Abstract
Aquatic bacterial pathogens can cause severe economic loss in aquaculture industry. An opportunistic pathogen, Aeromonas hydrophila is responsible for Motile Aeromonas Septicemia, leading to high mortality rates in fish. The present study was focused on the efficacy of Aloe barbadensis replacing fishmeal diets on hematological, serum biochemical, antioxidant, histopathological parameters, and disease resistance against A. hydrophila infection in Labeo rohita. Isonitrogenous fishmeal replaced diets (FMR) were prepared with varying levels of A. barbadensis at D1 (0%) (control), D2 (25%), D3 (50%), D4 (75%) and D5 (100%) then fed to L. rohita. After 60 days of post-feeding, the experimental fish were challenged with A. hydrophila. Blood and organs were collected and examined at 1- and 15-days post infection (dpi). The results demonstrated that on 1 dpi, white blood cells (WBC), total protein, cholesterol and low-density lipoprotein (LDL) levels were significantly increased in D3 diet fed groups. The D2 and D3 diet fed group showed decreasing trends of serum glutamic pyruvic transaminase (SGPT) and antioxidant enzymes activity on 15 dpi. The histopathological architecture results clearly illustrated that the D3 diet fed group had given a higher protective effect by reducing the pathological changes associated with A. hydrophila infection in liver, intestine and muscle. Higher percentage of survival rate was also observed in D3 diet fed group. Therefore, the present study suggested that the dietary administration of A. barbadensis up to 50% fishmeal replacement (D3 diet) can elicit earlier antioxidant activity, innate immune response and improve survival rate in L. rohita against A. hydrophila infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is one of the fastest growing food producing sectors that contributes to global food and nutritional security. Among the global aquatic production, the aquaculture sector reached a record of 49.2% in 2020 (FAO 2022). The global aquaculture production is dominated by freshwater finfish culture due to the species demand, easy to breed, and high tolerance of low level dissolved oxygen (Zhang et al. 2022). India is the second largest country after China in cultivable fish production. India's total production of finfish was 7.1 million tonnes, and inland finfish species account for 6.3 million tonnes (89.2%) (FAO 2020). Indian major carps such as catla, rohu and mrigal fish are of paramount interest. The freshwater fish rohu (Labeo rohita) is the most popular among carp species, accounting for about 35% of total carp production in the last decade and it is the most economically affordable, nutritious, and delicious fish with high market value in India and neighbouring countries (Devi et al. 2019 Sridhar et al. 2021b). The major drawback of the aquaculture industry is disease outbreaks, which affect productivity. Fishes are susceptible to pathogenic or non-pathogenic diseases. Non-pathogenic diseases are related to poor water quality, malnutrition, etc., and are not contagious. On the other hand, pathogenic diseases are more threatening because they are contagious and cause huge mortalities (Nasr-Eldahan et al. 2021).
Infectious diseases are caused by pathogens such as opportunistic bacteria (Haldar et al. 2010; Manchanayake et al. 2023), viruses (Pan et al. 2023), fungi (Dananjaya et al. 2017), and parasites (Zeng et al. 2023). Significant losses in aquaculture are attributed to outbreaks of bacterial diseases including vibriosis caused by Vibrio anguillarum (Kapetanović et al. 2022), redmouth disease caused by Yersinia ruckeri (Fajardo et al. 2022), enteric septicemia caused by Edwardsiella ictaluri (Yang et al. 2023), and septicemia caused by Aeromonas sp. (Zhao et al. 2023), and other more bacterial species (Chong et al. 2023). Recently, many reported fish diseases are related to Aeromonas species such as A. caviae (Xue et al. 2022), A. veronii (Reda et al. 2022), A. salmonicida (Moreau et al. 2023), A. hydrophila (Raissy et al. 2022), A. sobria (Almarri et al. 2023), and A. bestiarum (Fuentes-Valencia et al. 2022). Aeromonas are rod-shaped, gram-negative bacteria that are facultative anaerobes belong to the family Aeromonadaceae. These bacteria are abundant in nature and have high level of environmental adaptation, including brackish, estuarine and freshwater (Li et al. 2020). They have turned into prominent disease causing agents in fish and leads to 80% of mortality (Abd El Latif et al. 2019; Zhou et al. 2021). Among the species, A. hydrophila has been considered as the most harmful bacteria, causing hemorrhagic septicemia and epizootic ulcerative syndrome in cultured fishes (Marinho-Neto et al. 2019; Wang et al. 2023). The pathogenesis of infection was due to various virulent factors derived from A. hydrophila (El-Barbary 2010). The clinical symptoms include fin/tail rot, abdominal distension, and exophthalmia (Kumar et al. 2022a; Mursalim et al. 2022). It can also cause infections like endocarditis, gastroenteritis, peritonitis, and septicemia in humans (Chan et al. 2015; Chen et al. 2019; Matys et al. 2022).
In an intensive aquaculture farming, antibiotic treatments are essential to minimize bacterial infection related growth impairment and considerable economic losses. Antibiotics are administered to fish as part of their diet and also through baths and injections (Cabello et al. 2016; Nawaz et al. 2022). The constant presence of high levels of antibiotic residues in aquatic environments and sediments have the potential to affect normal flora and plankton in those niches, leading to shifts in microbiota diversity and causing deterioration of water quality (Cabello et al. 2016; Bhat et al. 2022). The longer use of antibiotics enable antimicrobial resistance gene exchange between bacteria (Muziasari et al. 2016; Watts et al. 2017; Okeke et al. 2022). The transfer of antibiotic residue and resistance genes from aquatic species and their habitats to terrestrial animals enhance the prevalence of drug-resistant bacteria (Santos and Ramos 2018). Vaccines could be a reasonable alternative for disease prevention in aquaculture. However, issues with inoculation and pathogen specificity limit their efficacy. Moreover, there are no commercial vaccines available for many bacterial infections (Wu et al. 2013; Ben Hamed et al. 2021; Pollard and Bijker 2021).
Another major obstacle in aquaculture is feed cost, fishmeal is considered as a prime feed ingredient for its high protein content, fatty acid and balanced amino acid profile. Nevertheless, researchers and feed manufacturers are looking for suitable dietary protein sources to replace fish meal because of its soaring price, higher demand and decline in supply (Ayiku et al. 2020). With increased accessibility, fewer hazards to aquatic habitats and lower cost, the use of plant-derived immunostimulants has become more popular in aquaculture over the last few years (Van Hai 2015; Mehrabi et al. 2019). Natural plant-based herbal immunostimulants or supplementation of herbal medicine is one of the alternative approaches of fishmeal and antibiotics due to its richness in various compounds, including phenols, tannins, alkaloids, terpenoids, and polysaccharides (Hoseinifar et al. 2020; Hosseini et al. 2022). In addition, plant based diets have antibacterial properties and also provide considerable growth, antioxidant, and immuno-modulation in fish (Panigrahi et al. 2021). These herbal supplements boost the immune system of cultured fish, making them more resistant to stress and diseases (Kumar et al. 2022b).
Aloe barbadensis belonging to the family of Liliaceae has been recognized as one of the most well-known medicinal plants with various applications (Gupta et al. 2012; Giannakoudakis et al. 2018; Yazarlu et al. 2021). It is a drought-resistant succulent, stemless herb, native to tropical and subtropical countries (Saljooghianpour and Javaran 2013; Hęś et al. 2019). Aloe barbadensis is characterized by its abundance in compounds with biological activity, namely mono-, di- polysaccharides, phenolic compounds, anthraquinones, minerals, water- and lipid- soluble vitamins, organic acids, and lipids (Folliero et al. 2022; Palaniyappan et al. 2023). Our previous study evaluating partial and complete fishmeal replacement by Aloe barbadensis with a concentration of 0% (D1), 25% (D2), 50% (D3), 75% (D4) and 100% (D5) experimental diets on freshwater fish Labeo rohita showed positive effects on growth, hematology, serum biochemistry, digestive and antioxidant enzyme activity (unpublished data). The previous literature suggests that, Aloe have the potential properties to act against aquatic pathogen in various fish species, such as Piaractus mesopotamicus (de Assis and Urbinati 2020), Cyprinus carpio (Khanal et al. 2021), Danio rerio (Mehrabi et al. 2020), Oncorhynchus mykiss (Mehrabi and Firouzbakhsh 2020). In light of this, the present study was aimed to investigate the protective effect of Aloe barbadensis diets replacing fishmeal against A. hydrophila infection in L. rohita fish.
Materials and methods
Experimental diets
Five experimental fishmeal replacement (FMR) diets were prepared to fulfil the nutrient requirement of L. rohita using ingredients such as fishmeal (FM), Aloe barbadensis (Ab), soy bean meal, ground nut oil cake, wheat flour, tapioca flour, corn flour, vitamin and mineral mix. The isonitrogenous diets were formulated according to Kari et al. (2021) to replace fishmeal gradually with the concentrations 0% (D1), 25% (D2), 50% (D3), 75% (D4) and 100% (D5) of A. barbadensis. All the ingredients were powdered, mixed thoroughly, autoclaved and prepared in small pellets. The formulation of experimental diets were given in Table 1. The prepared experimental diets were dried and stored at − 20 °C before being used.
Preparation of A. hydrophila
In order to determine the disease resistance rate of L. rohita after fed with fishmeal replaced A. barbadensis diets, the fish were challenged with A. hydrophila. The pathogenic bacteria A. hydrophila was cultured in Luria Bertani broth for 12 h at 37 °C. The overnight bacterial culture was then centrifuged at 7500 rpm for 10 min in a cooling centrifuge. The supernatant was discarded and the bacterial pellet was resuspended in PBS (Phosphate Buffer saline, pH 7.4). The bacterial suspension was adjusted to 0.5 absorbance or optical density (O.D.) at 456 nm corresponding to 1 × 107 colony forming unit (CFU/mL) using Synergy HT Multimode Reader (Bio Tek Instruments, Inc., Winooski, USA) (Devi et al. 2019).
