Abstract
Salt-affected soils cannot meet the needs of engineering projects due to their deficiency in providing desirable geotechnical properties. Cement stabilization is widely used to improve the engineering properties of salt-affected soils, but cement has many backward effects, especially on the environment, limiting its application as a binder. This study evaluates the potential effects of salt on protein-based biopolymer treated sand. The influence of salt content, biopolymer content, and curing time on the strength and stiffness development of salt-affected sand was explored with unconfined compressive strength (UCS) testing. The UCS results showed that an increase in casein biopolymer content led to an increase in the unconfined compressive strength and stiffness; however, the addition of salt had a reverse effect on UCS results. By adding 2% casein solution, the compressive strength reached 1021.34 kPa, which is significantly greater than that of untreated soil with a value close to zero. When the salt content rose from 0.5 to 10% (for 2% casein content), a substantial strength loss (more than 48%) was observed in the UCS value from 978 to 501 kPa. This might be due to the salt existence in soil which adversely affected the biopolymer connections by blocking the bonds and bridges with soil particles. This adverse effect was gradually mitigated by the biopolymer increment until adding 3.5% sodium caseinate, then a higher percentage of the biopolymer was involved in further enhancement of compressive strength. Microscopic observation revealed that sodium caseinate acted as a binding agent between soil particles, while salt disrupted the sodium caseinate performance. To evaluate the physical properties of the sandy soil, permeability and wind tunnel tests were conducted. The inclusion of sodium caseinate as a protein-based biopolymer resulted in lowering the hydraulic conductivity and increasing the erosion resistance of salt-affected sand. Curing time had positive effects on strength development, increasing the erosion resistance, and reducing the permeability. Overall, sodium caseinate could adequately improve the engineering properties of salt-affected sand.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to unrestrained emission of greenhouse gases, climate change has caused severe environmental problems such as desertification. Annually, 75 billion tons of fertile soil are degraded, costing around USD 42 billion (Middleton and Kang 2017). 44% of the total amount of soil degradation is accounted to wind erosion equal to around 5.05 × 106 km2 of the earth’s land, which is a crucial environmental issue against the advancement of the agricultural and livestock industries (Jiang et al. 2019). Wind erosion causes serious respiratory diseases, dust storms, disrupting commercial activities and transport, and deposits undesirable nutrients and salts (Middleton and Kang 2017).
Although cement is the most consumed binder in civil construction, because of its practicality, high strength, and economic cost (Jahandari et al. 2019; Miraki et al. 2021; Mohammadifar et al. 2022), there is a growing concern about its harmful impacts on the environment (Fatehi et al. 2018; Ghadir and Ranjbar 2018; Jahandari et al. 2021). In two ways, cement production causes the emission of carbon dioxide; the first way is related to the manufacturing process of clinker, and the second is about burning fossil fuels for making energy. The above-mentioned sources are responsible for 5–8% of global CO2 emissions (Ghadir et al. 2021; Shariatmadari et al. 2021). Also, cement can increase soil pH in a negative way, restrain plant growth, and restructure groundwater quality (Chang et al. 2016; Smitha and Rangaswamy 2020). Thus, the demand is rising for a new soil stabilizer to be compatible with the environment.
Salt-affected soils are a widespread problem across the world by encompassing about 952.2 million ha globally, especially in arid and semi-arid regions (Cherlet et al. 2015). From the geotechnical engineering point of view, saline soils pose major problems, such as differential settlement, low compressive strength, and low shear strength (Al-Amoudi et al. 1995; Horpibulsuk et al. 2012). The salt content of more than 3 wt.% was found to affect treated soil stability and slightly influence the maximum dry density (Li et al. 2016). An investigation by Xing et al. (2009) demonstrated that Cl− has a damaging effect on the strength of cement stabilized soil in both short and long terms (Xing et al. 2009). It has been indicated that a higher concentration of salt has an adverse effect on the elasticity modulus and compressive strength (Dingwen et al. 2013). The negative influence of organic matter on the strength of lime- and cement-treated soil could be decreased by the presence of salt in the soil (Jiang and Ontisuka 2004).
In the past decade, biological materials and methods such as microbial and enzyme induced calcite precipitations, biogass generation, bacterial biostimulation, as well as biopolymers have gained ever-increasing attention in geotechnical applications (Bahmani et al. 2019; Hosseinpour et al. 2021; Ramdas et al. 2020).
