCYP15C1 (AK289313, encoding cytochrome P450 monooxygenase), BmJHAMT (AB113578, encoding juvenile hormone acid methyltransferase), BmKr-h1 (AB360766, encoding Kruppel homolog 1), BmMet (AB359911, encoding methoprene-tolerant receptor), BmECR (D43943, encoding ecdysone receptor), BmUSP (AB182582, encoding RXR type hormone receptor), BmJHE (AY489292, encoding juvenile hormone esterase), BmJHEH (AY377854, encoding juvenile hormone epoxide hydrolase), BmJHDK (AY363308, encoding juvenile hormone diol kinase) and BmJHBP (AY286477, encoding juvenile hormone binding protein). Templates were cDNAs from the larval head or body without midgut, and PCR reactions were carried out in 25 cycles. BmActin3 was used as an internal control. The amplified products were detected by 1% agarose gel electrophoresis.
Quantitative RT-PCR
Quantitative RT-PCR was performed to measure the expression levels of BmFAMeT6 and BmJHAMT using the ABI Prism 7000 sequence detection system (Applied Biosystems, USA) with a SYBR Premix Ex-Taq kit (Takara). The primers designed for qRT-PCR are listed in Online resource 1: Table S4. The B. mori gene of translation initiation factor 4A (sw22934) was used as an internal control.
Western blotting
Concentrations of total proteins from the larval head of the third or fourth instar were estimated using the bicinchoninic acid (BCA). The sample with 30 μg protein was separated using 12% SDS-PAGE and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life Sciences). The BmFAMeT6 protein and tubulin protein on the membrane were detected with anti-BmFAMeT6 (1:1000 dilution) and anti-tubulin (1:5000 dilution), respectively, as primary antibody, and alkaline phosphatase-conjugated goat anti-rabbit IgG (1:2000 dilution, Sigma-Aldrich) as the secondary antibody.
Immunofluorescence analysis
The brain-corpora cardiaca-corpora allata complexes were taken out in pH 7.4 PBS and washed with wash buffer (0.1% Tween-20 in PBS) after removing the fat body and other sundries, then the complexes were fixed with 4% paraformaldehyde at 4 °C for 12 h. The fixed complexes were dialyzed with dialysate I (1 M pH 6.8 Tris, 10% Triton-X 100, 2% BSA, 0.05 M NaCl) at 4 °C for 2 days and dialysate II (4 volume anhydrous ethanol and 1 volume DMSO) at −20 °C for 4 h. Then the complexes washed with wash buffer to incubate it in polyclonal anti-BmFAMeT6 (1: 500) at 4 °C for 3 days. After rigorous washing, the tissues were incubated with Alexa Flour 488 (1:1000, Beyotime) at 4 °C for 3 days. Next, the tissues were counterstained the nuclei with DAPI (Sigma) for 3 h, and then washed with wash buffer before placing it in 50%, 60%, 70%, 80%, 90%, 100% ethanol gradients in turn for 5 min respectively. Finally, the stained tissues were transferred in 70% glycerol for microscopic observation under fluorescence after adding antibody quencher (Chen et al. 2019).
Results
Production of the near-isogenic lines of dominant trimolter
The larval molt times before wandering varies with strains, which is named “moltinism” in the silkworm. Larvae of standard B. mori strains undergo molting four times and thus have five larval instars, and these larvae are conventionally termed “tetramolter”. Similarly, larvae undergo molting three times and thus have four larval instars, and are termed “trimolter” (Zhou et al. 2017). HeiShan strain is a dominant trimolter whose offspring by mating with tetramolter show trimolter phenotype, and was used as the donor parent. And ZhongXian (the +M/+M) strain with tetramolter trait was used as the receptor parent in this work. To generate the near-isogenic lines of dominant trimolter, the moltinism trait in HeiShan strain was transferred into the receptor parent to displace its tetramolter traits. The specific process of transferring was as follows: HeiShan mated with the receptor parent to achieve the first transfer-crossing of dominant trimolter trait. The hybrid progeny with trimolter phenotype mated also with the receptor parent to continue the back-cross breeding. After five times of the back-cross breeding, the offspring with trimolter phenotype inbred to produce progenies. Then by inter-batch selection to above posterity, the group where all individuals had trimolter trait was chosen and then stabilized using self-crossing to establish new dominant trimolter strain (Fig. 1). Since the genetic composition of offspring is half from the father and the other half from the mother, the new dominant trimolter strain shared theoretically 96.875% similarity with the receptor parent on hereditary substance. So the new dominant trimolter strain (the M3/M3) and the receptor parent strain (the +M/+M) constituted the near-isogenic lines of dominant trimolter.