Experimental design and challenging study
The L. rohita fingerlings were obtained from Guna fish farm, Rapoosal, Pudukkottai, Tamil Nadu, India, and transported to laboratory in aerated polyethylene bags. They were acclimatized to laboratory conditions for 10 days with commercial feed. After acclimation to the experimental conditions, fish (7.22 ± 0.48 g) were randomly distributed into the experimental tanks in five groups with each group in triplicates. Fish were fed with experimental diets (D1, D2, D3, D4 and D5) at a rate of 5% total fish biomass twice a day (06:00 and 18:00 h) for 60 days. The water temperature was maintained at 27 ± 2 °C and pH at 7.1 ± 0.4 for the whole experimental period and the tanks were well aerated. The water was exchanged at a rate of 50% once a day. After 60 days of feeding, challenging study was carried out. Thirty healthy fish from each treatment tank, fed with different experimental diets (D1, D2, D3, D4 and D5) were randomly collected and subjected to artificial bacterial infection. The bacterial infection was induced by injecting 0.2 mL of 1 × 107 CFU/mL of A. hydrophila suspended in PBS intramuscularly (IM) above the caudal fin. The experimental fish from the control group (D1) was IM injected with 0.2 mL of PBS and A. hydrophila considered as negative control (NC) and positive control (PC) respectively. The respective experimental feed was given twice a day to the infected fish during the period of 15 days post infection (dpi). The schematic representation of experimental design was given in Fig. 1. The challenged fishes were monitored frequently for abnormal behaviour and clinical signs. External modifications were assessed, daily mortality was recorded and at the end of the challenging experiment, relative percentage survival (RPS) was calculated by using the following formula (Sattanathan et al. 2023).
Sample collection
After challenging with A. hydrophila, 5 fish were randomly collected from each tank on 1- & 15-dpi and euthanised using benzocaine (Yonar et al. 2019). The blood was collected from the fish branchial arch using 1 mL syringe and stored in heparinized and non-heparinized tubes separately (Carbonara et al. 2019). The heparinized blood samples were used for the analysis of hematological parameters. The non-heparinized blood samples were kept under room temperature for 2 h and centrifuged at 5000 rpm for 10 min; the serum was separated and stored at − 20 °C for serum biochemical studies. Further, the fish were dissected and organs (intestine, muscle and liver) were collected. For histopathological examination, some of the excised organs were stored in 10% formalin. The remaining organs were homogenized separately and stored at -20 °C until used for analysing antioxidant activities.
Hematological parameters
Hematological indices such as white blood cell (WBC), red blood cell (RBC), hemoglobin (HB), mean corpuscular hemoglobin (MCH), packed cell volume (PCV), mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were estimated by using 200 µL of blood sample through Fully Automatic Medonic M51, 5 part-Haematology Analyzer using Medonic M51-D diluent, L1 and L2 lyse reagents.
Serum biochemistry
Serum of 400 µL was used to analyse biochemical indices such as serum glutamic pyruvic transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), alkaline phosphatase (ALP), total proteins, glucose, triglycerides, cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), and creatinine levels by Erba XL 640 fully automated biochemistry analyzer (Erba Trans, India) using Erba’s biochemical kit procedures.
Immunological assay
Lysozyme activity
Lysozyme activity was determined by turbidimetric method as described by Ellis (1990) with slight modifications. Briefly, the bacterial suspension, containing sodium phosphate buffer (0.05 mM, 6.2 pH) and lyophilized Micrococcus lysodeikticus (0.03%), was prepared. Then, 250 μL of bacterial suspension was added to 20 µL of fish serum in a 96 well plate. The absorbance was read at 490 nm using Synergy HT Multimode Reader (Bio Tek Instruments, Inc.). Decrease in the absorbance of 0.001/min was considered as one unit of lysozyme activity.
Immunoglobulin M (IgM) assay
Total IgM activity of serum was measured based on the method of Sharma et al (2010). Briefly, after measuring the protein concentration of serum by Bradford (1976) method, 100 µL of polyethylene glycol (12% w/v) was added to 100 µL of serum and incubated under continuous shaking for 2 h. After incubation, the reaction mixture was centrifuged at 5000 rpm for 10 min. Then, the protein concentration of supernatant was determined. The total IgM level was calculated by subtracting the protein concentration of supernatant from the serum protein concentration and it was expressed as unit mg/mL.
Antioxidant assays
Superoxide dismutase (SOD) activity
The superoxide dismutase activity was determined using the method of Marklund and Marklund, (1974) with minor modifications. In brief, 50µL of the sample was added with 15µL of chloroform. The mixture was mixed well in a shaker for 15 min. The reaction mixture was then centrifuged at 13000 rpm for 15 min at 4ºC. Then, 15 µL supernatant was collected and 2 mL of 0.01 M EDTA buffer was added to it followed by 0.5 mL of pyrogallol. Finally, 1 mL of distilled water was added to the mixture. The absorbance was read at 420 nm using Synergy HT Multimode Reader (Bio Tek Instruments, Inc.). The enzyme activity was expressed in terms of units/mg protein in which one unit corresponds to the amount of enzyme that inhibited the auto-oxidation reaction by 50%.
Catalase (CAT) activity
The catalase activity was determined by the method of Sinha, (1972) with slight modifications. The reaction mixture contained 0.5 mL of hydrogen peroxide, 1 mL of phosphate buffer, 0.4 mL of distilled water, 0.1 mL of diluted homogenate (1:10). This mixture was incubated for 1 min at 37 ºC. Then, 2 mL of potassium dichromate acetic acid was added. The mixture was kept in boiling water bath for 10 min and the colour appeared was read at 570 nm using Synergy HT Multimode Reader (Bio Tek Instruments, Inc.). The activity of catalase was determined from the amount of hydrogen peroxide consumed and expressed as µmoles of hydrogen peroxide (H2O2) consumed/minute/mg protein.
Glutathione peroxidase (GPx) activity
The glutathione peroxidase activity was measured using the standard method described by Rotruck et al., (1973). In brief, the reaction mixture contains 0.5 mL of sodium phosphate buffer (0.3 M, pH 7), 0.1 mL of 10 mM sodium azide, 0.2 mL of 4 mM reduced glutathione, 0.1 mL of 2.5 mM hydrogen peroxide and 0.1 µL tissue homogenate. The reaction mixture was made up to 2 mL with distilled water and incubated at 37 ºC for 3 min. Then, 0.5 mL of 10% trichloroacetic acid (TCA) was added and centrifuged at 13000 rpm for 15 min in a cooling centrifuge. The supernatant was collected and 3 mL of disodium hydrogen phosphate (0.3 mM) was added. Finally, 0.5 mL of DTNB (5,5'-dithiobis-2-nitrobenzoic acid) was added into it. The colour developed was read against the blank at 412 nm in the Synergy HT Multimode Reader (Bio Tek Instruments, Inc.). The activity was expressed in terms of µg of glutathione utilized /minute/mg protein.
Histopathology
Histopathological analysis was carried out according to the method of (Krishnasamy Sekar et al. 2023). In order to prevent the tissue degradation and to maintain the intact structures, the tissues were fixed with 10% formalin initially. After 12 h, the fixatives were removed and washed with distilled water thoroughly to remove the fixative. Dehydration was done with various concentrations of ethanol (50, 70, 90 and 100%) for an hour. Chloroform was used to remove the alcohol residues. After infiltration, the tissue was embedded into the paraffin wax to give an external support while cutting. Then, the sections (5 μm) were made by Leica microtome (Leica Microsystems GmbH, Wetzlar, Germany), and stained with Hematoxylin and Eosin (H & E) solution. Finally, the histological sections were analyzed using microscope (Leica, DM750, Germany).
Statistical analysis
Experiments were performed in triplicates and the results were presented as mean ± standard deviation. The data were analysed by applying two-way ANOVA with Dunnett’s and Sidak’s multiple comparisons test using GraphPad Prism version 8 (GraphPad Software, Inc., San Diego, CA). The data were presented in the form of descriptive statistics through tables and graphs. *, **, ***, and **** indicate P-values of respectively ≤ 0.05, ≤ 0.01, ≤ 0.001 and ≤ 0.0001.
Results and discussion
Hematological parameters
Hematological parameters are an important diagnostic tool for examining the health status of fishes. Periodic hematological analysis provides simple way of evaluating chronic stress, diseases, reproductive dysfunction, and metabolic disorder, by environmental conditions both in the field and captive populations of fish species (Ahmed and Sheikh 2019). The results of hematological indices of the present study were given in Fig. 2. In this study, increased WBC count was observed in D3 diet fed group fish on 1 dpi (Fig. 2A), the count was significantly decreased on 15 dpi. In addition, the D3 diet fed group showed significant variations on both 1 & 15 dpi when comparing with D1. The groups with higher WBC count will fight infection more efficiently than other groups and these results are in line with Harikrishnan et al., (2020) in L. rohita after being fed with 2.5 and 5 g/kg of dried lemon peel diets. No significant variations were observed in MCH and MCV count between D1 and other diet fed groups on both 1 & 15 dpi except RBC which showed significant difference in D4 diet fed group on 1 dpi when comparing to D1 fed group (Fig. 2B, D, F). Nevertheless, significant variations in the count were observed between 1 & 15 dpi in some diet fed groups.