Biopolymers are degradable types of polymeric materials that are naturally formed in the environment (Chen et al. 2015; Plank 2005; Shariatmadari et al. 2020). Biopolymers have vast applications in food, medical, cosmetic, and constructive sectors (Fatehi et al. 2021; Schwark 2009). Using biopolymers in engineering dates back to ancient times, but with the advent of lignosulfonate in the 1920s, a new era of biopolymers was started in engineering (Fatehi et al. 2019; Hataf et al. 2018; Plank 2005). Several biopolymers have been examined for soil improvement purposes. Cellulose (from the plant's group), with 1.5 trillion tons of generation per year, is the most plentiful organic polymer and has several prospects for soil reinforcement because of its gelation features (Maher and Ho 1994; Sivakumar Babu and Vasudevan 2008). Furthermore, Xanthan gum has been applied to enhance soil stiffness, compressive strength, shear resistance, and altering dispersion characteristics of soil. Xanthan has shown to increase the compressive strength of soils to greater than 500% (Bonal et al. 2020; Fatehi et al. 2021; Latifi et al. 2016, 2017; Soldo and Miletić 2019). The incorporation of Beta-glucan and Xanthan gum into the silty soil improved erosion resistance to less than 1% (Chang et al. 2015). The hydraulic erosion also was improved to higher than 80% by using 0.5% xanthan gum and making a jelly layer on sand surface, which was more productive than employing 10% kaolinite clay. Also, the efficiency of other biopolymers, such as guar gum, chitosan, and sodium alginate, has been shown to improve the mechanical properties of soils (Arab et al. 2019; Dehghan et al. 2019; Khatami and O’Kelly 2013).
Protein-based biopolymers are known as natural polymers produced from dairy products. Globally, about 1 kg of 5 kg milk is spoiled and mostly disposed to landfills, negatively affecting the environment (Chang et al. 2018). Protein-based biopolymers, including casein and sodium caseinate, were utilized for strengthening sandy and silty soils. In this line, considerable growth was observed in the development of shear and compressive strengths. Higher than 600 kPa of compressive strength and 120 kPa undrained shear strength was obtained by employing only 1% of casein (Fatehi et al. 2018). Despite most of the polysaccharides, casein is not soluble in the water, and higher compressive strength under wet conditions was withstood by the casein-treated samples (Chang et al. 2018; Fatehi et al. 2018).
Although some studies have been conducted to evaluate the feasibility of using biopolymers in geotechnical engineering, the potential effect of salt on biopolymer-stabilized soil has not still been investigated. Therefore, the goal of this research is to study how NaCl can affect the geotechnical and physical characteristics of sandy soil. In this line, a series of laboratory experiments were conducted to evaluate the engineering performance of sodium caseinate-treated salty-affected sandy soil.
2 Materials and Methods
2.1 Soil Properties
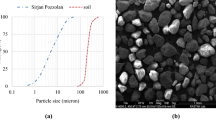
The soil sample was obtained from the casting industry in Firoozkooh district, northeast of Tehran, Iran. The soil is known as “Firoozkooh sand (No.161)” in the country because of its wide applications in industries. The sand has a specific gravity of 2.66, and it is classified as poorly graded sand (SP) based on the Unified Soil Classification System (USCS) (“ASTM D2487-17e1,” ASTM 2017). The grain size distribution curve of sand is shown in Fig. 1. The optimum moisture content and maximum dry density of the sand were obtained at 12.5 wt.% and 1.62 g/cm3, respectively, obtained from the modified proctor compaction method based on ASTM D1557 (“ASTM D1557-12e1,” ASTM 2012). Table 1 illustrates the physical properties of sand. The chemical composition of sand was determined by X-ray fluorescence (XRF) analysis, Table 2.
2.2 Sodium Caseinate Synthesis from the Bovine Milk
Casein constitutes approximately 80% of the total nitrogen in bovine milk (Huppertz et al. 2018). Casein usage is not limited to dairy products, and it has a variety of applications in the plastics, glues, and paper glazing industries (Huppertz et al. 2018). Casein has four main constituents that form casein micelles, with a diameter ranging from 50 to 300 nm (Holt et al. 2013). Among constituents, k-casein has a determinant role in many properties of the particles, especially their stability against aggregation (Dalgleish 1998; Holt et al. 2013). The method of obtaining casein from milk by isoelectric precipitation was developed by Huppertz et al. (2018). Acidification is the basis of the conversion of milk into curd and whey (De Kruif 1999). The casein used in this study was extracted from bovine milk through stages of precipitation, dewheying, washing, and drying, Fig. 2a (Mulvihill and Ennis 2003). Skim milk was preferred to achieve a better quality of casein; therefore, milk fat should be as low as possible (Mulvihill and Ennis 2003). Casein itself is not a suitable paste for making a homogenous mixture (Fatehi et al. 2018). Thus, 2% sodium hydroxide was added as a prevalent alkaline solution to form a pasty glue named sodium caseinate, Fig. 2b (Fatehi et al. 2018). Sodium caseinate has some distinct features. Unlike casein, sodium caseinate is water-soluble (Mulvihill and Ennis 2003). This study used sodium caseinate (casein solution) for soil treatment. Figure 2a and b show the synthesized casein and casein solution used in this study.
3 Soil Stabilization and Characterization Tests
3.1 Sample Preparation and Mechanical Characterization
In this study, the preparation of the soil-salt mixture was based on the International Standard ISO 11268 ("ISO 11268," ISO 1993). In the first step of sample preparation, the salt was dissolved with contents of 0.5, 2.5, 5, and 10 wt.% dry weight of sand in distilled water. NaCl was the dominant constituent of the salt, Table 3. In the second step, the soil was mixed with the solution. To ensure a homogenous soil-chemical compound mixture, each sample was stirred meticulously for about five minutes. In the third step, samples were kept in a sealed container at 20 °C for 15 days.