A flowsheet of generation of new dominant trimolter strain of B.mori by importing moltinism character from dominant trimolter strain (M3/M3, donor parent) into tetramolter strain (+M/+M, receptor parent). Black box indicates eliminated offspring; × indicates hybridization crossing; ⊕indicates self- crossing
Screening genes by differential expression between the M3/M3 and the +M/+M
JH modulates molting and growth of insects via stage-specific changes in titers and plays a leading role in maintaining larval morphology and characteristics (Riddiford 1994; Truman and Riddiford 1999; Riddiford et al. 2003; Muramatsu et al. 2008; Daimon et al. 2015; Zeng et al. 2017). And CYP15C1 (a key gene for JH biosynthesis) mutation (mod) showed that JH is not necessary for the first or second instar larvae of B.mori (Daimon et al. 2012). In order to find the genes causing moltinism difference between the near-isogenic lines of the dominant trimer (the M3/M3 and the +M/+M), the expressions of nineteen genes involved in JH biosynthesis (11 genes including BmACAT, BmHMGS, BmHMGR, BmMevK, BmMevPK, BmMevppD, BmIPPI, BmFPPS, BmJHAMT, BmCYP15C1 and BmFAMeT6), degradation (3 genes including BmJHE, BmJHEH and BmJHDK) and signal transmission (5 genes including BmJHBP, BmMet, BmECR, BmUSP, BmKr-h1) in the larval body without midgut were analyzed by RT-PCR in day 1 of the third instar (3L1D) and day 2 of the third instar (3L2D). BmFAMeT6 and BmJHAMT, but not the other seventeen genes, were obviously different in the band brightness of PCR products between the M3/M3 and the +M/+M (Fig. 2a). To verify the reliability of RT-PCR detection, the expression levels of BmFAMeT6 and BmJHAMT were analyzed by qRT-PCR. The results showed that the expression of BmFAMeT6 in M3/M3 was significantly increased in 3L1D and 3L2D compared with +M/+M, while BmJHAMT was only highly expressed in 3L2D instead of 3L1D (Fig. 2b, c). These data indicate that the expression of BmFAMeT6 is different between the near-isogenic lines of the dominant trimolter.
Expression of genes related to JH in both the M3/M3 and the +M/+M. a RT-PCR analysis for JH pathway-related genes. 1, 3 indicate the M3/M3; 2, 4 indicate the +M/+M. The red box indicates the difference-expression of BmFAMeT6 and BmJHAMT between strains. b qRT-PCR analysis for BmFAMeT6. c qRT-PCR analysis for BmJHAMT. Data represent the mean of three values, and error bars indicate S.E.M. * and ** indicate significance at p < 0.05 and p < 0.01, respectively. 3LD1 indicates day 1 of the third instar; 3LD2 indicates day 2 of the third instar
Cloning BmFAMeT6 and purifying expressed protein in vitro
In order to study the function of BmFAMeT6, we referenced KC888750 in GenBank and cloned the 706 bp cDNAs sequence containing the ORF reading frame of BmFAMeT6 from the larval head of the third instar of the M3/M3 (Fig. 3a). Furthermore, the complete coding sequence of BmFAMeT6 was cloned to the prokaryotic expression vector (Fig. 3a), and mass protein was induced to express by 0.2 mM IPTG (Fig. 3b). The molecular weight of the recombinant protein was about 49 kDa as estimated by electrophoresis (Fig. 3b, c), which is consistent with 49.2 kDa of the predicted weight of BmFAMeT6. The recombinant protein of BmFAMeT6 was purified in high yield and purity (Fig. 3d).