Effect of replacing fishmeal with Aloe barbadensis in Labeo rohita blood parameters on 1 and 15 days post infection (dpi) with Aeromonas hydrophila. (A) White blood cell (WBC); (B) Red blood cell (RBC); (C) Hemoglobin (HB); (D) Mean corpuscular hemoglobin (MCH); (E) Mean corpuscular hemoglobin concentration (MCHC); (F) Mean corpuscular volume (MCV); (G) Packed cell volume (PCV). *, **, ***, **** indicates significant difference to positive control (D1) at ≤ 0.05, ≤ 0.01, ≤ 0.001 and ≤ 0.0001 respectively. NC, negative control; D1-0% A. barbadensis (positive control); D2-25% A. barbadensis; D3-50% A. barbadensis; D4-75% A. barbadensis; D5-100% A. barbadensis replacing fishmeal; ns, non-significant
D3 diet fed fish showed significantly higher hemoglobin content on 1 dpi among all experimental diet fed groups compared to D1 (Fig. 2C). Increased level of hemoglobin suggests that iron consisting protein level was also increased. Hemoglobin prohibits anemia and favour for respiration (Das et al. 2022). On 15 dpi, no significant reduction was observed in all experimental groups except D5 diet fed group. It might be due to the swelling of cells (Vignesh et al. 2022) and our results were in line with that of Rathore et al., (2021) and Talpur et al., (2014). No significant variations were observed in MCHC except D4 & D5 diet fed groups which showed significantly decreased level compared to D1 on 15 dpi (Fig. 2E). The present study results were supported by the findings of Mbokane and Moyo, (2018), MCV represents the volume of the red blood corpuscle. On 1 & 15 dpi, the MCV values were comparatively similar in all groups and no significant variations were observed except D1 (Fig. 2F), whereas, a significant decrease in values were noted in D1 group on 15 dpi when compared to 1 dpi. The declining level of MCV may be imputed to the erythrocyte shrinking, which results microcytic anemia. Shrinking in erythrocytes occur due to insufficient synthesis of hemoglobin (Kumar et al. 2013). Similarly, no significant variations were observed in the levels of PCV except D3 diet fed group, which showed significantly increased value on 1 dpi comparing to D1 and other experimental diet fed groups (Fig. 2G).
Serum biochemistry
Serum biochemical study is also a crucial component in disease diagnosis. It is one of the ancillary diagnostic methods, many pathological abnormalities are reflected in serum well before clinical illnesses. Serum biochemical studies will give an insight information of internal environment of animals including fish (Ahmed and Sheikh 2019). Fish have a complex combination of serum proteins which are involved in a wide range of physiological functions in both healthy and diseased state and have an important role in understanding physiological parameters of fish (Fazio et al. 2013).
Serum glutamic pyruvic transaminase (SGPT) and serum glutamate oxaloacetate transaminase (SGOT) enzymes are found in hepatocyte cytosol. Due to the linkage of SGPT with liver parenchymal cells (Veerabadhran et al. 2023), it is highly present in liver and to a lesser amount in skeletal muscle and kidney (Saha and Bandyopadhyay 2020). The SGPT is a specific indicator of liver inflammation and damage (Nair et al. 2021). On the other hand, SGOT is commonly found in liver, skeletal muscle and heart. It also indicates the status of liver health. In this study, the concentration of SGPT is higher in liver. The optimum range of SGPT level in fish is usually between 5 and 45 U/L (Kumar et al. 2013). The current study results showed a significant increase in all the experimental diet fed fish groups on 1 dpi (Fig. 3A). The increasing level of these enzymes probably due to the liver damage caused by the pathogenic bacteria or oxidative stress (Hore et al. 2023; Kumar et al. 2022a). A significant SGPT decline was observed on 15 dpi in all the experimental diet fed groups when compared to 1 dpi. It indicates that the SGPT enzyme level increased during early stages of infection and reduced gradually. Moreover, significantly decreased values were observed in D2 & D3 diet fed group when compared to D1. Especially, the D3 diet fed group showed lower SGPT and SGOT enzymes level among the experimental diet fed groups. Significant differences in the SGOT enzyme levels were observed in D3 & D5 groups between 1 & 15 dpi. These values suggest that the diet D3 fed fish showed less infection than other diet fed groups (Fig. 3B). The decreasing trend of SGPT and SGOT was also documented in azadirachtin administered goldfish (Carassius auratus) against A. hydrophila (Kumar et al. 2013). In contrast, Haque et al., (2021) reported significantly higher SGPT and SGOT activity in Anabas testudineus (climbing perch) infected with Pseudomonas aeruginosa.
(A) Serum glutamic pyruvic transaminase (SGPT); (B) Serum glutamate oxaloacetate transaminase (SGOT); (C) Alkaline phosphatase (ALP) activity of Labeo rohita fed with Aloe barbadensis on 1 and 15-days post infection (dpi) with Aeromonas hydrophila. *, **, ***, **** indicates significant difference to positive control (D1) at ≤ 0.05, ≤ 0.01, ≤ 0.001 and ≤ 0.0001 respectively. NC, negative control; D1-0% A. barbadensis (positive control); D2-25% A. barbadensis; D3-50% A. barbadensis; D4-75% A. barbadensis; D5-100% A. barbadensis replacing fishmeal; ns, non-significant
The alkaline phosphatase (ALP) is a multifunctional enzyme that can serve as transphosphorylase in the alkaline pH and plays a major role in membrane transport and skeletal mineralization in aquatic animals (Bernet et al. 2001; Rahimikia 2017). ALP is one of the lysosomal enzymes, acts as an excellent antibacterial agent and protects fish during infection (Sridhar et al. 2022). Increased production of alkaline phosphatase activity is associated with the elevating enzyme production by macrophage cells (Zhu and Su 2022). In this study, between 1 & 15 dpi, no significant changes in ALP level were observed in all the experimental diet fed groups (Fig. 3C). The higher level of ALP enzyme was detected in D1, D4 and D5 diet fed groups. It shows that the ALP enzyme was produced in higher level in these particular groups to evade the severity of A. hydrophila infection. In contrast, D2 and D3 diet fed groups showed ALP enzyme levels similar to the negative control (NC) and significantly reduced when compared to D1. It suggests that the level of A. hydrophila infection was controlled in these groups. The similar increased trend was observed by Liang et al. 2020, at 48 h post infection in Onchidium reevesii against Vibrio harveyi. Gobi et al. 2018 also reported an increased level of ALP in dietary supplementation of probiotic Bacillus licheniformis Dahb1in tilapia (Oreochromis mossambicus) challenged with A. hydrophila.
One of the important components in serum is proteins. It consists of globulins and albumins (Tan et al. 2017). In fish, stronger innate immunity response is based on the level of serum/plasma total protein (Zhou et al. 2015). The increase in the level of serum total protein indicates enhanced antibody development, and several humoral constituents related to innate immune response (Schieber and Chandel 2014). In this study, the diets D2 and D3 fed fish showed higher protein level than the other experimental diet fed groups (Fig. 4A). It indicates that the A. barbadensis supplementation in these groups elevated the protein level and escalated the innate immune response in experimental fish. Medicinal herbs can directly strengthen the fish immunity by generating antibodies with response to the pathogenic organisms (Li et al. 2022). Reduced level of protein was observed by Vignesh et al., (2022) on Channa striata after infecting with A. hydrophila.
(A) Total protein; (B) Glucose; (C) Triglycerides level of Labeo rohita fed with Aloe barbadensis on 1 and 15-days post infection (dpi) with Aeromonas hydrophila. *, **, ***, **** indicates significant difference to positive control (D1) at ≤ 0.05, ≤ 0.01, ≤ 0.001 and ≤ 0.0001 respectively. NC, negative control; D1-0% A. barbadensis (positive control); D2-25% A. barbadensis; D3-50% A. barbadensis; D4-75% A. barbadensis; D5-100% A. barbadensis replacing fishmeal; ns, non-significant
Blood glucose level is a major factor to identify the second level stress conditions in fish (Ahmed and Sheikh 2019). Glucose acts as an indicator of hypoxic and environmental stress (Malini et al. 2018). The increased level of blood glucose indicates escalated levels of stress/infection (Citarasu et al. 2006). According to Patriche 2009, 40–90 mg/dL is the normal level of fish blood glucose. In our study there was a significant decrease in the level of serum glucose in D2 and D3 diet fed groups when compared to D1 (Fig. 4B) on 1 dpi. It might be the reason of slow pathogenesis caused by A. hydrophila due to the immunoprotective effect of A. barbadensis in these diet groups. Talpur and Ikhwanuddin 2013 stated that the blood glucose level was increased in the V. harveyi infected fish Asian seabass (Lates calcarifer) fed with 2 g/kg of neem leaf supplemented diet.
Glucose can also be formed from glycerol in triglycerides (Wen et al. 2021). Consequently, elevated levels of blood glucose likely due to the increase of triglycerides. The present results demonstrated the declined levels of glucose and triglycerides in the D2 and D3 diet fed groups and higher level of triglycerides were observed in D1 group, which acts as positive control (Fig. 4C). Our results were in agreement with the reports of Banihashemi et al. 2022 indicating increased levels of blood glucose and triglycerides in the Yersinia ruckeri exposed fishes.
Cholesterol is a lipid that is mainly produced by liver. The increased levels of cholesterol in serum is utilized by fish to assuage the excess energy demand during stress conditions. On 1 dpi, the cholesterol levels were increased in D1 diet fed group when compared to the other groups. Increasing cholesterol level was reported in Cyprinus carpio under stressful conditions (Abdel-Tawwab et al. 2022). During 15 dpi, the cholesterol levels were increased in all groups except D1 and D3 diet fed groups when compared to 1 dpi but it was vice versa in other experimental diet fed groups. The values were statistically significant comparing to D1 except 15 dpi on D5 group which showed no significant alterations. (Fig. 5A). In fish, cholesterol levels could be emblematic of common and traditional response to stress (Saha and Bandyopadhyay 2020).