To evaluate the effects of sodium caseinate on the properties of salt-affected sand, sodium caseinate powder was dissolved in water in the next step, and subsequently mixed with the salted soil. Various sodium caseinate contents of 2, 3.5, 5, and 6.5 wt.% of the soil were adopted in this study. For preparing the specimens, the mixture was kept in the mold for three days, after which they were demolded. Afterward, the samples were air-dried at room temperature (25 ± 2 °C) and relative humidity of 40 ± 2% and tested after 7, 14 and 28 days.
3.2 Unconfined Compression Strength (UCS) Test
Unconfined uniaxial compression testing was performed following ASTM D2166 ("ASTM D2166-16," ASTM 2016) using a universal testing machine on cylindrical samples with an inner diameter of 37 mm and a height/diameter ratio of 2.02. The axial strain rate was monitored at a rate of 0.5 mm/min. Three samples were prepared and tested for all measurements. To evaluate the curing effect on unconfined compressive strength (UCS), the samples were cured and tested after 7, 14 and 28 days. Secant modulus of elasticity (E50) was used to demonstrate the elastic stiffness of biopolymer-treated salt-affected sand by measuring the slope between the beginning and half of the failure stress. Table 4 summarizes the testing samples for UCS, permeability and wind tunnel tests.
3.3 Permeability Test
Permeability tests were conducted in accordance with ASTM D5084 ("ASTM D5084-16a," ASTM 2016) to determine the hydraulic conductivity of biopolymer-treated soils. Cylindrical samples with a diameter 70 mm * height 140 mm were prepared for the permeability tests. To obtain a B value (Skempton) of 0.95 or greater for considering samples as fully saturated, a back pressure of 240 kPa, under effective stress of 10 kPa was applied and then increased. After this stage, the water was entered into the sample from a tank elevated at a specific elevation to gratify the favorite hydraulic gradient. Time influence was considered on treated soils based on long- and short-term curing (7 and 28 days) to determine the optimum curing conditions (Table 4).
3.4 Wind Tunnel Test
Sand storm, as an outcome of wind erosion, releases sediment particles from the ground surface. Since bare land is most prone to sediment entrainment, these phenomena usually occur in arid and semi-arid areas such as the middle east (Zhou et al. 2020). The wind erosion experiment was carried out in a straight line forcing a wind tunnel with a test section size of 1.5 (length) * 0.8 (width) * 0.8 (height) m, Fig. 3. Steel boxes were used for this experiment (20 * 15 * 5 cm). For the fabrication of samples, a 4 cm layer of soil was placed on a tray, and a 1 cm layer of biopolymer-treated soil was placed as the upper layer. Salt and soil were mixed with biopolymer and then compacted on the tray. The samples were exposed to wind velocities of 50, 100, and 150 km/h for 5 min. Samples were placed in the central part of the tunnel that had a metallic hole to allow the installation of the samples. Sample preparation was based on the maximum and minimum strengths of the 28-day cured samples containing salt obtained from the UCS test. Furthermore, to compare the short-term and the long-term curing effects, the sample containing 10% salt and 2% casein solution was also tested after seven curing days (Table 4). Therefore, the effects of biopolymer content, salt content, velocity, and curing time on the erosion resistance of the soil were investigated by a series of wind tunnel tests.
3.5 FT-IR Analysis
Fourier transform infrared spectroscopy (FT-IR) testing was conducted using a Perkin Elmer System series 2000 spectrophotometer in a frequency range of 4000–400 cm−1, a resolution of 4 cm−1, and a scan speed of 0.5 cm/s to recognize the bands of casein solution. Aceton-washing was performed to pause the ongoing reaction in the sample. The potassium bromide (KBr) disc method was used for preparing the samples for FT-IR.
3.6 Microscopic Analysis
Microscopic observation was conducted to assess the interactions of salt and sodium caseinate with soil particles. This analysis provides data about the size, shape, and aggregation of samples. To visualize the inter-particle structure, optical and scanning electron microscopy (SEM) images of untreated sand, salt-affected sand (10% salt), 6.5% sodium caseinate treated sand without salt, and 6.5% sodium caseinate treated soil that contains 10% salt at 28 days of curing were recorded using a Dino-Lite digital microscope and TESCAN VEGA instrument, respectively.