Production of recombinant BmFAMeT6, cloning, and in vitro expression, and purification. a clone and expression vector construction of BmFAMeT6 ORF sequence. b inducing expression of BmFAMeT6 protein by 0.2 mM IPTG. c Detection of BmFAMeT6 protein in supernatant and pellet of cell lysate. d Effect of various concentrations of imidazole eluate in the purification of BmFAMeT6 protein. The red arrow indicates the target band
Knockdown of BmFAMeT6 in larvae of the dominant trimer
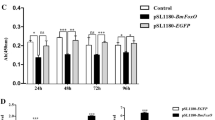
The dsRNA of BmFAMeT6 (dsBmFAMeT6) was injected into the larvae of the M3/M3 at the second molting process. Each larva was injected with 2 μL solution at 0 h and 3 μL solution at 12 h after entering the molting process. The control larvae were injected with dsEGFP or ddH2O under the same conditions and each group consisted of 30 individuals. There were 24, 25, and 22 living individuals in the ddH2O, the dsEGFP and the dsBmFAMeT6 group, respectively (Table 1). Interestingly, the survival individuals with dsBmFAMeT6 entered the third molting process, the mid-term of fourth instar and the fourth molting process, while most of the survival larvae with ddH2O or dsEGFP were in the late third instar, the third molting process and the first half of the final instar at 116 h, 141 h, and 229 h after injection, respectively (Fig. 4a-c). The larvae in the dsBmFAMeT6 group shortened the duration of the third and fourth instars and showed an extra molt to the fifth instar. And qRT-PCR analysis displayed that the expression of BmFAMeT6 was substantially down-regulated in the larvae injected dsBmFAMeT6 (Fig. 4d). Further, the two control groups, the dsEGFP group and the ddH2O group, were similar in the results. These suggested that shortening the time span in the third and fourth instars, increasing the number of larval molts, and converting trimolter to tetramolter were induced by the decrease of the BmFAMeT6 expression, and BmFAMeT6 was probably related to the moltinism in the M3/M3. Also, there were very few larvae having five instars in the dsEGFP group or the ddH2O group, which was similar to the investigation from the group of un-injected larvae and should be caused by environmental factors.
Effect of injecting dsBmFAMeT6 into the larvae of the M3/M3 at the second molting process. a larvae at 116 h after injection. b larvae at 141 h after injection. c larvae at 229 h after injection. The side length of each small dark square grid under larvae represents 1 cm. d Expression levels of BmFAMeT6 in the injected larvae by qRT-PCR. Data represent the mean of three values, and error bars indicate S.E.M. *** indicate significance at p < 0.001
To corroborate whether BmFAMeT6 influence the moltinism of dominant trimolter, RNAi was applied to three other dominant trimolter strains S4, S6, and S11 to record the molt times before the onset of spinning. There were 30 larvae for dsFAMeT6 and 50 larvae for ddH2O per strain. Results showed that the tetramolter rates in living individuals were 70.59% and 23.81%, 71.43% and 25.58%, 72.73% and 18.18% in the dsFAMeT6 group and the ddH2O group in S4, S6 and S11 strain, respectively (Table 2). Compared with the control group, the tetramolter rate of the experimental groups was increased about 3 times, 2.8 times, and 4 times in S4, S6, and S11 strains, respectively. These indicated that injection of dsBmFAMeT6 into several other dominant trimolter strains also could increase the larval molt times and transform trimolter to tetramolter. This is similar to the investigation from the M3/M3.
Detection of BmFAMeT6 mRNA and protein in the larval head
We analyzed the presence of mRNA and the encoded protein of BmFAMeT6 in the larval head of 3L1D, 3L2D, day 1 of the fourth instar (4L1D) and day 2 of the fourth instar (4L2D) in both the M3/M3 and the +M/+M. RT-PCR analysis showed that the BmFAMeT6 transcript appeared in all samples although with differences in the levels. The expressions of the BmFAMeT6 in the M3/M3 were high in 3L1D and 3L2D, but low in 4L1D and 4L2D. In the +M/+M, the expressions of the BmFAMeT6 were moderate in 3L1D, 3L2D, 4L1D and 4L2D (Fig. 5a). The results of Western blotting indicated that the protein signals of BmFAMeT6 were strong in 3L1D and 3L2D of the M3/M3, 3L1D and 4L1D of the +M/+M, but weak in 4L1D and 4L2D of the M3/M3, 3L2D and 4L2D of the +M/+M (Fig. 5b). The third instar was more than the fourth instar on the BmFAMeT protein signal as well as the mRNA level in the M3/M3. And the protein signals were stronger on the first day than the second day within all observed instars, excepting the fourth instar (the final larval instar) of the M3/M3 where signals were equally weak in both periods.