(A) Cholesterol; (B) High density lipoprotein (HDL); (C)Low density lipoprotein (LDL); (D) Creatinine levels of Labeo rohita fed with Aloe barbadensis on 1 and 15-days post infection (dpi) with Aeromonas hydrophila. *, **, ***, **** indicates significant difference to positive control (D1) at ≤ 0.05, ≤ 0.01, ≤ 0.001 and ≤ 0.0001 respectively. NC, negative control; D1-0% A. barbadensis (positive control); D2-25% A. barbadensis; D3-50% A. barbadensis; D4-75% A. barbadensis; D5-100% A. barbadensis replacing fishmeal; ns, non-significant
Chen et al. 2020 stated that the serum HDL mainly collect excess phospholipids and cholesterol and reverse them to liver. The elevated levels of HDL activity nullify the ROS (reactive oxygen species) produced from oxidative stress (Zahran et al. 2020). In our study, lower level of HDL was observed in D2 and D3 diet fed groups (Fig. 5B) which suggests that the lesser oxidative stress was generated in these particular groups post challenged with A. hydrophila. Contrarily, increasing level of HDL was reported by Gabriel et al., (2015a) on GIFT – tilapia (Oreochromis niloticus) fed with A. barbadensis against Streptococcus iniae. Adeli et al., (2021) reported the elevated level of HDL in the Oncorhynchus mykiss (rainbow trout) fed with Aloysia thriphylla. The LDL levels were similar in D3 and NC group on 1 dpi and decreased values were noted in other experimental diet fed groups (Fig. 5C). Gabriel et al., (2015a), mentioned that, LDL content is usually correlated with the levels of free radicals which can lead to damage cells, DNA and tissues. The present study demonstrated that the Aloe barbadensis supplementation diets positively altered the LDL levels of the experimental fish. The similar decreasing trend of LDL was also observed in Labeo rohita fed with Geotrichum candidum (Amir et al. 2019).
Creatinine is a kind of protein which is produced by the breakdown of creatinine phosphate in the muscle and discharged into blood for energy. Creatinine acts as a helpful marker for assessing the health of fish gill and kidney. It constitutes more than 50% of nitrogenous waste eliminated by fish kidney and kidney filtration capability is measured by the level of creatinine (Pastorino et al. 2022). In this study, D1 diet fed group showed increased creatinine level than other experimental diet fed groups. The D5 diet fed group showed significant difference in creatinine level between 1 & 15 dpi (Fig. 5D). In addition, significantly decreased creatinine levels were observed on 1 dpi in all the experimental diet fed groups when compared with D1 diet fed group. Mahboub et al., (2022) reported increased level of creatinine in the Nile tilapia (Oreochromis niloticus) post infected with Burkholderia cepacia. Increased level of creatine was recorded in Aeromonas hydrophila infected Nile tilapia (O. niloticus) fed with micro algae Spirulina, Chlorella diet (Fadl et al. 2017).
Immunological assay
According to previous studies, the medicinal plants and their extracts can ameliorate the specific and non-specific immune response of various fish species such as Poecilia reticulata (Ahmadniaye Motlagh et al. 2020), Pelteobagrus fulvidraco (Hou et al. 2022), Achycentron canadum (Lee et al. 2020) and Oncorhynchus mykiss (Mehrabi et al. 2020). In line with these previous reports, this current study investigated that whether the administration of Aloe barbadensis can enhance the humoral immunity of experimental fish Labeo rohita by analyzing the activity of immunological parameters such as lysozyme and IgM.
Lysozyme activity
Usually, neutrophils, submucosal glands and macrophages secrete lysozyme enzymes (Ganz 2006; Dotta et al. 2014). It serves a crucial role in innate immunity by protecting against pathogens (Sridhar et al. 2021a) and also used as a biomarker (Das et al. 2015; Sutthi et al. 2020). Bacterial lysis, stimulation of complement system and phagocytosis are accomplished by the enzyme lysozyme. The declining activity of this enzyme might be due to the reduction in the count or function of macrophages and neutrophils (Farag et al. 2021). In this present study, increased level of lysozyme activity was observed in the D3 diet fed fish group in both 1 & 15 dpi (Table 2). The results revealed that the supplementation of A. barbadensis positively modulates in D3 diet fed group. Plant based ingredients usually contain antinutritional factors and it was reflected in the higher inclusion diets (D4 & D5). Identical results were obtained by Sutthi et al., (2020), in which 0.25 g/kg inclusion of ethanolic extract of Apium graveolens leaves diet fed fish Labeo chrysophekadion showed elevated levels of lysozyme activity and declined in higher inclusion diets against Aeromonas hydrophila infection. Teoh and Loo, (2022) mentioned that lysozyme enzyme protects the fish by rupturing the peptidoglycan layer of pathogenic bacterial cell wall. Gabriel et al., (2015b) reported that higher lysozyme activity was observed in the aloe vera inclusion diet fed GIFT tilapia post infected with Streptococcus iniae. Significant increase in the level of lysozyme was also noted after A. hydrophila infection on Piaractus mesopotamicus fed with 1 and 2% aloe vera inclusion diets (Zanuzzo et al. 2017). El-Kassas et al. (2022) observed elevated levels of lysozyme activity on A. hydrophila infected Oreochromis niloticus fed with Moringa oleifera leaf powder at 48 h of post infection.
Immunoglobulin M (IgM) assay
IgM is a major fish immune component in serum (Zhou et al. 2021). IgM is a main constituent in humoral immune system of teleost and indicator of fish health (Yeganeh et al. 2021). IgM is believed to serve a crucial role in various immune processes including phagocytosis, toxins and pathogenic viruses, bacteria neutralization, and opsonization in the affected fish (Magnadottir 2010; Yonar et al. 2019). In our study, IgM level was increased in D3 diet fed group (Table 2). Higher Aloe barbadensis inclusion diets (D4 & D5) showed no significant variations on 1 & 15 dpi when compared with D1. The increased IgM levels are related to augmented fish immunity. Das et al., (2013) reported the correlation between immunoglobulin levels and proteins. The present study revealed a notable higher IgM content in experimental diet D3 fed group fishes and suggesting that the fishmeal replaced with A. barbadensis at 50% may enhance the immunity of Labeo rohita. The inclusion of plant-derived compounds in fish feed increase the non-specific immune responses in fish (Zhang et al. 2020). Increased level of IgM level was reported by Adeli et al., (2021) on rainbow trout (Oncorhynchus mykiss) juveniles fed with Lemon verbena (Aloysia thriphylla).
Antioxidant activity
Common oxidative stress could make fish susceptible to diseases by creating an imbalance between the generation and removal of ROS (Sheikhzadeh et al. 2012; Yuan et al. 2019). An antioxidant is a type of compound that stabilizes, scavenges, and suppresses the generation of oxidants and free radicals. External stress is directly responsible to stimulate the antioxidant system (Ding et al. 2022).
Superoxide dismutase (SOD)
SOD is an important antioxidant enzyme (metalloenzyme) that converts superoxide radicals into hydrogen peroxide and molecular oxygen (O2). It acts as the defence mechanism against free radicals (Weydert and Cullen 2010) and protects cells from oxidative damage due to external stimuli. It is also a crucial component in the first line of protection in fish against invading pathogens (Wang et al. 2016). Kaur et al. (2020) reported elevated level of SOD activity in Aeromonas veronii challenged Labeo rohita supplemented with turmeric powder. In the current study, lesser SOD activity was observed in D2 & D3 groups compared to other diet fed groups though they did not show any significant alterations (Table 3). Similar level of SOD was recorded in liver, intestine and muscle. Dotta et al., (2018) also observed no significant variations in SOD and GPx activity in A. hydrophila infected Nile tilapia fed with the 1% extracts of propolis and Aloe barbadensis. In this study, no significant variations were observed between 1 & 15 dpi in all the analyzed organs. When comparing to the negative control, all the experimental diet fed fish showed increased SOD enzyme level which indicates that the treated fish have experienced some disruption in the antioxidant defense system. In contrast, no significant variation was observed between the infected and uninfected silver cat fish (Rhamdia quelen) with A. caviae (Baldissera et al. 2018).
Catalase activity
Catalase (CAT) is a primary antioxidant enzyme found in almost all living organisms. It catalyzes the decomposition of hydrogen peroxide into water and oxygen and helps to protect cells from oxidative stress caused by ROS (Weydert and Cullen 2010). All the experimental diet fed groups showed increased catalase activity than negative control and implies that A. barbadensis significantly modulates the immunity of Labeo rohita. Significant higher antioxidant activity was noted in D1 & D4 diet fed fish on both 1 & 15 dpi irrespective of tissues (Table 3). An increase in the catalase enzyme activity denotes an accumulation of H2O2 (Garcia et al. 2011). The decreased catalase activity was observed in D2 and D3 diet fed fish on 1 & 15 dpi in all the tissues indicating lesser infection in these diets fed groups. Especially in the liver, the D3 diet fed fish showed significantly decreased activity on 1 & 15 dpi when compared with D1. This result suggests that the inclusion of A. barbadensis enhanced the L. rohita immunity. Elevated level of CAT activity was observed in licorice (Abdel-Tawwab and El-Araby 2021) and mulberry leaves (Neamat-Allah et al. 2021) fed Oreochromis niloticus against A. hydrophila.
Glutathione peroxidase activity
Another crucial antioxidant enzyme is GPx (Glutathione peroxidase) which converts hydrogen peroxide into water and oxygen and reduce peroxide radicals into alcohols and oxygen (Weydert and Cullen 2010). It acts as a strong free radical and lipid peroxidase scavenging enzyme. All the three tissues analyzed in this study showed higher GPx activity in D1, D4 & D5 diet fed groups similarly to the catalase activity (Table 3). There were no significant variations in the tissues between 1 & 15 dpi. Significantly decreased GPx activity was observed in D2 and D3 group when compared with D1. Sahreen et al., (2021) reported the elevated levels of SOD, CAT and GPx activity in Indian catfish, Wallago attu against Isoparorchis hypselobagri infection and indicated that the host organism had adaptive changes to the pathogens or infections.
The results of the antioxidant assays conclude that the antioxidant enzymes (SOD, CAT, GPx) studied in the present work showed increased levels on 1 dpi than 15 dpi. When comparing all the experimental diet fed groups, D4 showed elevated levels of antioxidant enzymes. Whereas, decreased activity was observed in D3 followed by D2 diet fed group. The activation of these enzyme cause deleterious effects to ROS (Halliwell 2012). Antioxidant activity such as SOD, CAT, GPx was significantly influenced in the D1 due to the stress caused by the severe infection of Aeromonas hydrophila. The phytoconstituents like phenols, flavonoids, vitamins, etc., in the extracts of medicinal plants impede oxidation process (Rajasekaran et al. 2006; Hoseinifar et al. 2020). Study by Vicente et al., (2019) also reported that the phytoconstituents of orange peel enriched diets influenced the antioxidant enzyme activity in Nile tilapia Oreochromis niloticus.
