Abstract
Clean drinking water is a necessity and recycling stormwater and greywater has been more appealing in the recent decades to provide filtered water for drinking water and non-drinking water purposes. Biosand filters have been a popular filtering system of filtering potable water in developing countries, due to their simplicity in construction, reliability in operation and availability of constituent materials. The sand filtration system consists of diffuser rock, two sizes of gravel, and sand, which the container is constructed from concrete or plastic. Sand filtration system tend to struggle in removing specific chemicals such as nitrate and phosphate. Therefore, innovative materials can be added to the sand filtration system to increase the chances of chemical removal. Innovative materials such as biochar and bentonite clay were used to analyze their effectiveness in sand filtration system experiments. Biochar and bentonite clay have unique characteristics such as absorbing capacity and activated carbon, which remove suspended solids, chemicals, and toxins from contaminated water. Constant head permeability tests were used to simulate the sand filtration system in the lab. 15 tests were completed with different filter media arrangements, which incorporated two types of sand (coarse and fine), two sizes of gravel (small and large), biochar, and sodium bentonite clay. Contaminated water was created by simulating greywater by adding chemicals to reverse osmosis water. The pH, sulfate, chloride, phosphate and nitrate ion concentration removal were analyzed based on the contaminated water and filtered water. The hydraulic conductivity, filtration rate and pressure loss were also analyzed for each test. The tests that had the most effective result in terms of chemical ion removal, pH neutralization and filtration rate was the arrangement that included large and small gravel, fine and coarse sand, and biochar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biosand filtration systems are widely used to convert dirty contaminated water into clean drinking water. This filtration system is known for removing contaminants such as chemicals, toxin, total suspended solids, and bacteria. However, disinfection methods must be completed afterwards to ensure the highest water quality (CAWST 2009). Some example of these disinfection methods includes boiling and ultraviolet disinfection. Sand filtration systems can also convert contaminated water into non-drinking purposed water. For example, greywater from household showers and dishwashers could be filtered without disinfection and the filtered water could be used in the heating/cooling system of a household. The sand filtration system operations are usually simple, mostly gravity-driven; however, pumps can be used if necessary (Elliot et al. 2008). The sand filters are usually housed in concrete or plastic. The contents in the filter are a diffuser plate, sand, and fine and course gravel (Kennedy et al. 2012). The diffuser plate is a metal plate with slits to allow the water to flow evenly into the system and ensures that the biofilm is undisturbed. Diffuser rock was used as a diffuser plate in the sand filtration system. The sand removes total suspended solids and chemicals (such as iron) from the water (Bradley et al. 2011). The gravel is used to ensure the filtration process runs smoothly without any clogging issues. A very important characteristic that the sand filtration system must have to achieve the highest quality of filtration is the formation of the biofilm layer, which is also known as the schmutzdecke. The biofilm layer is made up of bacteria, fungi, larvae and other organic matter. This layer can take up to 2–3 weeks to form (Kennedy et al. 2012), usually on top of the sand layer. This layer traps bacteria, viruses, and suspended solids by reducing the pore sizes of the sand (Elliot et al. 2011). This in turn reduces the chances of the bacteria and solids from entering the filter, which removes the contaminants from the filtered water. However, over time, the pore size of the sand will become very small and reduce the filtration rate drastically and could ultimately clog the system (Lynn et al. 2013). Therefore, maintenance must be required to regularly clean the systems and regenerate the biofilm layer. Innovative materials such as biochar and bentonite clay could be added to the sand filtration systems, to improve performance with respect to the effectiveness to remove chemicals, toxins, and suspended solid from the contaminated water. Bentonite clay has a very low hydraulic conductivity, swelling characteristics and self-sealing capacity, which makes it ideal to be a barrier (Sarkar et al. 2017). Additionally, small portions of bentonite clay can be added to the sand filtration system to absorb chemicals (such as nitrate, phosphate, and chloride) and block suspended solids from entering the filtered water (Karakaya et al. 2011). Biochar has been used as a filter media for urban storm runoff. Reddy et al. (2014) completed an experiment to analyze the effectiveness of removing contaminants from storm water. It was determined that the pH change was not significant, however the removals of total suspended solids, nitrate and lead was significant, which increased by up to 86 percent. Therefore, bentonite clay and biochar are important materials to be considered in a sand filtration system.