4 Results and Discussion
4.1 FT-IR
FT-IR test was carried out for assessing the validity of synthesized sodium caseinate compared to the FT-IR spectrum of sodium caseinate in the previous studies (Zhao et al. 2018), Fig. 4. O–H stretching vibration mode of sodium caseinate was observed in a wavenumber of 3430 cm−1 (Zhao et al. 2018). Also, asymmetrical and symmetrical vibrations of C–H bonds showed absorption peaks in wavenumber of 2930 cm−1 and 2820 cm−1, respectively. The absorption peak of sodium caseinate in wavenumber of 1680 cm−1 could be related to the protein bands of amide I. Moreover, the stretching vibration of amide II was detected in a wavenumber of 1563 cm−1. Absorption peaks observed in the range of 1400–1500 cm−1 were in accordance with bending vibration of N–H bands in sodium caseinate structure. The absorption peak in the range of 1000–1300 cm−1 was related to the bending vibration of C–H bonds. Besides, a wide peak in wavenumber of fewer than 700 cm−1 was related to aromatic ring in sodium caseinate structure. The results of the FT-IR test verify the accurate synthesize of sodium caseinate in this study.
Comparison of FTIR spectra of sodium caseinate synthesized in this study and sodium caseinate synthesized in previous studies (Zhao et al. 2018)
4.2 Interaction of Sodium Caseinate with Salt-Affected Soil
The optical image was used to grasp the effect of the casein solution on the soil more accurately. As shown in Fig. 5a, depicting the optical image of untreated sand, the particles of sandy soil stand freely without cohesion in their natural states. Figure 5b indicates the compacted untreated salt-affected sand (for 10% salt content) after 28 days. It is evident that particles are in closer proximity in comparison to the intact state. Also, it can be observed from Fig. 5c and d, demonstrating the optical images of 28 days cured sodium caseinate-treated sand (6.5% sodium caseinate without salt) and sodium caseinate treated salt-affected sand (6.5% sodium caseinate and 10% salt), respectively, that casein solution acted as a binder and caused particles to stick together (red circles as shown in Fig. 5c and d).
Among biopolymers, sodium caseinate has at least one connected amino acid containing nonpolar side chains that make protein-based biopolymers to be more resistant to water (Némethy and Scheraga 1962). When casein solution infiltrates the soil, it begins to encompass and makes a smooth cover over soil particles, which results in the formation of inter-particle bonding as well as sodium caseinate-soil conglomerates (Chang et al. 2018; Fatehi et al. 2018). The most influential factors in forming strong bindings between sodium caseinate and soil are the solution concentration, pH, and the type of interactions, such as Van der Waals bonds, hydrogen bonds, electrostatic interactions, and complex bonds between activated protein groups (Chang et al. 2018; Fatehi et al. 2018).
For sodium caseinate concentrations lower than 12%, there is comparatively a low viscous solution with Newtonian behaviour (Chang et al. 2018). But when the solution concentration exceeds 12%, casein solution behaves pseudoplastic, and stronger binding is expected to be formed (Chang et al. 2018). Casein is rich in amine groups, phosphate groups, and carboxylic acid, which can form bonds and bridges between soil particles and the ions through various mechanisms such as polar interaction (because of the hydrolysis of amino acid by the alkaline) and electrostatic interactions. The entry of alkaline into the casein chains leads to the increase in pH, formation of the complex structure of joining sodium to casein phosphate, and generating more charges so that strong bonds are formed.
When a salt-affected soil is a host for casein solution, the biopolymer is not able to act as effective as before due to the presence of salt. The precipitated salt in the soil matrix prevents the biopolymer solutions from infiltrating the soil freely, and a non-uniform biopolymer distribution might occur, as can be seen from Fig. 5d. Also, NaCl causes a reduction in pH of the casein solution, according to (Zhao and Corredig 2015). Furthermore, the addition of salt decreases the total phosphate contents, and the ions exchange reduces the number of available calcium ions, which results in the reduction of electrostatic charges in the caseinate solution structure so that fewer electrostatic and chemical interactions would be formed.
The SEM images were utilized to grasp the effect of the casein solution and salt on soil properties in a better way. Figure 6 shows Scanning Electron Microscope (SEM) images of untreated and casein treated salt-affected sand (6.5% sodium caseinate and 10% salt) at 28 days of curing.). Because of the long polymeric chain in casein biopolymer, covalent bond, van der Waals forces, and hydrogen bonding could exist at the interface of the particle and casein. Casein solution interaction with saline soil particles has several phases. When casein reacts with sodium hydroxide, sodium caseinate is produced. Casein solution forms a sol (type of colloids). The sol coats soil’s grains and provides more contact surfaces for soil particles (red circles as shown in Fig. 6b–d). After being spread on the surface, sol drenches the surfaces and adheres to particles. When water pours out of soil, solid protein remains, causing particles to cling to each other. As a matter of fact, polar interaction (due to hydrolysis of amino acid chains by sodium hydroxide) and hydrogen bonds (between particles and casein) are two major contributors to the saline soil improvement. It is worthy to be noted that after the treatment there is no obvious trace of salt particles in SEM images. Authors believe that the mentioned occurrence could be related to casein solution. When casein is added to saline soil, it might dissolve the salt. As salt content increases, it disrupts the casein solution performance and efficiency which results in a weaker glue-type agent.