Histopathology
Histopathology is a well-established method to determine the qualitative alterations in the organs caused by biological infectious agents/chemicals and the patterns of recovery (Forouhar Vajargah et al. 2018; Abraham et al. 2021). The histopathological lesions clearly demonstrate the interactions of bacterial virulence factors with the host immune system. The bacterium Aeromonas cause cellular membrane rupture by producing hemolysin and aerolysin, resulted in cell death (Hussain Bhat et al. 2021). In this study, severe infection was observed on 1 dpi in all the experimental diets fed fish groups (Fig. 6). D3 and D4 group fish showed lesser damage than other experimental diets fed fish groups.
The histopathological photomicrographs of liver, intestine and muscle of Labeo rohita fed with Aloe barbadensis on 1 day post infection (dpi) with Aeromonas hydrophila. H-hepatocytes, DV-diffused vacuolation, S-sinusoid, SV-sinusoidal vacuolation, N-necrosis, NH-necrotic hepatocyte, CV-central vein, LP-lamina propria, VI-villi, SM-submucosa, E-tunica mucosa, M-tunica muscularis, S-tunica serosa, GC-goblet cell, MB-muscle bundle, SMB-space between muscle bundle, IMFS-intra myofibril space. NC, negative control; D1-0% A. barbadensis (positive control); D2-25% A. barbadensis; D3-50% A. barbadensis; D4-75% A. barbadensis; D5-100% A. barbadensis replacing fishmeal
In liver at 1 dpi, the D1 diet fed group showed severe necrosis of hepatocytes and extreme diffused hepatic vacuolation. The D2 diet fed group showed moderate necrosis of hepatocytes and vacuolation. The D3 diet fed group revealed slight necrosis of hepatocytes with severe diffused vacuolation. The D4 diet fed group showed very slight necrosis of hepatocytes with high diffused vacuolation. The D5 diet fed group exhibited diffused vacuolization and necrosis of hepatocytes and sinusoidal dilation. On 15 dpi (Fig. 7), the D3 and D5 diet fed fish liver showed mild vacuolation and necrosis of hepatocytes and blood sinusoid were clearly seen when compared to 1 dpi. The D4 diet fed group on 15 dpi showed severe diffused vacuolation and moderate necrosis of hepatocytes. Islam et al., (2013) demonstrated structural abnormalities like focal necrosis, massive atrophy, hepatic abscess, hemorrhagic area and vacuolation in the liver of Heteropneustes fossilis (Asian stinging catfish) infected with Aeromonas hydrophila. Vinosha et al., (2020) reported histopathological alterations like necrosis, degraded hepatocyte and vacuolations in liver, muscle and gill tissue of A. hydrophila infected Nile tilapia. Whereas, sulfated galactan from Halymenia dilatata treated fish groups showed positively upgraded tissue structures.
The histopathological photomicrographs of liver, intestine and muscle of Labeo rohita fed with Aloe barbadensis on 15 days post infection (dpi) with Aeromonas hydrophila. H-hepatocytes, DV- diffused vacuolation, S-sinusoid, SV-sinusoidal vacuolation, N-necrosis, NH-necrotic hepatocyte and CV-central vein, LP-lamina propria, VI-villi, SM-submucosa, E-tunica mucosa, M-tunica muscularis, S-tunica serosa, GC-goblet cell, MB-muscle bundle, SMB-space between muscle bundle, N-necrosis, IMFS-intra myofibril space. NC, negative control; D1-0% A. barbadensis (positive control); D2-25% A. barbadensis; D3-50% A. barbadensis; D4-75% A. barbadensis; D5-100% A. barbadensis replacing fishmeal
At 1 dpi, D1 group showed the infected intestine with complete degeneration along with severe necrosis, and sloughing of lamina mucosa. The D2 and D3 diet fed fish intestine showed partial sloughing of intestinal villi and moderate necrosis. The same modifications were reported by Hoseini et al., (2022) in the intestine of Cyprinus carpio exposed to copper and polyvinyl chloride microparticles. Sloughing of intestinal villi was observed in the Aeromonas infected fishes such as Cyprinus carpio (Di et al. 2017), Oreochromis niloticus (Assane et al. 2022), Ictalurus punctatus (Abdelhamed et al. 2017), and Labeo rohita (Sidiq et al. 2023). Blood innervates the submucosa's connective tissue, causing submucosa congestion that eventually results in haemorrhage with leukocyte infiltration and sloughing of the epithelial submucosa (Hussain Bhat et al. 2021). D4 and D5 diet fed fish intestine on 1 dpi showed severe necrosis and degeneration along the length of intestinal villi. In the case of 15 dpi, degeneration and necrosis were observed in D4 and D5 diet fed fish intestine. Intact structure was observed in the D2 and D3 diet fed groups.
In the case of muscle histoarchitectural analysis, D4 and D5 diet fed group fish showed severe necrosis and atrophy of muscle bundles. Abdel-Latif et al. (2022) reported the slight edema, loss of muscle striation in A. hydrophila challenged Nile tilapia. Necrosis was reported by ELbialy et al. (2023) in Oreochromis niloticus infected with A. hydrophila. Intact and clear structures were noted in the negative control, D2 and D3 diet fed fish on 1 dpi (Fig. 6). Necrosis level was also decreased in those group fish on 15 dpi (Fig. 7). It suggests the reduction in the infection rate on 15 dpi. In this current study, Aloe barbadensis inclusion diet D2 & D3 reduced the pathological changes associated with A. hydrophila infection in all examined tissues with an obvious effect in D3 diet fed group (50% FMR). In the same context, Ostaszewska et al., (2008) have showed an improvement in organ structures in silver bream (Vimba vimba) fed on natural plant feed additives.
Survival rate
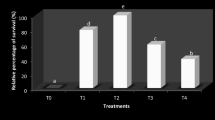
The survival percentage of fish challenged with A. hydrophila after fed with different concentrations of A. barbadensis diet was showed in Fig. 8. In this study, D3 diet fed group showed higher survival rate (80%) followed by D2 (75%), D5 (65%), D1 (50%) diet fed group and the least survival percentage was obtained in D4 (40%) diet fed group. Giri et al., (2023) reported higher survival percentage on A. hydrophila infected Cyprinus carpio fed with Bougainvillea glabra leaf inclusion diets and indicated that the dietary supplementation of herbal immunostimulants increase the disease resistance mechanism in fish. Administration of Mentha piperita enhanced the survival of Labeo rohita against A. hydrophila infection (Padala et al. 2021). Among the experimental diet fed groups challenged with A. hydrophila infection, D3 diet fed group showed increased lysozyme and IgM activity, better antioxidant activity and higher survival rate which indicates that the Aloe barbadensis inclusion at the rate of 50% replacing fishmeal enhance the disease resistance by elevating the immune activity of Labeo rohita.
Relative percentage survival of Labeo rohita fed with Aloe barbadensis replacing fishmeal post challenged with Aeromonas hydrophila. NC, negative control; D1-0% A. barbadensis (positive control); D2-25% A. barbadensis; D3-50% A. barbadensis; D4-75% A. barbadensis; D5-100% A. barbadensis replacing fishmeal
Conclusion
The findings of the current study demonstrated that L. rohita fed with A. barbadensis replacing 50% of fishmeal (D3 diet group) exhibited effective immune protection and increased disease resistance to A. hydrohila infection. However, higher level of A. barbadensis replacing fishmeal (75 & 100%—D4 and D5 group respectively) diets had a potential risk of decreasing the disease resistance and survival rate of the fish L. rohita. Therefore, based on the results obtained in this study, Diet 3 (50% of A. barbadensis replacement in fishmeal) could be considered as strategy to increase the disease resistance against A. hydrohila associated disease occurrences in L. rohita farming. A. barbadensis could enhance the fish immunity against aquatic disease problems through its sustainable, non-drug-resistant, non-noxious, and non-negligible characteristics.
Data availability
All data and materials are included in this published article.