The goal of this paper was to analyze the effectiveness of using a selection of filter media that are implemented in the sand filtration system and ultimately choose the appropriate filter media layer arrangement, based on certain characteristics. These materials were analyzed based on the change in chloride, sulfate, nitrate and phosphate ion concentration and pH of the contaminated and filtered water, and hydraulic conductivity of the filter media. The contaminated water that were used in these experiments were decided to be simulated greywater which was completed by adding specific chemicals to reverse osmosis (RO) water. The materials that were decided to be used for this experiment were two types of sand (coarse and fine), two sizes of gravel (large and small), biochar, and sodium bentonite clay.
2 Materials and Methods
The filter media that are generally used in a sand filtration system are sand, two sizes of gravel and diffuser rock. The sand is primarily used as the filtering medium to remove suspended solids and chemicals. However, the gravel is used to prevent clogging in the system. The filter media that were proposed to be used in this experiment to analyze the effectiveness of sand filtration systems were sand, gravel, biochar, and bentonite clay. The biochar and bentonite clay have distinctive properties (high absorbing capacity and low hydraulic conductivity) that can make them valuable additions to the system. The filter media used in experimenting are presented in Fig. 1.
2.1 Sand
Two types of sand were used to analyze the change in filtration based on the sand size and shape. The particle size distribution was determined for the two types of sand by performing a sieve analysis following the ASTM C136-14 (2014) standard. A plot of cumulative percent passing versus particle size was created. From this plot, the effective size (D10) and uniformity coefficient (Cu) were determined. The D10 is the diameter from the particle size distribution curve at 10% finer. The coarse sand had an D10 of 0.13 mm and Cu of 5 while the fine sand had an D10 of 0.19 mm and Cu of 3.16. Based on research, for sand filtration systems the D10 should be 0.15–0.20 mm and Cu should be 1.5–2.5 (Elliot et al. 2008). These recommended values have a direct relationship to the desired flowrate of the sand filtration system which is 0.5–1.1 L/min. Exceeding the desired flowrate can create craters in the filter media and reduce the efficiency of the filter. Therefore, the D10 values are within the range with some tolerances. However, the Cu values are outside the range which must be considered a factor if inadequate filtration rate occurs. The specific gravity of both sands was determined following the ASTM C128-15 (2015) standard. The specific gravity of the coarse sand was determined to be 2.68. The specific gravity of the fine sand was determined to be 2.8. Based on the conditions for well graded sand, both sands were poorly graded.
2.2 Gravel
Two sizes of gravel are generally added to the biosand filtration system to prevent clogging in the outlet tube of the filter and provides structural support of the sand layer. The specific gravity of the gravel was determined following the ASTM C127-15 (2015). The larger gravel had a size of 10 mm with a specific gravity of 2.63 while the smaller gravel had a size of 4.5 mm with a specific gravity of 2.56.
2.3 Biochar
Biochar is a charcoal that is created from plant matter through pyrolysis (combustion in the absence of oxidizing agents to remove various volatile impurities) and is used in a range of applications, including ground sequestration to remove carbon dioxide from the atmosphere, use in fertilizers, among others. Biochar attracts water due to it large surface area and structure. This results in achieving nutrients for plants and reducing fertilizers flowing into groundwater (Mohan et al. 2014). Biochar is known to have the ability to hold chemicals, toxins, and bacteria from water (Reddy et al. 2014). Activated biochar was used in the experiments to reduce the importation of materials. The particle size of biochar used in testing ranged from 0.25 to 2 mm. The specific gravity of the biochar used was determined to be 0.7 following ASTM C128-15 (2015) standards.
2.4 Bentonite Clay
Bentonite clay is primarily used for construction as a barrier to stop contaminated water from seeping into aquifers. However, small portions of bentonite clay were added to the sand filtration system to analyze the effectiveness of removing chemicals, heavy metals and toxins from water. The portion added was a small portion (5% in weight) to reduce the chance of clogging the system due to bentonite clay having a very low hydraulic conductivity. Sodium bentonite (Wyoming bentonite) was used in experimenting. The composition of the bentonite is about 70% montmorillonite content with illite and quartz (Sarkar et al. 2017). The average particle size ranges from 15 to 20 μm and the specific gravity of 1.74. The pH of the sodium bentonite is also more alkaline compared to calcium bentonite which is more neutral (Karakaya et al. 2011). The contaminated water is generally acidic; therefore, the sodium bentonite will ideally neutralize the pH of the water more significantly.