4.3 Unconfined Compressive Strength and Secant Modulus of Elasticity
After treatment, the UCS of samples was evaluated in terms of salt content, biopolymer content, and curing time. In Fig. 7, the UCS of the sodium caseinate treated specimens cured at 7, 14 and 28 days are compared. As it can be seen from Fig. 7a, the incorporation of the casein solution increased the UCS of the soil samples regardless of the salt content; as the biopolymer content increased, considerable growth in UCS values was observed. By adding 2% casein solution, the compressive strength reached 1021.34 kPa, which is significantly greater than that of untreated soil with a value close to zero. Casein sticks the unbounded sand particles together through a process of coating and making bridges, so that most of the applied shear force is undergone by casein polymeric chains. Also, chemical interactions between the charged surfaces of finer particles and casein has a contribution to the increment of UCS strength (Chang et al. 2018; Fatehi et al. 2018).
When the salt was added to the biopolymer-soil mixture, the UCS strength decreased. This is due to the reason that the presence of salt reduced the attraction of soil particles to form a bond with casein solution gel. For instance, when the salt content rose from 0.5 to 10% (for 2% casein content), a substantial strength loss (more than 48%) was observed in the UCS value from 978 to 501 kPa, Fig. 7b. Figure 7c shows that by adding 6.5% casein solution, the compressive strength of the soil reached 2139.54 kPa, which was the highest strength achieved in UCS tests in the present study. In the case of constant salt and variable sodium caseinate content, the growth speed in UCS was much higher from 3.5 to 5% in comparison to lower amounts. This difference might be due to the salt existence in soil which adversely affected the biopolymer connections by blocking the bonds and bridges with soil particles. This adverse effect was gradually mitigated by the biopolymer increment until adding 3.5% sodium caseinate, then a higher percentage of the biopolymer was involved in further enhancement of compressive strength. But from 5 to 6.5%, the biopolymer content became less effective and reached the optimal content of effective biopolymer. The typical strength progression with curing time for the sample containing 0.5% salt and 2% biopolymer is shown in Fig. 8. According to Fig. 8, it is obvious that curing time had a positive effect on sample strength development. The compressive strength of the biopolymer treated soil is highly dependent on the moisture content. The reason is that the presence of moisture delays the formation of chemical bonds between biopolymer-biopolymer and biopolymer-soil and stronger biopolymer polymeric chains are formed in dry conditions. On the other hand, as poorly graded sand has negligible compressive strength, most of the strength in biopolymer treated sand is obtained from biopolymer bonding. Over time, the dehydration process leads to reduction in the moisture content and a higher compressive strength is expected to obtain. It is noteworthy that a considerable growth of compressive strength in samples was achieved on the 14th day of curing (96%), which indicates that before the 28th day of curing, most of the treatment process had elapsed. Figure 9 shows a typical failure of soil specimens in UCS tests.
Figure 10 illustrates the secant modulus of elasticity (E50) of samples before and after treatment on the 7th day of curing. As shown, a remarkable increase was achieved in the stiffness of the salt-affected sand after biopolymer treatment By comparing Fig. 10a and b, it can be observed that casein solution content had a positive effect on increasing the stiffness, although salt content acted in the reverse order. Overall, treatment by a higher content of biopolymer brought about a change in ductility and enhanced brittleness. This increase in stiffness is because casein molecules are placed among sand and salt grains and limit their interactions. The binding capacity of the added biopolymer overcomes the negative impact of the existing salt and increases the stiffness of the mixture by keeping the solid grains together (Chang et al. 2018; Varzi et al. 2016). Figure 11 shows the stress–strain curve of sodium caseinate treated salt-affected soil samples on the 7th day of curing. Table 5 summarizes the mixture of soil samples in Fig. 11.
4.4 Permeability
The effects of salt content and casein solution concentration on the permeability coefficient are shown in Fig. 12. Figure 12a demonstrates that the permeability coefficient was reduced as salt content increased. This is due to the fact that salt could fill the pores of the soil, although the reduction was not remarkable. It can be seen that adding the casein solution reduced the permeability, Fig. 12b. For instance, the permeability coefficient of soil containing 0.5% salt and 6.5% sodium caseinate on the 7th day of curing was 1.70 × 10−3 cm/s, which was significantly less than that of untreated salt-affected sand (with 0.5% salt) with the magnitude of 8.8 × 10−3 cm/s, Fig. 12a and b. The reduction in permeability is because the casein solution absorbs water and slows down water transport throughout the soil matrix with its water retention capability. Sodium caseinate biopolymer tends to absorb water because of its hydrophilic property and carrying negative charges. So, water and biopolymer molecules interact through different mechanisms leading to hydrogen bonding between hydroxide and hydrogen. Also, the absorbed water by dried biopolymer increases the film volume existing in the soil mass pores which results in the reduction in coefficient of permeability. The results are in good agreement with previous studies that emphasized the clogging effects of viscous biopolymer hydrogels (Cabalar et al. 2017; Ivanov and Chu 2008). Furthermore, results showed that more salt and casein solution content (6.5% sodium caseinate and 10% salt) in soil did not lead to a further reduction in permeability. The accumulation of salt particles prevented the casein solution from acting as an effective binder. Moreover, a longer curing time generally achieved a lower permeability, as indicated in Fig. 12b. As an instance, it can be seen that the coefficient of permeability of 2% casein solution-mixed salt-affected soil (with 10% salt) reduced from 3.1 × 10−3 cm/s at 7 days of curing to 11.8 × 10−4 cm/s at 28 days of curing.