References
Abd El Latif AM, Elabd H, Amin A et al (2019) High mortalities caused by Aeromonas veronii:identification, pathogenicity, and histopathologicalstudies in Oreochromis niloticus. Aquac Int 27:1725–1737. https://doi.org/10.1007/s10499-019-00429-8
Abdelhamed H, Ibrahim I, Baumgartner W et al (2017) Characterization of Histopathological and Ultrastructural Changes in Channel Catfish Experimentally Infected with Virulent Aeromonas hydrophila. Front Microbiol 8:1519. https://doi.org/10.3389/fmicb.2017.01519
Abdel-Latif HMR, Shukry M, Abd-elaziz RA (2022) Clinico-pathological findings and expression of inflammatory cytokines, apoptosis, and oxidative stress-related genes draw mechanistic insights in Nile tilapia reared under ammonia-N exposure and Aeromonas hydrophila challenge. Fish Shellfish Immunol 127:1–12. https://doi.org/10.1016/j.fsi.2022.06.001
Abdel-Tawwab M, Abdulrahman NM, Ahmad VM et al (2022) Effects of dietary oak (Quercus aegilops L.) acorn on growth performance, somatic indices, and hemato-biochemical responses of common carp, Cyprinus carpio L., at different stocking densities. J Appl Aquac 34:877–893. https://doi.org/10.1080/10454438.2021.1902450
Abdel-Tawwab M, El-Araby DA (2021) Immune and antioxidative effects of dietary licorice (Glycyrrhiza glabra L.) on performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to Aeromonas hydrophila infection. Aquaculture 530:735828. https://doi.org/10.1016/j.aquaculture.2020.735828
Abraham TJ, Julinta RB, Roy A et al (2021) Dietary therapeutic dose of oxytetracycline negatively influences the antioxidant capacity and immune-related genes expression in Nile tilapia Oreochromis niloticus (L.). Environ Toxicol Pharmacol 87:103685. https://doi.org/10.1016/j.etap.2021.103685
Adeli A, Shamloofar M, Akrami R (2021) Dietary effect of Lemon Verbena (Aloysia triphylla) extract on growth performance, some haematological, biochemical, and non-specific immunity and stocking density challenge of rainbow trout juveniles (Oncorhynchus mykiss). J Appl Anim Res 49:382–390. https://doi.org/10.1080/09712119.2021.1990069
Ahmadniaye Motlagh H, Safari O, Selahvarzi Y et al (2020) Non-specific immunity promotion in response to garlic extract supplemented diets in female Guppy (Poecilia reticulata). Fish Shellfish Immunol 97:96–99. https://doi.org/10.1016/j.fsi.2019.12.007
Ahmed I, Sheikh ZA (2019) Hematological and serum biochemical parameters of five freshwater snow trout fish species from river Jhelum of Kashmir Himalaya, India. Comp Clin Pathol 28:771–782. https://doi.org/10.1007/s00580-019-02909-y
Almarri SH, Khalil AA, Mansour AT, El-Houseiny W (2023) Antioxidant, Immunostimulant, and Growth-Promoting Effects of Dietary Annona squamosa Leaf Extract on Nile Tilapia, Oreochromis niloticus, and Its Tolerance to Thermal Stress and Aeromonas sobria Infection. Animals 13:746. https://doi.org/10.3390/ani13040746
Amir I, Zuberi A, Kamran M et al (2019) Evaluation of commercial application of dietary encapsulated probiotic (Geotrichum candidum QAUGC01): Effect on growth and immunological indices of rohu (Labeo rohita, Hamilton 1822) in semi-intensive culture system. Fish Shellfish Immunol 95:464–472. https://doi.org/10.1016/j.fsi.2019.11.011
Assane IM, Prada-Mejia KD, Gallani SU et al (2022) Enterogyrus spp. (Monogenea: Ancyrocephalinae) and Aeromonas jandaei co-infection associated with high mortality following transport stress in cultured Nile tilapia. Transbound Emerg Dis 69:e276–e287. https://doi.org/10.1111/tbed.14295
Ayiku S, Shen J, Tan B et al (2020) Effects of reducing dietary fishmeal with yeast supplementations on Litopenaeus vannamei growth, immune response and disease resistance against Vibrio harveyi. Microbiol Res 239:126554. https://doi.org/10.1016/j.micres.2020.126554
Baldissera MD, Souza CF, Parmeggiani B et al (2018) The disturbance of antioxidant/oxidant balance in fish experimentally infected by Aeromonas caviae: Relationship with disease pathophysiology. Microb Pathog 122:53–57. https://doi.org/10.1016/j.micpath.2018.06.011
Banihashemi EA, Soltanian S, Gholamhosseini A, Banaee M (2022) Effect of microplastics on Yersinia ruckeri infection in rainbow trout (Oncorhynchus mykiss). Environ Sci Pollut Res 29:11939–11950. https://doi.org/10.1007/s11356-021-16517-3
Ben Hamed S, Tapia-Paniagua ST, Moriñigo MÁ, Ranzani-Paiva MJT (2021) Advances in vaccines developed for bacterial fish diseases, performance and limits. Aquac Res 52:2377–2390. https://doi.org/10.1111/are.15114
Bernet D, Schmidt H, Wahli T, Burkhardt-Holm P (2001) Effluent from a Sewage Treatment Works Causes Changes in Serum Chemistry of Brown Trout (Salmo trutta L.). Ecotoxicol Environ Saf 48:140–147. https://doi.org/10.1006/eesa.2000.2012
Bhat RAH, Khangembam VC, Thakuria D et al (2022) Antimicrobial activity of an artificially designed peptide against fish pathogens. Microbiol Res 260:127039. https://doi.org/10.1016/j.micres.2022.127039
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cabello FC, Godfrey HP, Buschmann AH, Dölz HJ (2016) Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect Dis 16:e127–e133. https://doi.org/10.1016/S1473-3099(16)00100-6
Carbonara P, Alfonso S, Zupa W et al (2019) Behavioral and physiological responses to stocking density in sea bream (Sparus aurata): Do coping styles matter? Physiol Behav 212:112698. https://doi.org/10.1016/j.physbeh.2019.112698
Chan JFW, Lau SKP, To KKW et al (2015) Middle East Respiratory Syndrome Coronavirus: Another Zoonotic Betacoronavirus Causing SARS-Like Disease. Clin Microbiol Rev 28:465–522. https://doi.org/10.1128/CMR.00102-14
Chen F, Sun J, Han Z et al (2019) Isolation, Identification and Characteristics of Aeromonas veronii From Diseased Crucian Carp (Carassius auratus gibelio). Front Microbiol 10:2742. https://doi.org/10.3389/fmicb.2019.02742
Chen X, Xie J, Liu Z et al (2020) Modulation of growth performance, non-specific immunity, intestinal morphology, the response to hypoxia stress and resistance to Aeromonas hydrophila of grass carp (Ctenopharyngodon idella) by dietary supplementation of a multi-strain probiotic. Comp Biochem Physiol Part C Toxicol Pharmacol 231:108724. https://doi.org/10.1016/j.cbpc.2020.108724
Chong C-M, Shakir MZ, Lai K-S, et al (2023) Chapter 5 - Microbes and fish diseases. In: Mathew J, Jose MS, E.k. R, Kumar A (eds) Recent Advances in Aquaculture Microbial Technology, Academic Press, pp 65–102. https://doi.org/10.1016/B978-0-323-90261-8.00009-2
Citarasu T, Sivaram V, Immanuel G et al (2006) Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeus monodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol 21:372–384. https://doi.org/10.1016/j.fsi.2006.01.002
Dananjaya SHS, Udayangani RMC, Shin SY et al (2017) In vitro and in vivo antifungal efficacy of plant based lawsone against Fusarium oxysporum species complex. Microbiol Res 201:21–29. https://doi.org/10.1016/j.micres.2017.04.011
Das A, Nakhro K, Chowdhury S, Kamilya D (2013) Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish Shellfish Immunol 35:1547–1553. https://doi.org/10.1016/j.fsi.2013.08.022
Das R, Raman RP, Saha H, Singh R (2015) Effect of Ocimum sanctum Linn. (Tulsi) extract on the immunity and survival of Labeo rohita (Hamilton) infected with Aeromonas hydrophila. Aquac Res 46:1111–1121. https://doi.org/10.1111/are.12264
Das S, Pradhan C, Pillai D (2022) Dietary coriander (Coriandrum sativum L) oil improves antioxidant and anti-inflammatory activity, innate immune responses and resistance to Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 108486. https://doi.org/10.1016/j.fsi.2022.108486
de Assis RWS, Urbinati EC (2020) Physiological activity of Aloe vera in pacu (Piaractus mesopotamicus) inoculated with Aeromonas hydrophila. Fish Physiol Biochem 46:1421–1430. https://doi.org/10.1007/s10695-020-00800-0
Devi G, Harikrishnan R, Paray BA et al (2019) Effect of symbiotic supplemented diet on innate-adaptive immune response, cytokine gene regulation and antioxidant property in Labeo rohita against Aeromonas hydrophila. Fish Shellfish Immunol 89:687–700. https://doi.org/10.1016/j.fsi.2019.04.036
Di G, Li H, Zhang C et al (2017) Label-free proteomic analysis of intestinal mucosa proteins in common carp (Cyprinus carpio) infected with Aeromonas hydrophila. Fish Shellfish Immunol 66:11–25. https://doi.org/10.1016/j.fsi.2017.04.025
Ding Y, Song X, Yu Z (2022) Transcriptome profiles of genes related to growth and virulence potential in Vibrio alginolyticus treated with modified clay. Microbiol Res 262:127095. https://doi.org/10.1016/j.micres.2022.127095
Dotta G, de Andrade JIA, Garcia P et al (2018) Antioxidant enzymes, hematology and histology of spleen in Nile tilapia fed supplemented diet with natural extracts challenged with Aeromonas hydrophila. Fish Shellfish Immunol 79:175–180. https://doi.org/10.1016/j.fsi.2018.05.024
Dotta G, de Andrade JIA, Tavares Gonçalves EL et al (2014) Leukocyte phagocytosis and lysozyme activity in Nile tilapia fed supplemented diet with natural extracts of propolis and Aloe barbadensis. Fish Shellfish Immunol 39:280–284. https://doi.org/10.1016/j.fsi.2014.05.020