2.5 Synthetic Greywater
The contaminated water was made by using chemicals to simulate the properties of greywater. The greywater from showers and washing machines were the designated source and comparability for the synthetic greywater. There were a low concentration (trial) greywater and synthetic greywater (high concentration) which the chemicals are listed in Table 1 and Abed and Scholz (2016) respectively. The chemical that were added were based on research and the availability of chemicals in the lab that was provided (Pidou et al. 2008; Meehan and Maxey 2009). A trial batch of synthetic greywater was produced for the daily individual and layered sample tests. The trial batch of synthetic greywater was used for these tests because it was important to analyze the hydraulic conductivity, pH, and chemical composition without concerning about biofilm formation. This combination of chemicals was determined to be the most efficient in terms of water quality and time to produce the trial greywater. The high concentration synthetic greywater was used in the biofilm tests to achieve the highest chances of producing a biofilm layer on the sand and biochar. The chemicals were added to 18 L of RO water. The cellulose and humic acid sodium salt provided the organic matter. The presence of iron, zinc and copper provided a source of metals for the synthetic greywater. The secondary effluent was lake water from the Okanagan Lake in Kelowna, B.C. collected from Gyro Beach area. The lake water provided microorganisms and pathogens to the synthetic water which is a necessity for biofilm creation in a sand filtration system. A water sample from Gyro Beach was collected by a Castanet analysist and the sample was analyzed by Caro Analytical Lab in Kelowna. The microorganisms present in the lake water can be found in Kelly (2018). The reading limits are the minimum units that are used when measuring the microorganism content in the lake water. The reading limit for the total coliforms and E. coli are 1 CFU/100 mL. Therefore, the minimum microorganism value the reading device can detect is 1 CFU/100 mL. The results for the total coliforms and E. coli are less than 1 CFU/100 mL (Kelly 2018). Therefore, there was no detection of coliforms or E. coli in the lake water sample. The presence of these microorganism is very minimal or non-present in the lake water which provides an inadequate source of microorganisms for the synthetic greywater recipes.
2.6 Experimental Set-up and Methodology
The biosand filtration system was simulated in a lab using the constant head permeability testing apparatus and procedure based on ASTM D2434 (2006) standards. The constant head permeability test uses a utility pump to provide water to a constant level tank which provides a constant flow to a permeameter cell (ASTM 2006). The permeameter cell is where different filter media sample arrangements are molded. There are inlet and outlet tanks which store the contaminated and filtered water, respectively. A manometer is connected to two different ports on the permeameter cell which is used to determine the head change (pressure loss) throughout the layers. Valves are used to control the flow and remove air bubbles from the manometer. The apparatus set-up drawing can be seen in Fig. 2.
There were two replicates of fifteen tests that were completed. The average of the two replicates results were studied. Each test had a different arrangement in terms of the filter media layers. These tests arrangements were chosen based on previous research and through consultation with industry contacts. There were individual tests which only included one filter medium. This was completed for small and large gravel, coarse and fine sand, and biochar. There were tests that had more than one filter medium layer added. These tests included different arrangements of small and large gravel, coarse and fine sand, biochar, and bentonite clay. There were tests that a small portion (5% of weight) of bentonite clay were added to large and small gravel, and fine and coarse sand. Flushing 18 L of synthetic greywater was completed for each test. Finally, there was a biofilm test completed. This entailed flushing 18 L of synthetic greywater through the system daily for 30–50 days to analyze the creation of the biofilm layer above the sand and its effectiveness to remove chemicals, bacteria and viruses. The filter media arrangement for the biofilm test was determined based on which of the fifteen tests performed most successfully. There was a pause period between daily discharges of greywater. The pause period allowed for required oxygen to be provided to the sand layer to produce a biofilm layer. The pause period was for 20–24 h. During the pause period, a 5 cm standing layer of synthetic greywater above the sand layer was required to prevent drying of the filter media. (CAWST 2009). The full list of tests can be seen in Table 2. The table displays the layer thickness and oven-dried mass of the filter media for each test. Tests 10, 11, 12, and 13 have small portions of sodium bentonite clay added to the filter media at a measurement of 5 percent of the filter media oven-dried mass. The thickness values shown in the table are the filter media thicknesses after compaction is completed. The compaction process was completed by placing the oven-dried filter media into the permeameter cell and compacting the filter media with a compaction rod which weighed approximately 50 g. The compacting included 10 handheld gravity blows to the filter media throughout the diameter of the permeameter cell. Compaction was completed to ensure the filter media would resemble accurately in a real-world sand filtration system. The only filter medium that was not compacted for testing was the bentonite clay. The bentonite clay has a very low hydraulic conductivity compared to the other filter media which further compaction would result in higher chance of the system clogging. The height of the permeameter cell is 23 cm.
The procedure for the constant head permeability test that was followed was based on the ASTM D2434 (2006) code. However, there were some modifications made, due to having a slightly different set-up and to ensure that the system runs smoothly without any air bubbles.
Prior to running the test, the following steps were taken:
-
Measure and record the inner diameter and length between the manometer ports of the permeameter cell.