4.5 Wind tunnel
Figure 13 represents the results of wind erosion of samples at different velocities. Two different Y-axes were used in this figure as it was not possible to indicate soil mass loss before and after treatment in one axis due to the significant differences in their values. As seen, the salt-affected sample experienced a dramatic soil mass loss in the wind tunnel test at different velocities, but a significant reduction was observed in the soil mass loss by stabilization with casein solution. Although higher biopolymer concentration led to a decline in mass loss, 2% and 6.5% sodium caseinate content did not show a considerable difference in resistance against erosion. Thus, a small portion of casein solution content is sufficient to prevent a salt storm, which is more hazardous than a dust storm. Besides, in 7 days of curing, an acceptable performance against surface erosion was demonstrated by the treatment. Also, as expected, 28 days of curing had less soil mass loss than 7 days as biopolymer reached its maximum productivity by losing almost all the moisture. Soil mass loss in the sample of 2% casein solution-mixed salt-affected soil (with 10% salt and velocity of 150 km/h) was reduced from 0.84% for 7 days curing time to 0.26% after 28 days. In fact, added casein solution increased the soil’s inter-particle strength. In other words, after drying, the soil surface formed a homogenous layer that was almost tough, without any cracks.
5 Conclusions
In the current study, the physical and geotechnical properties of salt-affected soil stabilized with biopolymer (sodium caseinate) were evaluated by a series of laboratory explorations. The following conclusions can be obtained from the results of the tests.
-
Optical images were used to visualize the effects of salt and sodium caseinate on the inter-particle structures of the soil. Results showed that casein solution was spread on saline soil particles and formed strong bonding, which caused interlocking between salt-affected soil particles.
-
The results of the unconfined compression strength showed that substantial development of strength was achieved by the inclusion of sodium caseinate biopolymer in the salt-affected sand. An increase in sodium caseinate content led to an increase in the compressive strength of salt-affected sand, although when salt content increased, the UCS of salt-affected sand decreased. As time passed, casein solution-treated soil demonstrated a further increase in the UCS. The stiffness of the samples was also increased considerably after treatment by casein solution to a level of at least 6 times higher compared to the untreated samples.
-
A significant decrease in permeability was observed by adding the casein solution into the salt-affected soil regardless of the salt content. This could be attributed to the hydrophilic essence of the casein solution aided to slow down water transport by absorbing it. Permeability of the casein solution treated sand reduced by increasing the curing period, which indicates that longer curing time caused a further reduction in permeability because of the growth of the bonds.
-
The wind tunnel test results indicated that the salt-affected sand experienced a significant soil mass loss at different velocities, but the inclusion of 2% casein solution was enough to form a well-structured resistant layer on the soil surface that can withstand high wind velocity. Experiments also revealed that samples in the short-term curing demonstrated a considerable resistance against erosion.
Overall, the casein solution can be suitably used as an alternative to cement to stabilize salt-affected soils due to their environmentally-friendly traits. However, further studies in diverse conditions need to be performed to fundamentally evaluate the role of sodium caseinate in geotechnical engineering applications.
Data availability
Enquiries about data availability should be directed to the authors.