El-Barbary MI (2010) Some clinical, microbiological and molecular characteristics of Aeromonas hydrophila isolated from various naturally infected fishes. Aquac Int 18:943–954. https://doi.org/10.1007/s10499-009-9315-x
ELbialy ZI, Atef E, Al-Hawary II et al (2023) Myostatin-mediated regulation of skeletal muscle damage post-acute Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus L.). Fish Physiol Biochem 49:1–17. https://doi.org/10.1007/s10695-022-01165-2
El-Kassas S, Aljahdali N, Abdo SE et al (2022) Moringa oleifera Leaf Powder Dietary Inclusion Differentially Modulates the Antioxidant, Inflammatory, and Histopathological Responses of Normal and Aeromonas hydrophila-Infected Mono-Sex Nile Tilapia (Oreochromis niloticus). Front Vet Sci 9:918933. https://doi.org/10.3389/fvets.2022.918933
Ellis AI (1990) Lysozyme Assays. Tech Fish Immunol 1:101–103
Fadl SE, ElGohary MS, Elsadany AY et al (2017) Contribution of microalgae-enriched fodder for the Nile tilapia to growth and resistance to infection with Aeromonas hydrophila. Algal Res 27:82–88. https://doi.org/10.1016/j.algal.2017.08.022
Fajardo C, Santos P, Passos R et al (2022) Functional and Molecular Immune Response of Rainbow Trout (Oncorhynchus mykiss) Following Challenge with Yersinia ruckeri. Int J Mol Sci 23:3096. https://doi.org/10.3390/ijms23063096
FAO (2020) The State of World Fisheries and Aquaculture 2020. 244. FAO. https://doi.org/10.4060/ca9229en
FAO (2022) The State of World Fisheries and Aquaculture 2022. 266. https://doi.org/10.4060/cc0461en
Farag MR, Alagawany M, Taha HSA et al (2021) Immune response and susceptibility of Nile tilapia fish to Aeromonas hydrophila infection following the exposure to Bifenthrin and/or supplementation with Petroselinum crispum essential oil. Ecotoxicol Environ Saf 216:112205. https://doi.org/10.1016/j.ecoenv.2021.112205
Fazio F, Marafioti S, Torre A et al (2013) Haematological and serum protein profiles of Mugil cephalus: effect of two different habitats. Ichthyol Res 60:36–42. https://doi.org/10.1007/s10228-012-0303-1
Folliero V, Dell’Annunziata F, Roscetto E et al (2022) Rhein: A novel antibacterial compound against Streptococcus mutans infection. Microbiol Res 261:127062. https://doi.org/10.1016/j.micres.2022.127062
Forouhar Vajargah M, Mohamadi Yalsuyi A, Hedayati A, Faggio C (2018) Histopathological lesions and toxicity in common carp (Cyprinus carpio L. 1758) induced by copper nanoparticles. Microsc Res Tech 81:724–729. https://doi.org/10.1002/jemt.23028
Fuentes-Valencia MA, Osornio-Esquivel JL, Martínez Palacios CA et al (2022) Bacterial and parasite co-infection in Mexican golden trout (Oncorhynchus chrysogaster) by Aeromonas bestiarum, Aeromonas sobria, Plesiomonas shigelloides and Ichthyobodo necator. BMC Vet Res 18:137. https://doi.org/10.1186/s12917-022-03208-5
Gabriel NN, Qiang J, He J et al (2015a) Dietary Aloe vera supplementation on growth performance, some haemato-biochemical parameters and disease resistance against Streptococcus iniae in tilapia (GIFT). Fish Shellfish Immunol 44:504–514. https://doi.org/10.1016/j.fsi.2015.03.002
Gabriel NN, Qiang J, Ma XY et al (2015b) Dietary Aloe vera improves plasma lipid profile, antioxidant, and hepatoprotective enzyme activities in GIFT-tilapia (Oreochromis niloticus) after Streptococcus iniae challenge. Fish Physiol Biochem 41:1321–1332. https://doi.org/10.1007/s10695-015-0088-z
Ganz T (2006) LYSOZYME. In: Laurent GJ, Shapiro SD (eds) Encyclopedia of Respiratory Medicine. Academic Press, Oxford, pp 649–653
Garcia LO, Becker AG, Bertuzzi T et al (2011) Oxidative stress parameters in silver catfish (Rhamdia quelen) juveniles infected with Ichthyophthirius multifiliis and maintained at different levels of water pH. Vet Parasitol 178:15–21. https://doi.org/10.1016/j.vetpar.2010.12.039
Giannakoudakis DA, Hosseini-Bandegharaei A, Tsafrakidou P et al (2018) Aloe vera waste biomass-based adsorbents for the removal of aquatic pollutants: A review. J Environ Manage 227:354–364. https://doi.org/10.1016/j.jenvman.2018.08.064
Giri SS, Kim SG, Woo KJ et al (2023) Effects of Bougainvillea glabra leaf on growth, skin mucosal immune responses, and disease resistance in common carp Cyprinus carpio. Fish Shellfish Immunol 132:108514. https://doi.org/10.1016/j.fsi.2022.108514
Gobi N, Vaseeharan B, Chen J-C et al (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol 74:501–508. https://doi.org/10.1016/j.fsi.2017.12.066
Gupta M, Kiran S, Gulati A et al (2012) Isolation and identification of phosphate solubilizing bacteria able to enhance the growth and aloin-A biosynthesis of Aloe barbadensis Miller. Microbiol Res 167:358–363. https://doi.org/10.1016/j.micres.2012.02.004
Haldar S, Maharajan A, Chatterjee S et al (2010) Identification of Vibrio harveyi as a causative bacterium for a tail rot disease of sea bream Sparus aurata from research hatchery in Malta. Microbiol Res 165:639–648. https://doi.org/10.1016/j.micres.2009.12.001
Halliwell B (2012) Free radicals and antioxidants: updating a personal view. Nutr Rev 70:257–265. https://doi.org/10.1111/j.1753-4887.2012.00476.x
Haque S, Bandyopadhyay PK, Mondal K (2021) Studies on Growth, Behavior and Blood Profile in Anabas testudineus Infected with Pseudomonas aeruginosa. Proc Zool Soc 74:19–27. https://doi.org/10.1007/s12595-020-00330-w
Harikrishnan R, Thamizharasan S, Devi G et al (2020) Dried lemon peel enriched diet improves antioxidant activity, immune response and modulates immuno-antioxidant genes in Labeo rohita against Aeromonas sorbia. Fish Shellfish Immunol 106:675–684. https://doi.org/10.1016/j.fsi.2020.07.040
Hęś M, Dziedzic K, Górecka D et al (2019) Aloe vera (L.) Webb.: Natural Sources of Antioxidants – A Review. Plant Foods Hum Nutr 74:255–265. https://doi.org/10.1007/s11130-019-00747-5
Hore M, Saha R, Bhaskar S et al (2023) Oxidative stress responses in Puntius sarana collected from some environmentally contaminated areas of River Mahananda. Ecotoxicology, Malda, West Bengal. https://doi.org/10.1007/s10646-023-02630-1
Hoseini SM, Sinha R, Fazel A et al (2022) Histopathological damage and stress- and immune-related genes’ expression in the intestine of common carp, Cyprinus carpio exposed to copper and polyvinyl chloride microparticle. J Exp Zool Part Ecol Integr Physiol 337:181–190. https://doi.org/10.1002/jez.2555
Hoseinifar SH, Jahazi MA, Mohseni R et al (2020) Effects of dietary fern (Adiantum capillus-veneris) leaves powder on serum and mucus antioxidant defence, immunological responses, antimicrobial activity and growth performance of common carp (Cyprinus carpio) juveniles. Fish Shellfish Immunol 106:959–966. https://doi.org/10.1016/j.fsi.2020.09.001
Hosseini H, Pooyanmehr M, Foroughi A et al (2022) Remarkable positive effects of figwort (Scrophularia striata) on improving growth performance, and immunohematological parameters of fish. Fish Shellfish Immunol 120:111–121. https://doi.org/10.1016/j.fsi.2021.11.020
Hou T, Liu H, Li C (2022) Traditional Chinese herb formulas in diet enhance the non-specific immune responses of yellow catfish (Pelteobagrus fulvidraco) and resistance against Aeromonas hydrophila. Fish Shellfish Immunol 131:631–636. https://doi.org/10.1016/j.fsi.2022.10.050
Hussain Bhat RA, Thakuria D, Dubey MK et al (2021) Lethal dose and histopathological alterations induced by Aeromonas salmonicida in experimentally challenged common carp. Cyprinus Carpio Microb Pathog 158:105110. https://doi.org/10.1016/j.micpath.2021.105110
Islam M, Mostafa K, Rashid M (2013) Histopathological Studies of Experimentally Infected Shing, Heteropneustes fossiliswith Aeromonas hydrophila Bacteria. Progress Agric 19:89–96. https://doi.org/10.3329/pa.v19i1.17359
Kapetanović D, VardićSmrzlić I, Gavrilović A et al (2022) Characterization of Vibrio Populations from Cultured European Seabass and the Surrounding Marine Environment with Emphasis on V. anguillarum. Microorganisms 10:2159. https://doi.org/10.3390/microorganisms10112159
Kari ZA, Kabir MA, Mat K, Rusli ND, Razab MKAA, Ariff NSNA, Edinur HA, Rahim MZA, Pati S, Dawood MA, Wei LS (2021) The possibility of replacing fish meal with fermented soy pulp on the growth performance, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus). Aquaculture Reports 21:100815
Kaur A, Holeyappa SA, Bansal N et al (2020) Ameliorative effect of turmeric supplementation in feed of Labeo rohita (Linn.) challenged with pathogenic Aeromonas veronii. Aquac Int 28:1169–1182. https://doi.org/10.1007/s10499-020-00518-z
Khanal M, Lamichhane S, Bhattarai A et al (2021) Extract of Aloe vera (Aloe barbadensis Miller) Enhances the Growth, Protein Contents, and Gastrosomatic Index (GaSI) of Common Carp Cyprinus carpio. J Nutr Metab 2021:e8029413. https://doi.org/10.1155/2021/8029413
Krishnasamy Sekar R, Arunachalam R, Anbazhagan M et al (2023) Accumulation, Chronicity, and Induction of Oxidative Stress Regulating Genes Through Allium cepa L. Functionalized Silver Nanoparticles in Freshwater Common Carp (Cyprinus carpio). Biol Trace Elem Res 201:904–925. https://doi.org/10.1007/s12011-022-03164-z