-
Determine which filter media samples will be used for testing.
-
Thoroughly wash the filter media samples to ensure they are saturated and clean.
-
Weigh each filter media sample in the steel bowls. Pour the desired amount into the permeameter cell and add a steel screen mesh between samples. Complete compaction process. Measure the thickness of each layer. Weigh the remaining sample in the steel bowls.
-
After all the samples are added to the permeameter cell, secure the cell by placing the steel plate on it with the provided fasteners.
-
Add the chemicals into the water storage tank to make the water contaminated.
-
Pour 18 L of the contaminated water in the inlet tank.
-
Complete the pH and IC testing to determine the results of the contaminated water.
During operation of the testing apparatus, the following steps were taken:
-
Plug-in and turn on the utility pump using an extension cord. Ensure all valves are open besides the outlet valve from the permeameter cell.
-
Once the permeameter cell has filled with water, close the inlet valve to permeameter cell. Open the air-release valve on top of the permeameter cell to release the air build-up inside and reduce the air bubbles in the manometer lines.
-
Once the water level in the constant level tank is at the level where the overflow line is releasing water back into the inlet tank, open the inlet and outlet valves of the permeameter cell very slowly. This will lower the chance of having air bubbles in the manometer.
-
When the heads of the manometer remain constant, record the two head values.
-
Using the stopwatch and 200 mL graduated cylinder, record the time it takes for the outlet line of the permeameter cell to fill the 200 mL graduated cylinder.
-
After all the contaminated water filters through the permeameter cell and into the outlet tank, the pH and IC testing are completed for the filtered water and compared to the results of the contaminated water.
The pressure loss and filtration rate are determined based on the results directly given by the constant head permeability test. The pressure loss is the change in head from the two manometer readings. The filtration rate is determined by dividing the 200 mL by the time it takes to fill the 200-mL graduated cylinder. The hydraulic conductivity is calculated using the Eq. 1 (ASTM 2006). Q (in cm3) is the 200-mL volume, L (in cm) is the distance between the manometer ports on the permeameter cell, A (in cm2) is the cross-sectional area of the filter media layers, h (in cm) is the change in head from the manometer, t (in seconds) is the time to fill the 200-mL graduated cylinder, and k (in cm/s) is the hydraulic conductivity. Q, A, and L remained constant for each test which were 200 cm3, 101.59 cm2, and 11.5 cm, respectively. The h and t are the changing parameters in the hydraulic conductivity calculation.
The pH testing is completed before and after the testing is done. A pH probe is used to determine the pH of the contaminated water and filtered water. The results are compared and analysed. The pH is required to be around neutral (7) or alkaline (above 7) to be classified as a success. Acidic (less than 7) is not ideal in filtering water that will be used for drinking or other non-drinking purposes (for example, heating or cooling system). A pH that is too alkaline however can have a high hardness which is not ideal for drinking water. For heating and cooling systems, alkaline pH between 8 and 9 are recommended (Pidou, et al. 2008). Therefore, the ideal cases for the biosand filtration system is to have pH values between 7 and 9.
Ion Chromatography (IC) testing was completed to determine the concentration of the contaminated water and filtered water. IC testing is the most common application used to determining ion concentrations of drinking water (Haddad et al. 2000). The process includes samples being passed through an ion-exchange column (Michalski 2006; Jackson 2001). The ions that are separated in the column and eluents transfer into the suppressor. The suppressor reduces the conductivity of the eluent and increase the conductivity of the sample ion which produces a detection signal. A graph is generated for the detection signal versus time. Based on ion standards that are ran through the system, the ion compound is determined based on the analysis time comparison. The ion concentrations are calculated based on a linear best-fit line equation from the area under the detection signal curve and time of the ion standards. Popular eluents include HCl, H2SO4, and HNO3 with organic modifications (Michalski 2006).
The ion chromatography was generated using software, Chromeleon, for the 15 tests and additional biofilm test. Blank (Type 1 water) were used in between the 15 mL samples to balance and effectively achieve the results. The chromatographs that were generated had two peaks in the graphs. Standards were required to be created to determine which chemical ions were present and represented the peaks. Sodium (Na+), calcium (Ca2+), magnesium (Mg2+), and any ions in kaolin could not be analyzed using the IC test software. Therefore, for the trial (low concentration) greywater, chloride (Cl−) and sulfate (SO42−) standards were the only standards created. The standards were created by adding different portions of sodium chloride (NaCl) and magnesium sulfate (MgSO4) based on the molecular mass to provide four parts per millions (ppm) values. These ppm values were determined based on the concentrations added for each test. The ppm values that were chosen were 10, 50, 200, 500. The IC test was completed for the standards and tests were generated for the ppm versus area under the peak. The time of the peak compared to the test results concluded that the first peak was chloride and the second peak was sulfate. An equation of a line was generated for the chloride and sulfate standard graphs which were the equations used to determine the ppm for the chloride and sulfate for each test. The equations to determine the chloride and sulfate concentrations (in mg/L) can be seen below (Eqs. 2, 3) where Ac is the area under the chromatograph peaks.