Location BmFAMeT6 in the brain - corpus cardiacum - corpus allatum complex
The corpora allata is a very small ellipsoid tissue connected to the brain through the corpus cardiacum in the larval head. In order to reduce the loss of small organs with low visibility in the immunohistochemical experiment, the brain with attached corpora cardiaca-allata were analysed as wholemounts. Clear fluorescence was only observed in the corpora allata but not in the brain or corpus cardiacum at 4L1D and 4L2D in the +M/+M (Fig. 6a, b). No fluorescence signals in the corpora allata were found at day 1 of the fifth instar (5L1D) and day 2 of the fifth instar (5L2D) in the +M/+M (Fig. 6c, d), and at 4L1D and 4L2D in the M3/M3 (Fig. 6e, f). The fourth instar of the M3/M3 such as the fifth instar of the +M/+M is the last larval instar, where no BmFAMeT6 fluorescence signals were found.
Immunofluorescence analysis of BmFAMeT6 in the brain - corpus cardiacum – corpus allatum complex. a 4LD1 of the +M/+M. b 4LD2 of the +M/+M. c day 1 of the fifth instar (5LD1) of the +M/+M. d day 2 of the fifth instar (5LD2) of the +M/+M. e 4LD1 of the M3/M3. f 4LD2 of the M3/M3. g 5LD1 of the +M/+M for DAPI. h 5LD2 of the +M/+M for DAPI. i 4LD1 of the M3/M3 for DAPI. j 4LD2 of the M3/M3 for DAPI. The red arrow indicates the fluorescence signal. The red box indicates the magnified area. Scale bar 200 μm
Discussion
The silkworm is an important economic insect, its moltinism is closely related to many important economic characters such as cocoon yield, cocoon silk quality and cocoon filament size (Chen 2006). Near-isogenic lines, due to the small genetic difference, have great advantages in studying the genetic information of important characters and were widely used in animals and plants (Shen et al. 2001; Miao et al. 2005;). Here, we generated the near-isogenic lines of dominant trimolter using classical transfer hybridization in silkworm breeding. These lines provide an excellent resource for the study of the molecular mechanism of moltinism regulation that underlies plasticity in the number of larval instars.
In the silkworm, the number of larval instars in a strain is generally fixed although can be modified by the environment and genetic signals (Shen et al. 2001; Miao et al. 2005;). So far, several signaling molecules (FOXO, JHE, CYP15C1, Met, JHAMT, Kr-h1 and E93) have been shown to be involved in the regulation of moltinism (Shinoda and Itoyama 2003; Tan et al. 2005; Konopova and Jindra 2007; Daimon et al. 2012; Zeng et al. 2017). In this study, the BmFAMeT6 expression had an obvious distinction between the near-isogenic lines of the dominant trimolter (the M3/M3 and the +M/+M), suggesting that BmFAMeT6 is probably related to the moltinism of dominant trimolter. Especially, knockdown of BmFAMeT6 in the M3/M3 induced extra molting and convert trimolter to tetramolter. And injecting dsBmFAMeT6 could promote the tetramolter rate to increase significantly in several other dominant trimolter strains. These demonstrate that BmFAMeT6 is relevant to the moltinism of dominant trimolter. In crustaceans, many studies reveal that FAMeT plays an important role in reproduction (Wainwright et al. 1998; Zapata et al. 2003; Duan et al. 2014; Buaklin et al. 2015). However, FAMeT mRNA is constitutively expressed throughout the molt cycle in M. ensis (Gunawardene et al. 2002), and its level is higher in pre-molt than inter-molt in the mud crabs Scylla olivacea (Herbst, 1796) (Decapoda) (Sunarti et al. 2016). The shrimps can’t develop to the final stage of molt cycle when FAMeT is knocked down in the white shrimp, Litopenaeus vannamei Boone, 1931 (Decapoda) (Hui et al. 2008). By RNAi silence of FAMeT down-regulates the expression of the ecdysone receptor gene (EcR) and silence of EcR decreases the expression of FAMeT as well in the giant freshwater prawn, Macrobrachium rosenbergii De Man, 1879 (Decapoda) (Qian and Liu 2019). These indicate that FAMeT is also interrelated to the molt of crustacean.