References
Al-Amoudi OSB, Asi IM, EI-Naggar ZR (1995) Stabilization of an arid, saline sabkha soil using additives. Q J Eng Geol Hydrogeol 28(4):369–379
Arab MG, Mousa R, Gabr A, Azam A, El-Badawy S, Hassan A (2019) Resilient behavior of sodium alginate-treated cohesive soils for pavement applications. J Mater Civ Eng 31(1):04018361
ASTM D1557-12e1 (ASTM 2012). In standard test methods for laboratory compaction characteristics of soil using modified effort (56,000 ft-lbf/ft3 (2700 kN-m/m3)). ASTM International, West Conshohocken, PA
ASTM D2166-16 (ASTM 2016) In standard test method for unconfined compressive strength of cohesive soil. ASTM International, West Conshohocken, PA
ASTM D5084–16a (ASTM 2016) In standard test methods for measurement of hydraulic conductivity of saturated porous materials using a flexible wall permeameter. ASTM International, West Conshohocken, PA
ASTM D2487-17e1 (ASTM 2017) In standard practice for classification of soils for engineering purposes (Unified Soil Classification System). ASTM International, West Conshohocken, PA
Bahmani M, Fatehi H, Noorzad A, Hamedi J (2019) Biological soil improvement using new environmental bacteria isolated from northern Iran. Environ Geotechn 1–13
Bonal N, Prasad A, Verma A (2020) Use of biopolymers to enhance the geotechnical properties of coal mine overburden waste. Géotech Lett 10(2):179–185
Cabalar A, Wiszniewski M, Skutnik Z (2017) Effects of xanthan gum biopolymer on the permeability, odometer, unconfined compressive and triaxial shear behavior of a sand. Soil Mech Found Eng 54(5):356–361
Chang I, Im J, Cho G-C (2016) Introduction of microbial biopolymers in soil treatment for future environmentally-friendly and sustainable geotechnical engineering. Sustainability 8(3):251
Chang I, Im J, Chung M-K, Cho G-C (2018) Bovine casein as a new soil strengthening binder from diary wastes. Constr Build Mater 160:1–9
Chang I, Im J, Prasidhi AK, Cho G-C (2015) Effects of Xanthan gum biopolymer on soil strengthening. Constr Build Mater 74:65–72
Chen R, Lee I, Zhang L (2015) Biopolymer stabilization of mine tailings for dust control. J Geotech Geoenviron Eng 141(2):04014100
Cherlet M, Reynolds J, Hutchinson C, Hill J, von Maltitz G, Sommer S, Fensholt R, Horion S, Shepherd G, Weynants M (2015) World atlas of desertification mapping land degradation and sustainable land management opportunities-introductory brochure
Dalgleish D (1998) Casein micelles as colloids: surface structures and stabilities. J Dairy Sci 81(11):3013–3018
De Kruif C (1999) Casein micelle interactions. Int Dairy J 9(3–6):183–188
Dehghan H, Tabarsa A, Latifi N, Bagheri Y (2019) Use of xanthan and guar gums in soil strengthening. Clean Technol Environ Policy 21(1):155–165
Dingwen Z, Libin F, Songyu L, Yongfeng D (2013) Experimental investigation of unconfined compression strength and stiffness of cement treated salt-rich clay. Mar Georesour Geotechnol 31(4):360–374
Fatehi H, Abtahi SM, Hashemolhosseini H, Hejazi SM (2018) A novel study on using protein based biopolymers in soil strengthening. Constr Build Mater 167:813–821
Fatehi H, Bahmani M, Noorzad A (2019) Strengthening of dune sand with sodium alginate biopolymer. In: Geo-congress 2019: soil improvement
Fatehi H, Ong DE, Yu J, Chang I (2021) Biopolymers as green binders for soil improvement in geotechnical applications: a review. Geosciences 11(7):291
Ghadir P, Ranjbar N (2018) Clayey soil stabilization using geopolymer and Portland cement. Constr Build Mater 188:361–371
Ghadir P, Zamanian M, Mahbubi-Motlagh N, Saberian M, Li J, Ranjbar N (2021) Shear strength and life cycle assessment of volcanic ash-based geopolymer and cement stabilized soil: a comparative study. Transp Geotech 31:100639
Hataf N, Ghadir P, Ranjbar N (2018) Investigation of soil stabilization using chitosan biopolymer. J Clean Prod 170:1493–1500
Holt C, Carver J, Ecroyd H, Thorn D (2013) Invited review: caseins and the casein micelle: their biological functions, structures, and behavior in foods. J Dairy Sci 96(10):6127–6146
Horpibulsuk S, Suddeepong A, Chinkulkijniwat A, Liu MD (2012) Strength and compressibility of lightweight cemented clays. Appl Clay Sci 69:11–21
Hosseinpour Z, Najafpour-Darzi G, Latifi N, Morowvat M, Manahiloh KN (2021) Synthesis of a biopolymer via a novel strain of Pantoea as a soil stabilizer. Transp Geotech 26:100425
Huppertz T, Fox P, Kelly A (2018) The caseins: structure, stability, and functionality. In: Proteins in food processing. Elsevier, pp 49–92
ISO 11268 (ISO 1993) In soil quality—effects of pollutants on earthworms (Eisenia fetida)—part 1: determination of acute toxicity using artificial soil substrate
Ivanov V, Chu J (2008) Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev Environ Sci Bio/technol 7(2):139–153
Jahandari S, Saberian M, Zivari F, Li J, Ghasemi M, Vali R (2019) Experimental study of the effects of curing time on geotechnical properties of stabilized clay with lime and geogrid. Int J Geotech Eng 13(2):172–183