Using the synthetic (high concentration) greywater solution, more chemicals standards needed to be considered when running the IC test. The ions that were detected and used as standards at 10, 50, 200, and 500 ppm concentrations were chloride (Cl−), sulfate (SO42−), phosphate (PO43−), and nitrate (NO32−). The IC test was completed for the standards and tests were generated for the ppm versus area under the peak. The time of the peak compared to the test results concluded that the first peak was chloride, second peak was nitrate, third peak was sulfate, and the final peak was phosphate. An equation of a line was generated for the ion standard graphs which were the equations used to determine the ppm for the ions for each test. The equations to determine the ion concentrations (in mg/L) can be seen below (Eqs. 4–7) where Ac is the area under the chromatograph peaks.
Scanning Electron Microscopy (SEM) analysis was completed to determine if a biofilm layer formed on the sand and biochar at a microscopic level. The biofilms on the biosand filtration system are not visible for the eye (CAWST 2009). Therefore, the microscope is needed to physically see the biofilm form on the sand and biochar. The SEM testing is completed after a sample is collected from the testing apparatus every week. The sample (sand and biochar) is then cell fixed to ensure the biofilm remains intact until the SEM testing is completed. The cell fixation procedure includes adding a 1.25% glutaraldehyde solution to the sample for 1 h and then removal before drying in ethanol solutions (30, 50, 95%) at 20-min intervals. The sample was then dried for 12 h before SEM imaging. The SEM imaging involves de-magnetizing using a platinum coating on the sample and placing it in a microscopy arrangement. Electrons deflect off the sample and the beam produce the images. The biofilm layer should be seen at the 10 μm-scale. The chemical composition can also be determined for the sand and biochar.
3 Results and Discussion
The individual sample testing was completed to analyze the hydraulic conductivity of each material and the effect the individual sample has on the contaminated filtration process. The layer sample testing was completed to determine which layering sequence provides the most efficient filtration in terms of hydraulic conductivity, filtration rate, pressure loss, pH, and chemical composition change.
3.1 Hydraulic Conductivity and Filtration Rate
The filtration rate, pressure loss (change in head), and hydraulic conductivity for the 15 tests can be seen in Table 3. Based on the results, once sand or bentonite clay are added to the layer arrangement, the hydraulic conductivity and flowrate decreased. Sand and bentonite clay are known for having a lower hydraulic conductivity compared to gravel and biochar. The pressure loss is also a lot higher for the tests involving sand. Tests 12 and 13 are not included in the table, due to clogging occurring in the system, which would provide a value of zero for the results. Based on the biosand filtration system manual (CAWST 2009), The recommended filtration rate range is between 0.5 and 1.1 L/minute. Based on the results from the experiment, the filtration rate ranges between 0.06 to 4.5 L/minute. As expected, the filtration rate of the experiment varies significantly depending on the materials used and compaction applicability and often exceeds the design recommendations for general sand filtration systems, which is not ideal. For long term use, this can create craters and separation of the filter media which could ultimately affect the filtration of the contaminated water. The tests with the sand and biochar filter media provide the most adequate filtration rate excluding the tests with bentonite.
3.2 pH Analysis
The pH results for the 15 tests can be seen in Table 4. Based on the results, all the pH results for the filtered water tend to increase towards the neutral/basic range which is ideal. Tests 3, 8, and 9 are the best tests in terms of getting the pH of the treated water to the neutral range. Tests 5, 10 and 13 are best tests in terms of getting the pH of the treated water between 8 and 9 which is recommended for heating and cooling systems. Tests with bentonite clay and biochar tend to increase the pH into the 8 and 9 range while the sand-biochar-gravel arrangement tend to increase the pH into the neutral range. There was no pH for the filtered water for the test with coarse sand and 5% bentonite clay due to the system clogging which resulted in having no discharge to run the pH test.