Immunohistochemical analysis has confirmed the presence of FAMeT in the corpus allatum of D. melanogaster ring gland (Burtenshaw et al. 2008). The FAMeT is highly expressed in the corpus allatum of N. lugens (Liu et al. 2010) and S. gregaria (Marchal et al. 2011). In D. citri, the FAMeT mRNA is detected in the head-thorax containing the corpora allata (Van Ekert et al. 2015). Our data exhibited that BmFAMeT6-specific fluorescence was strictly limited to the corpora allata, and BmFAMeT6 transcript and protein were discovered in the larval head containing corpus allatum in silkworm. Therefore, the BmFAMeT6 is expressed in the corpus allatum of the silkworm, what is concordand with other studied insects.
The JH titer is high at the beginning of each instar and then debase to a low level, and gradually decrease from the early larval stage to the early stage of the final instar and then disappear (Furuta et al. 2013; Meng et al. 2015). The concentration of JH titers in the dominant trimolter is high during the second instar (Meng et al. 2015). We tested BmFAMeT6 mRNA and protein at the early stage of the third and fourth instar in the larval head with corpus allatum. The results show that the expression level of BmFAMeT6 was higher at the beginning of each instar and decreased gradually from the early larval stage to the final instar, which was consistent with the change of JH titers. And the BmFAMeT6 fluorescence signal was located on the corpus allatum at the fourth instar (an un-last instar stage) of the +M/+M. These suggested that there seemed to be a correlation between BmFAMeT6 expression and JH titers, which was similar to the report from C. capitata (Van Ekert et al. 2015).
In the silkworm, precocious larval-pupal transitions could be induced by the loss of or low levels of JH (Fukuda 1944; Tan et al. 2005; Daimon et al. 2012; Wang et al. 2012). The trimolting phenotype in the dominant trimolter is most likely due to the greater concentration of JH titers during the second larval instar (Meng et al. 2015). This indicates that the disorder of JH titers is associated with moltinism mutation. Our immunohistochemical analysis revealed that BmFAMeT6 is specifically expressed in the corpus allatum, the only organ producing and secreting JHs in the Bombyx larva. BmFAMeT6 expression probably had a close correlation with the JH synthesis and secretion. Particularly, the reduction of BmFAMeT6 expression induced extra molt to turn trimolter to tetramolter. These indicated that descending BmFAMeT6 could change the larval molt times in the dominant trimolter, which might be due to the close relationship between BmFAMeT6 and JH titers. We will try to elucidate the relationship between BmFAMeT6 and JH titers in further research.
Conclusion
Our results provide new information for moltinism regulation in dominant trimolter of silkworm, and display the possible biological function of FAMeT in Lepidoptera for the first time.