Jahandari S, Tao Z, Saberian M, Shariati M, Li J, Abolhasani M, Kazemi M, Rahmani A, Rashidi M (2021) Geotechnical properties of lime-geogrid improved clayey subgrade under various moisture conditions. Road Mater Pavement Des 1–19
Jiang C, Zhang H, Zhang Z, Wang D (2019) Model-based assessment soil loss by wind and water erosion in China’s Loess Plateau: dynamic change, conservation effectiveness, and strategies for sustainable restoration. Glob Planet Change 172:396–413
Jiang CML, Ontisuka K (2004) Influence of humic acid and salt concentration on lime-stabilized ariake clays and microstructure research. Chin J Geotech Eng 27(2):281–286
Khatami HR, O’Kelly BC (2013) Improving mechanical properties of sand using biopolymers. J Geotech Geoenviron Eng 139(8):1402–1406
Latifi N, Horpibulsuk S, Meehan CL, Abd Majid MZ, Rashid ASA (2016) Xanthan gum biopolymer: an eco-friendly additive for stabilization of tropical organic peat. Environ Earth Sci 75(9):825
Latifi N, Horpibulsuk S, Meehan CL, Abd Majid MZ, Tahir MM, Mohamad ET (2017) Improvement of problematic soils with biopolymer—an environmentally friendly soil stabilizer. J Mater Civ Eng 29(2):04016204
Li M, Chai S, Du H, Wang C (2016) Effect of chlorine salt on the physical and mechanical properties of inshore saline soil treated with lime. Soils Found 56(3):327–335
Maher M, Ho Y (1994) Mechanical properties of kaolinite/fiber soil composite. J Geotech Eng 120(8):1381–1393
Middleton N, Kang U (2017) Sand and dust storms: Impact mitigation. Sustainability 9(6):1053
Miraki H, Shariatmadari N, Ghadir P, Jahandari S, Tao Z, Siddique R (2021) Clayey soil stabilization using alkali-activated volcanic ash and slag. J Rock Mech Geotech Eng
Mohammadifar L, Miraki H, Rahmani A, Jahandari S, Mehdizadeh B, Rasekh H, Samadi P, Samali B (2022) Properties of lime-cement concrete containing various amounts of waste tire powder under different ground moisture conditions. Polymers 14(3):482
Mulvihill D, Ennis M (2003) Functional milk proteins: production and utilization. In: Advanced dairy chemistry—1 Proteins. Springer, pp 1175–1228
Némethy G, Scheraga HA (1962) Structure of water and hydrophobic bonding in proteins. I. A model for the thermodynamic properties of liquid water. J Chem Phys 36(12):3382–3400
Plank J (2005) Applications of biopolymers in construction engineering. Biopolym Online Biol Chem Biotechnol Appl 10
Ramdas VM, Mandree P, Mgangira M, Mukaratirwa S, Lalloo R, Ramchuran S (2020) Review of current and future bio-based stabilisation products (enzymatic and polymeric) for road construction materials. Transp Geotech 100458
Schwark F (2009) Influence factors for scenario analysis for new environmental technologies–the case for biopolymer technology. J Clean Prod 17(7):644–652
Shariatmadari N, Hasanzadehshooiili H, Ghadir P, Saeidi F, Moharami F (2021) Compressive strength of sandy soils stabilized with alkali-activated volcanic ash and slag. J Mater Civ Eng 33(11):04021295
Shariatmadari N, Reza M, Tasuji A, Ghadir P, Javadi AA (2020) Experimental study on the effect of chitosan biopolymer on sandy soil stabilization. In: E3S Web of conferences
Sivakumar Babu G, Vasudevan A (2008) Strength and stiffness response of coir fiber-reinforced tropical soil. J Mater Civ Eng 20(9):571–577
Smitha S, Rangaswamy K (2020) Effect of biopolymer treatment on pore pressure response and dynamic properties of silty sand. J Mater Civ Eng 32(8):04020217
Soldo A, Miletić M (2019) Study on shear strength of xanthan gum-amended soil. Sustainability 11(21):6142
Varzi A, Raccichini R, Marinaro M, Wohlfahrt-Mehrens M, Passerini S (2016) Probing the characteristics of casein as green binder for non-aqueous electrochemical double layer capacitors’ electrodes. J Power Sources 326:672–679
Xing H, Yang X, Xu C, Ye G (2009) Strength characteristics and mechanisms of salt-rich soil–cement. Eng Geol 103(1–2):33–38
Zhao T, Liu F, Duan X, Xiao C, Liu X (2018) Physicochemical properties of lutein-loaded microcapsules and their uptake via caco-2 monolayers. Molecules 23(7):1805
Zhao Z, Corredig M (2015) Changes in the physico-chemical properties of casein micelles in the presence of sodium chloride in untreated and concentrated milk protein. Dairy Sci Technol 95(1):87–99
Zhou Z, Zhang Z, Zou X, Zhang K, Zhang W (2020) Quantifying wind erosion at landscape scale in a temperate grassland: Nonignorable influence of topography. Geomorphology 370:107401
Acknowledgements
The authors would like to acknowledge the National Elites Foundation of Iran. Also, we would like to appreciate the efforts of Dr Afshin Asadi. This study was supported by Chem Concrete Pty Ltd (No. 120/3F1397) and MatSoil Company (No. 01A/2021).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nouri, H., Ghadir, P., Fatehi, H. et al. Effects of Protein-Based Biopolymer on Geotechnical Properties of Salt-Affected Sandy Soil. Geotech Geol Eng 40, 5739–5753 (2022). https://doi.org/10.1007/s10706-022-02245-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-022-02245-z