3.3 Chemical Composition of the Water
Based on the 15 tests and additional biofilm test chromatographs, the concentrations of sulfate (Table 5) and chloride (Table 6) can be seen. The concentrations tend not to decrease significantly. This could be due to the high filtration rates, which makes it, so the water is not in the filter medium for a long enough time to remove more chemicals. The bentonite clay tests increase in concentration of sulfate and chloride, which is not acceptable. The elementary analysis from the bentonite clay used has percentages of chloride and sulfate ions which is a reasoning for the increase in concentrations of these ions passing through this filter medium. These test filter media arrangements will not be used in the sand filtration system. Based on the results, tests 8 and 9 (large gravel, small gravel, coarse/fine sand, and biochar) removed the most of sulfate concentrations (maximum of 20% removal rate) while remaining almost constant chloride concentration (maximum of 4% removal rate). Individual test, such as fine sand and biochar, performed similarly in removing both sulfate and chloride. However, adding only one material to the sand filtration system is impractical, due to the durability and issues the system may have without gravel. For example, the system may clog and not run smoothly without large and small gravel. Therefore, tests 8 and 9 filter media layer arrangements should be considered for the sand filtration system. Test 12 had no results for the filtered water, due to the bentonite acting as a barrier and clogging the system, which resulted in having no discharge filtered water to test. Based on the World Health Organization for drinking water, there is no health-based guideline limit for chloride and sulfate. However, it is recommended to have chloride and sulfate concentrations less than 250 mg/l due to it effecting the taste (WHO 2017). Therefore, the chlorine and sulfate ion concentrations meet the requirements of the World Health Organization.
Based on the hydraulic conductivity, filtration rate, pressure loss, pH and chemical concentration results, tests 8 and 9 are most effective layer arrangements. Test 8 consists of large gravel, small gravel, coarse sand and biochar while Test 9 consists of large gravel, small gravel, fine sand, and biochar. Therefore, these arrangements must be considered for being used in the sand filtration system and will be further examined in the biofilm testing.
3.4 Biofilm Testing
The biofilm testing is essential to analyze the changing of the filtering characteristics throughout the sand filtration system lifetime. Based on the layered sample tests discussed in the previous section, the tests that should be included for the biofilm test are tests 8 and 9 (large gravel, small, gravel, coarse or fine sand, and biochar). The biofilm testing was completed based on two scenarios, which are using the trial water (sodium chloride, calcium chloride, magnesium sulfate and kaolin) and using the synthetic greywater solution. This was completed to analyze the experimental parameters based on different synthetic greywater solutions (low concentration and high concentration). The hydraulic conductivity, chemical composition change, pH, microscopic imaging and biofilm formation were analyzed for these two synthetic greywater solutions.
The hydraulic conductivity for the trial (low concentration) biofilm test can be seen in Table 7 over the 30-day period. The hydraulic conductivity is fluctuating slightly throughout the 30 days. However, the hydraulic conductivity stays roughly around the same area in terms of values. If the biofilm forms, the hydraulic conductivity of the system should decrease significantly. Therefore, this is the first sign that the biofilm layer has not formed over the 30-day period.
The hydraulic conductivity for the synthetic greywater biofilm test can be seen in Table 8 over the 50-day period. The hydraulic conductivity decreases significantly throughout the 50 days. For example, the hydraulic conductivity decreases 94 percent comparing Day 1–50. If the biofilm forms, the hydraulic conductivity of the system should decrease significantly. Therefore, it is possible that a biofilm has formed based on the hydraulic conductivity values. However, there is also possible that suspended solid in the contaminated water filled the voids in the system to lower the hydraulic conductivity.
The chemical concentration of the chloride and sulfate ions for the trial (low concentration) biofilm test can be seen in Tables 9 and 10 over the 30-day period intervals. The chemical concentration for the chloride and sulfate ions mostly increased after filtration slightly (up to 5%). This is not a significant increase in chloride and sulfate. However, this does show that the duration of time does not increase the chemical removal efficiency of chloride and sulfate. The biofilm formation should increase the chemical removal efficiency due to trapping contaminates from entering the filter. Therefore, this is another critical sign that the biofilm formation did not occur over the 30-day period. The sulfate and chloride meet the less than 250 mg/l requirement of the World Health Organization (WHO 2017).