References
Buaklin A, Jantee N, Sittikankaew K, Chumtong P, Janpoom S, Menasveta P, Klinbunga S, Khamnamtong B (2015) Expression and polymorphism of farnesoic acid O-methyltransferase (FAMeT) and association between its SNPs and reproduction-related parameters of the giant tiger shrimp Penaeus monodon. Aquaculture 441:106–117. https://doi.org/10.1016/j.aquaculture.2015.02.021
Burtenshaw SM, Su PP, Zhang JR, Tobe SS, Dayton L, Bendena WG (2008) A putative farnesoic acid O-methyltransferase (FAMeT) orthologue in Drosophila melanogaster (CG10527): relationship to juvenile hormone biosynthesis? Peptides 29(2):242–251. https://doi.org/10.1016/j.peptides.2007.10.030
Chen P (2006) Differences in growth and main economic characters between trimolters and tetramolters of silkworm. J Southwest Agric Univ 28(4):648–6504
Chen P, Li T, Zhang L, Chen X, Li YT (2019). A treatment method of micro organ. Chinese Invention Patent. Announcement No, CN107560917B
Daimon T, Kozaki T, Niwa R, Kobayashi I, Furuta K, Namiki T, Uchino K, Banno Y, Katsuma S, Tamura T, Mita K, Sezutsu H, Nakayama M, Itoyama K, Shimada T, Shinoda T (2012) Precocious metamorphosis in the juvenile hormone-deficient mutant of the silkworm, Bombyx mori. PLoS Genet 8(3):13. https://doi.org/10.1371/journal.pgen.1002486
Daimon T, Uchibori M, Nakao H, Sezutsu H, Shinoda T (2015) Knockout silkworms reveal a dispensable role for juvenile hormones in holometabolous life cycle. Proc Natl Acad Sci USA 112(31):E4226–E4235. https://doi.org/10.1073/pnas.1506645112
Duan YF, Liu P, Li JT, Wang Y, Li J, Chen P (2014) A farnesoic acid O-methyltransferase (FAMeT) from Exopalaemon carinicauda is responsive to Vibrio anguillarum and WSSV challenge. Cell Stress Chaperones 19(3):367–377. https://doi.org/10.1007/s12192-013-0464-5
Fukuda S (1944) The hormonal mechanism of larval molting and metamorphosis in the silkworm. J Fac Sci Tokyo Imp Univ 6:477–532 http://ci.nii.ac.jp/naid/10008670790. Accessed 18 March 2020
Furuta K, Ichikawa A, Murata M, Kuwano E, Shinoda T, Shiotsuki T (2013) Determination by LC-MS of juvenile hormone titers in hemolymph of the silkworm, Bombyx mori. Biosci Biotechnol Biochem 77(5):988–991. https://doi.org/10.1271/bbb.120883
Gunawardene Y, Chow BKC, He JG, Chan SM (2001) The shrimp FAMeT cDNA is encoded for a putative enzyme involved in the methylfarnesoate (MF) biosynthetic pathway and is temporally expressed in the eyestalk of different sexes. Insect Biochem Mol Biol 31(11):1115–1124. https://doi.org/10.1016/s0965-1748(01)00060-1
Gunawardene Y, Tobe SS, Bendena WG, Chow BKC, Yagi KJ, Chan SM (2002) Function and cellular localization of farnesoic acid O-methyltransferase (FAMeT) in the shrimp, Metapenaeus ensis. Eur J Biochem 269(14):3587–3595. https://doi.org/10.1046/j.1432-1033.2002.03048.x
Holford KC, Edwards KA, Bendena WG, Tobe SS, Wang ZW, Borst DW (2004) Purification and characterization of a mandibular organ protein from the American lobster, Homarus americanus: a putative farnesoic acid O-methyltransferase. Insect Biochem Mol Biol 34(8):785–798. https://doi.org/10.1016/j.ibmb.2004.04.003
Hui JHL, Tobe SS, Chan SM (2008) Characterization of the putative farnesoic acid O-methyltransferase (LvFAMeT) cDNA from white shrimp, Litopenaeus vannamei: evidence for its role in molting. Peptides 29(2):252–260. https://doi.org/10.1016/j.peptides.2007.08.033
Konopova B, Jindra M (2007) Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci USA 104(25):10488–10493. https://doi.org/10.1073/pnas.0703719104
Laufer H, Borst D, Baker FC, Carrasco C, Sinkus M, Reuter CC, Tsai LW, Schooley DA (1987) Identification of a juvenile hormone-like compound in a crustacean. Science 235(4785):202–205. https://doi.org/10.1126/science.235.4785.202
Liu S, Zhang C, Yang B, Gu J, Liu Z (2010) Cloning and characterization of a putative farnesoic acid O-methyltransferase gene from the brown planthopper, Nilaparvata lugens. J Insect Sci 10(1):103. https://doi.org/10.1673/031.010.10301
Marchal E, Zhang J, Badisco L, Verlinden H, Hult EF, Van Wielendaele P, Yagi KJ, Tobe SS, Vanden Broeck J (2011) Final steps in juvenile hormone biosynthesis in the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol 41(4):219–227. https://doi.org/10.1016/j.ibmb.2010.12.007
Meng M, Cheng D, Peng J, Kang L, Wang Y, Qian W, Xia Q (2013) Identification and expression pattern analysis of farnesic acid methyltransferase (FAMeT) gene in silkworm, Bombyx mori. Sci Seric 39(4):680–688
Meng M, Liu C, Peng J, Qian W, Qian H, Tian L, Li J, Dai D, Xu A, Li S, Xia Q, Cheng D (2015) Homeodomain protein scr regulates the transcription of genes involved in juvenile hormone biosynthesis in the silkworm. Int J Mol Sci 16(11):26166–26185. https://doi.org/10.3390/ijms161125945
Miao XX, Xu SJ, Li MH, Li MW, Huang JH, Dai FY, Marino SW, Mills DR, Zeng PY, Mita K, Jia SH, Zhang Y, Liu WB, Xiang H, Guo QH, Xu AY, Kong XY, Lin HX, Shi YZ, Lu G, Zhang XL, Huang W, Yasukochi Y, Sugasaki T, Shimada T, Nagaraju J, Xiang ZH, Wang SY, Goldsmith MR, Lu C, Zhao GP, Huang YP (2005) Simple sequence repeat-based consensus linkage map of Bombyx mori. Proc Natl Acad Sci USA 102(45):16303–16308. https://doi.org/10.1073/pnas.0507794102
Muramatsu D, Kinjoh T, Shinoda T, Hiruma K (2008) The role of 20-hydroxyecdysone and juvenile hormone in pupal commitment of the epidermis of the silkworm, Bombyx mori. Mech Dev 125(5–6):411–420. https://doi.org/10.1016/j.mod.2008.02.001
Nagaraju GPC (2007) Is methyl farnesoate a crustacean hormone? Aquaculture 272(1):39–54. https://doi.org/10.1016/j.aquaculture.2007.05.014
Qian Z, Liu X (2019) Elucidation of the role of farnesoic acid O-methyltransferase (FAMeT) in the giant freshwater prawn, Macrobrachium rosenbergii: possible functional correlation with ecdysteroid signaling. Comp Biochem Physiol A Mol Integr Physiol 232:1–12. https://doi.org/10.1016/j.cbpa.2019.03.003
Riddiford LM (1994) Cellular and molecular actions of juvenile-hormone I. General considerations and premetamorphic actions. Adv Insect Physiol 24:213–274. https://doi.org/10.1016/S0065-2806(08)60084-3
Riddiford LM, Hiruma K, Zhou X, Nelson CA (2003) Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochem Mol Biol 33(12):1327–1338. https://doi.org/10.1016/j.ibmb.2003.06.001
Shen L, Courtois B, McNally KL, Robin S, Li Z (2001) Evaluation of near-isogenic lines of rice introgressed with QTLs for root depth through marker-aided selection. Theor Appl Genet 103(1):75–83. https://doi.org/10.1007/s001220100538
Shinoda T, Itoyama K (2003) Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci USA 100(21):11986–11991. https://doi.org/10.1073/pnas.2134232100
Sunarti Y, Soejoedono RD, Mayasari NLPI, Tahya AM (2016) RNA expression of farnesoic acid O-methyl transferase in mandibular organ of intermolt and premolt mud crabs Scylla olivacea. AACL Bioflux 9(2):270–275 http://www.bioflux.com.ro/docs/2016.270-275.pdf. Accessed 18 March 2020
Tan A, Tanaka H, Tamura T, Shiotsuki T (2005) Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc Natl Acad Sci USA 102(33):11751–11756. https://doi.org/10.1073/pnas.0500954102
Truman JW, Riddiford LM (1999) The origins of insect metamorphosis. Nature 401(6752):447–452. https://doi.org/10.1038/46737