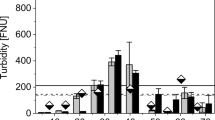
The chemical concentration changes of the ions for the synthetic greywater (high concentration) biofilm test can be seen in Table 11 over the 50-day period intervals. Based on the results, the samples collected from Day 1 had significant decrease in chemical ion concentrations after the water flowed through the system. The phosphate had a 100 percent removal rate while chloride had an 86.34 percent removal rate and sulfate and nitrate had removal rates below 50 percent. Samples collected after Day 1 had significantly lower chemical ion removal rates. For example, the sulfate concentration rates become very minimal (1–4%) compared to the Day 1 removal rate of 44.85 percent. Figure 3 shows the filtered concentration of the ions throughout the 50-day testing period. The first day filtered concentrations are significantly lower than the rest of the sample results. After Day 1, the filtered concentrations stayed reasonably constant. Therefore, the sand filtration system that was tested has very impressive short-term performance however it falters when given a long-term duration. The chloride and sulfate concentration meet the less than 250 mg/l requirement from the World Health Organization for drinking water. The requirement for nitrate is to be less than 50 mg/l which is met and there is no requirement for phosphate (WHO 2017). Therefore, all the chemical ions measured in the synthetic greywater meet the requirement of the World Health Organization.
The pH of the trial (low concentration) and synthetic (high concentration) greywater were recorded before and after the water passed through the system. The pH was analyzed for the biofilm tests to determine whether time duration of testing affects the pH in terms of acid or alkaline content. For the treated water, the preferred results would be neutral or being on the alkaline spectrum. A high acid content would result in poor treated water that should not be used for any treated water applications. Tables 12 and 13 show the before and after filtration pH values for the trial and synthetic greywaters, respectively. As seen from both tables, the pH of the contaminated water starts in the acidic range and then is filtered which increase the pH towards the neutral and basic range. The pH of the filtered water is acceptable to be used as recycled water for irrigation and other non-drinking purposes. In terms of long-term use (30–50 days), the pH change does not improve and remains comparable to initial pH changes. Therefore, the biofilm tests effluent waters pH remains reasonably constant (pH of 6–7).
3.5 SEM Imaging
The microscopic imaging was completed to analyze the bacteria (biofilm layering) content that formed on the sand and biochar throughout the testing process. Samples were collected from the top layer of the sand initially, after Day 1, and every week until Day 50. On the final day, two separate samples were collected from different layers of the sand as well as a biochar sample. After the samples were collected, a cell fixation process was completed to ensure the possible biofilm stayed intact. The samples were then taken for SEM analysis. Based on the images produced, there were signs that a biofilm (or bacteria) had formed on the sand or biochar samples that were collected. There was presence of carbon, nitrogen, and phosphorus contents in the filter media. As seen in Fig. 4, the SEM images of a sand sample collected is shown for Day 1 and Day 50 of testing. The nitrogen content increases to 14.1%in the small spot outlined in the figure. The nitrogen increase does not show until Day 50 and the section is very small even at the 10kx magnification. The chemical content presence throughout the 50 days are very randomized. For example, on Day 28 there is nitrogen content while in Day 35 there is no presence of nitrogen. This causes uncertainty in trusting the results of chemical concentrations. Based on the microstructure of the sample, there are bacteria-like shapes. However, they are very small at the 10kx magnification. The cell fixation of the sample based on literature could create conflicts in analyzing the biofilm formation. The cell fixation process could disrupt or distort the samples biofilm (Joubert et al. 2008). Usually the biofilm is made up of 97% of water (Sutherland 2001). The dehydration of the sample is known to affect the quality and structural integrity of the biofilm sample. Therefore, there was success (although randomized) and uncertainty in forming a biofilm when running the sand filtration system over a 50-day period.
4 Conclusion
Biosand filtration systems are primarily used to convert dirty contaminated water into clean drinking water. The purpose of this paper was to understand the filter media that should be used in biosand filtration systems. Innovative filtering materials that are known to remove chemicals, toxins and bacteria were considered for sand filtration system, such as biochar and bentonite clay to become more successful. The pH, hydraulic conductivity, filtration rate, and chemical composition were analyzed for the different tests that were completed. The filter media arrangement that involved small and large gravel, fine or coarse sand, and biochar had the most effective results and should be used in the sand filtration systems. The addition of biochar to the sand filtration system improved the pH change and decreased the chemical composition of the contaminated water. However, the addition of sodium bentonite clay improved the pH change and increase the chemical composition of the contaminated water. The bentonite clay is ideal to be used as a barrier, however the material may further contaminate the water if used in the sand filtration system. The biofilm tests were successful in reducing the hydraulic conductivity and filtration rate of the system. However, the chemical removal of the biofilm tests was unsuccessful, due to increasing in concentrations of chloride and sulfate ions after the filtering is completed for the trial water. For the synthetic greywater, the short-term chemical removal efficiency was impressive however it faltered with long-term use. All the chemical ion removal met the requirement set out by the World Health Organization for drinking water. The trial (low concentration) test with the test 9 filter media arrangement (large gravel, small gravel, fine sand, and biochar) was unsuccessful to form a biofilm layer on the sand or biochar. This could be a result from not using any microorganisms, organics or inorganic materials in the greywater solution. Although, the synthetic greywater solution (high concentration) test with the test 8 filter media arrangement (large gravel, small gravel, coarse sand, and biochar) was successful in having signs of a biofilm forming on the sand layer in terms of filtration rate and hydraulic conductivity. In conclusion, a biofilm was formed on the sand and biochar layers of the sand filtration system using the synthetic greywater recipe.
Data availability
The data will be available upon email request.
References
Abed SN, Scholz M (2016) Chemical simulation of greywater. Environ Technol 37(13):1631–1646
ASTM International (2006). ASTM D2434–68: Standard test method for permeability of granular soils (constant head). ASTM International, pp. 1–6
ASTM International (2014). ASTM C136–14: Standard test method for sieve analysis of fine and coarse aggregates. ASTM International, pp. 1–5
ASTM International (2015). ASTM C127–15: Standard test method for relative density (specific gravity) and absorption of coarse aggregate. ASTM International, pp. 1–5
ASTM International (2015). ASTM C128–15: Standard method for relative density (specific gravity) and absorption of fine aggregate. ASTM Int pp. 3–6
Bradley I, Straub A, Maraccini P, Markazi S, Nguyen TH (2011) Iron oxide amended biosand filters for virus removal. Water Res 45:4501–4510
CAWST. (2009). Biosand filter manual: Design, construction, installation, operation and maintenance. Calgary: Centre for Affordable Water and Sanitation Technology
Elliot MA, Stauber CE, Koksal F, DiGiano FA, Sobsey MD (2008) Reductions of E. coli, echovirus type 12 and bacteriophages in an intermittently operated household-scale slow sand filter. Water Res 42:2662–2670
Elliot MA, DiGiano FA, Sobsey MD (2011) Virus attenuation by microbial mechanisms during the idle time of a household slow sand filter. Water Res 45:4092–4102
Haddad, P. R., Jackson, P. E. (2000). Ion chromatography: Principles and applications. J Chromatograph Library 46
Jackson PE (2001) Determination of inorganic ions in drinking water by ion chromatography. Trends Anal Chem 20:320–329
Joubert ED, Pillay B (2008) Visualization of the microbial colonization of a slow sand filter using an environmental scanning electron microscope. Electron J Biotechnol 11(2):119
Karakaya MC, Karakaya N, Bakir S (2011) Some properties and potential applications of the Na- and Ca-bentonites of ordu (N.E. Turkey). Appl Clay Sci 54:159–165
Kelly, A. (2018). How clean is the lake? Retrieved from https://www.castanet.net/news/Kelowna/225282/How-clean-is-the-lake
Kennedy TJ, Hernandez EA, Morse AN, Anderson TA (2012) Hydraulic loading rate effect on removal rates in a biosand filter: a pilot study of three conditions. Water Air Soil Pollut 223:4527–4537
Lynn TJ, Wanjugi P, Harwood VJ, Ergas SJ (2013) Dynamic performance of biosand filters. Am Water Works Assoc 105(10):587–595
Meehan B, Maxey A (2009) Potential impacts of using greywater for domestic irrigation. RMIT Enivonmental Science Department
Michalski R (2006) Ion chromatography as a reference method for determination of inorganic ions in water and wasterwater. Crit Rev Anal Chem 36:107–127
Mohan D, Sarswat A, Ok YS, Pittman CU Jr (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent: a critical review. Biores Technol 160:191–202
Pidou M, Avery L, Stephenson T, Jeffrey P, Parsons SA, Liu S, Memon FA, Jefferson B (2008) Chemical solutions for greywater recycling. Chemosphere 71:147–155
Reddy KR, Xie T, Dastgheibi S (2014) Evaluation of biochar as a potential filter media for the removal of mixed contaminants from urban storm water runoff. J Environ Eng 140(12):1–10
Sarkar G, Dey A, Siddiqua S (2017) Preparation of Wyoming bentonite nanoparticles. Environ Geotech. https://doi.org/10.1680/jenge.15.00001
Sutherland IW (2001) The biofilm matrix: an immobilized but dynamic microbial environment. Trends Microbiol 9(5):222–227
WHO. (2017). Guidelines for drinking-water quality. World Health Organization, 4th Edition
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no Conflicts of interest.
Informed consent
All the authors have their consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Emslie, D., Siddiqua, S., Crawford, B. et al. Biofilm Formation and Effectiveness of Biosand Filtration Systems with Typical and Innovative Filter Media. Geotech Geol Eng 40, 1251–1266 (2022). https://doi.org/10.1007/s10706-021-01960-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-021-01960-3