Van Ekert E, Shatters RG, Rouge P, Powell CA, Smagghe G, Borovsky D (2015) Cloning and expressing a highly functional and substrate specific farnesoic acid o-methyltransferase from the Asian citrus psyllid (Diaphorina citri Kuwayama). FEBS OpenBio 5:264–275. https://doi.org/10.1016/j.fob.2015.03.012
Vannini L, Ciolfi S, Spinsanti G, Panti C, Frati F, Dallai R (2010) The putative-farnesoic acid O-methyl transferase (FAMeT) gene of Ceratitis capitata: characterization and pre-imaginal life expression. Arch Insect Biochem Physiol 73(2):106–117. https://doi.org/10.1002/arch.20344
Vieira CU, Bonetti AM, Simoes ZLP, Maranhao AQ, Costa CS, Costa MCR, Siquieroli ACS, Nunes FMF (2008) Farnesoic acid O-methyl transferase (FAMeT) isoforms: conserved traits and gene expression patterns related to caste differentiation in the stingless bee, Melipona scutellaris. Arch Insect Biochem Physiol 67(2):97–106. https://doi.org/10.1002/arch.20224
Wainwright G, Webster SG, Rees HH (1998) Neuropeptide regulation of biosynthesis of the juvenoid, methyl farnesoate, in the edible crab, Cancer pagurus. Biochem J 334:651–657. https://doi.org/10.1042/bj3340651
Wang HB, Ali SM, Moriyama M, Iwanaga M, Kawasaki H (2012) 20-hydroxyecdysone and juvenile hormone analog prevent precocious metamorphosis in recessive trimolter mutants of Bombyx mori. Insect Biochem Mol Biol 42(2):102–108. https://doi.org/10.1016/j.ibmb.2011.11.002
Wen D, Rivera-Perez C, Abdou M, Jia Q, He Q, Liu X, Zyaan O, Xu J, Bendena WG, Tobe SS, Noriega FG, Palli SR, Wang J, Li S (2015) Methyl farnesoate plays a dual role in regulating Drosophila metamorphosis. PLoS Genet 11(3). https://doi.org/10.1371/journal.pgen.1005038
Zapata V, Greco LSL, Medesani D, Rodriguez EM (2003) Ovarian growth in the crab Chasmagnathus granulata induced by hormones and neuroregulators throughout the year. In vivo and in vitro studies. Aquaculture 224(1–4):339–352. https://doi.org/10.1016/s0044-8486(03)00226-6
Zeng B, Huang Y, Xu J, Shiotsuki T, Bai H, Palli SR, Huang Y, Tan A (2017) The FOXO transcription factor controls insect growth and development by regulating juvenile hormone degradation in the silkworm, Bombyx mori. J Biol Chem 292(28):11659–11669. https://doi.org/10.1074/jbc.M117.777797
Zhang H, Tian L, Tobe S, Xiong Y, Wang S, Lin X, Liu Y, Bendena W, Li S, Zhang YQ (2010) DrosophilaCG10527 mutants are resistant to juvenile hormone and its analog methoprene. Biochem Biophys Res Commun 401(2):182–187. https://doi.org/10.1016/j.bbrc.2010.09.019
Zhou A, Zhang C, Gong D, Xiao W, Chen Y, Zou B, Xiao J (2017) Breeding of sex-limited marking trimolter silkworm new materials and preparation of hybrid combination. Sci Seric 43(6):1045–1049
Funding
This work was supported by the Basic research and frontier exploration projects of Chongqing [grant numbers cstc2018jcyjAX0075]; and the Subsidy fund for the development of National Silk in Chongqing [grant number CQ2018JSCE05].
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Ping Chen. Performed the experiments: Liang Zhang, Tian Li, Yan Li, Xiu-Zhi Li, Rong Xiong, Dong-Sheng Yan. Wrote the manuscript: Ping Chen.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing or financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 141 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Li, X., Li, T. et al. Farnesoic acid methyltransferase 6 (BmFAMeT6) interrelates with moltinism of dominant trimolter in silkworm, Bombyx mori. Biologia 76, 2231–2240 (2021). https://doi.org/10.1007/s11756-021-00707-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00707-y