Kumar S, Choubey AK, Srivastava PK (2022a) The effects of dietary immunostimulants on the innate immune response of Indian major carp: A review. Fish Shellfish Immunol 123:36–49. https://doi.org/10.1016/j.fsi.2022.02.039
Kumar S, Raman RP, Kumar K et al (2013) Effect of azadirachtin on haematological and biochemical parameters of Argulus-infested goldfish Carassius auratus (Linn. 1758). Fish Physiol Biochem 39:733–747. https://doi.org/10.1007/s10695-012-9736-8
Kumar V, Das BK, Swain HS, et al (2022b) Outbreak of Ichthyophthirius multifiliis associated with Aeromonas hydrophila in Pangasianodon hypophthalmus: The role of turmeric oil in enhancing immunity and inducing resistance against co-infection. Front Immunol 13. https://doi.org/10.3389/fimmu.2022.956478
Lee PT, Chen HY, Liao ZH et al (2020) Effects of three medicinal herbs Bidens pilosa, Lonicera japonica, and Cyathula officinalis on growth and non-specific immune responses of cobia (Rachycentron canadum). Fish Shellfish Immunol 106:526–535. https://doi.org/10.1016/j.fsi.2020.07.032
Li M, Wei D, Huang S et al (2022) Medicinal herbs and phytochemicals to combat pathogens in aquaculture. Aquac Int 30:1239–1259. https://doi.org/10.1007/s10499-022-00841-7
Li T, Raza SHA, Yang B et al (2020) Aeromonas veronii Infection in Commercial Freshwater Fish: A Potential Threat to Public Health. Animals 10:608. https://doi.org/10.3390/ani10040608
Liang W, Wu R, Yang T et al (2020) Effect of pathogenic bacteria on a novel C-type lectin, hemocyte and superoxide dismutase/ alkaline phosphatase activity in Onchidium reevesii. Fish Shellfish Immunol 102:185–194. https://doi.org/10.1016/j.fsi.2020.04.001
Magnadottir B (2010) Immunological Control of Fish Diseases. Mar Biotechnol 12:361–379. https://doi.org/10.1007/s10126-010-9279-x
Mahboub HH, Elsheshtawy HM, Sheraiba NI et al (2022) Dietary black cumin (Nigella sativa) improved hemato-biochemical, oxidative stress, gene expression, and immunological response of Nile tilapia (Oreochromis niloticus) infected by Burkholderia cepacia. Aquac Rep 22:100943. https://doi.org/10.1016/j.aqrep.2021.100943
Malini DM, Madihah AAF, Arista S (2018) Increased Blood Glucose Level on Pelagic Fish as Response to Environmental Disturbances at East Coast Pangandaran, West Java. IOP Conf Ser Earth Environ Sci 166:012011. https://doi.org/10.1088/1755-1315/166/1/012011
Manchanayake T, Salleh A, Amal MNA et al (2023) Pathology and pathogenesis of Vibrio infection in fish: A review. Aquac Rep 28:101459. https://doi.org/10.1016/j.aqrep.2022.101459
Marinho-Neto FA, Claudiano GS, Yunis-Aguinaga J et al (2019) Morphological, microbiological and ultrastructural aspects of sepsis by Aeromonas hydrophila in Piaractus mesopotamicus. PLoS ONE 14:e0222626. https://doi.org/10.1371/journal.pone.0222626
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Matys J, Turska-Szewczuk A, Gieroba B et al (2022) Evaluation of Proteomic and Lipidomic Changes in Aeromonas-Infected Trout Kidney Tissue with the Use of FT-IR Spectroscopy and MALDI Mass Spectrometry Imaging. Int J Mol Sci 23:12551. https://doi.org/10.3390/ijms232012551
Mbokane EM, Moyo NAG (2018) Alterations of haemato-biochemical parameters pre and post-challenge with Aeromonas hydrophila and survival of Oreochromis mossambicus fed Moringa oleifera-based diets. Fish Shellfish Immunol 83:213–222. https://doi.org/10.1016/j.fsi.2018.09.017
Mehrabi Z, Firouzbakhsh F (2020) Short-term effects of feeding powdered Aloe vera (Aloe barbadensis) and nettle (Urtica dioica) on growth performance and stimulation of innate immune responses in rainbow trout (Oncorhynchus mykiss). Comp Clin Pathol 29:441–449. https://doi.org/10.1007/s00580-019-03068-w
Mehrabi Z, Firouzbakhsh F, Rahimi-Mianji G, Paknejad H (2019) Immunostimulatory effect of Aloe vera (Aloe barbadensis) on non-specific immune response, immune gene expression, and experimental challenge with Saprolegnia parasitica in rainbow trout (Oncorhynchus mykiss). Aquaculture 503:330–338. https://doi.org/10.1016/j.aquaculture.2019.01.025
Mehrabi Z, Firouzbakhsh F, Rahimi-Mianji G, Paknejad H (2020) Immunity and growth improvement of rainbow trout (Oncorhynchus mykiss) fed dietary nettle (Urtica dioica) against experimental challenge with Saprolegnia parasitica. Fish Shellfish Immunol 104:74–82. https://doi.org/10.1016/j.fsi.2020.05.050
Moreau E, Pineau L, Bachelet F et al (2023) Time effect of experimental infection on Rainbow trout (Oncorhynchus mykiss) by immersion with Aeromonas salmonicida subsp. salmonicida. Fish Shellfish Immunol 135:108664. https://doi.org/10.1016/j.fsi.2023.108664
Mursalim MF, Budiyansah H, Raharjo HM et al (2022) Diversity and antimicrobial susceptibility profiles of Aeromonas spp. isolated from diseased freshwater fishes in Thailand. J Fish Dis 45:1149–1163. https://doi.org/10.1111/jfd.13650
Muziasari WI, Pärnänen K, Johnson TA, et al (2016) Aquaculture changes the profile of antibiotic resistance and mobile genetic element associated genes in Baltic Sea sediments. FEMS Microbiol Ecol 92. https://doi.org/10.1093/femsec/fiw052
Nair AV, Leo Antony M, Praveen NK et al (2021) Evaluation of in vitro and in vivo potential of Bacillus subtilis MBTDCMFRI Ba37 as a candidate probiont in fish health management. Microb Pathog 152:104610. https://doi.org/10.1016/j.micpath.2020.104610
Nasr-Eldahan S, Nabil-Adam A, Shreadah MA et al (2021) A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquac Int 29:1459–1480. https://doi.org/10.1007/s10499-021-00677-7
Nawaz M, Gao T, Huang K et al (2022) Pathogenicity, diagnosis, prevention strategies and immune response of bacterium Nocardia seriolae: A critical review. Aquac Res 53:4901–4918. https://doi.org/10.1111/are.15988
Neamat-Allah ANF, Mahmoud EA, Mahsoub Y (2021) Effects of dietary white mulberry leaves on hemato-biochemical alterations, immunosuppression and oxidative stress induced by Aeromonas hydrophila in Oreochromis niloticus. Fish Shellfish Immunol 108:147–156. https://doi.org/10.1016/j.fsi.2020.11.028
Okeke ES, Chukwudozie KI, Nyaruaba R et al (2022) Antibiotic resistance in aquaculture and aquatic organisms: a review of current nanotechnology applications for sustainable management. Environ Sci Pollut Res 29:69241–69274. https://doi.org/10.1007/s11356-022-22319-y
Ostaszewska T, Dabrowski K, Hliwa P et al (2008) Nutritional regulation of intestine morphology in larval cyprinid fish, silver bream (Vimba vimba). Aquac Res 39:1268–1278. https://doi.org/10.1111/j.1365-2109.2008.01989.x
Padala D, Marakini GN, Kokkam Valappil A et al (2021) Effect of dietary peppermint (Mentha piperita) on growth, survival, disease resistance and haematology on fingerlings of rohu (Labeo rohita). Aquac Res 52:2697–2705. https://doi.org/10.1111/are.15120
Palaniyappan S, Sridhar A, Arumugam M, Ramasamy T (2023) Bioactive Analysis of Antibacterial Efficacy and Antioxidant Potential of Aloe barbadensis Miller Leaf Extracts and Exploration of Secondary Metabolites Using GC–MS Profiling. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-023-04565-z
Pan Y, Huang J, Li Y et al (2023) Dynamic immune response in the spleens of rainbow trout (Oncorhynchus mykiss) to infectious hematopoietic necrosis virus revealed by transcriptome and immune-related genes expression analysis. Aquac Rep 29:101473. https://doi.org/10.1016/j.aqrep.2023.101473
Panigrahi A, Naveenkumar R, Das RR (2021) Immunoprophylactic Measures in Aquaculture. In: Pandey PK, Parhi J (eds) Advances in Fisheries Biotechnology. Springer Nature Singapore, Singapore, pp 263–288
Pastorino P, Bergagna S, Vercelli C et al (2022) Changes in Serum Blood Parameters in Farmed Rainbow Trout (Oncorhynchus mykiss) Fed with Diets Supplemented with Waste Derived from Supercritical Fluid Extraction of Sweet Basil (Ocimum basilicum). Fishes 7:89. https://doi.org/10.3390/fishes7020089
Patriche T (2009) THE IMPORTANCE OF GLUCOSE DETERMINATION IN THE BLOOD OF THE CYPRINIDS. Sci Pap Anim Sci Biotechnol 42:102–106
Pollard AJ, Bijker EM (2021) A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 21:83–100. https://doi.org/10.1038/s41577-020-00479-7
Rahimikia E (2017) Analysis of antioxidants and serum biochemical responses in goldfish under nickel exposure by sub-chronic test. J Appl Anim Res 45:320–325. https://doi.org/10.1080/09712119.2016.1190732
Raissy M, Ghafarifarsani H, Hoseinifar SH et al (2022) The effect of dietary combined herbs extracts (oak acorn, coriander, and common mallow) on growth, digestive enzymes, antioxidant and immune response, and resistance against Aeromonas hydrophila infection in common carp. Cyprinus Carpio Aquaculture 546:737287. https://doi.org/10.1016/j.aquaculture.2021.737287
Rajasekaran S, Ravi K, Sivagnanam K, Subramanian S (2006) Beneficial Effects of Aloe Vera Leaf Gel Extract on Lipid Profile Status in Rats with Streptozotocin Diabetes. Clin Exp Pharmacol Physiol 33:232–237. https://doi.org/10.1111/j.1440-1681.2006.04351.x
Rathore SS, Murthy HS, Mamun MA-A et al (2021) Nano-selenium Supplementation to Ameliorate Nutrition Physiology, Immune Response, Antioxidant System and Disease Resistance Against Aeromonas hydrophila in Monosex Nile Tilapia (Oreochromis niloticus). Biol Trace Elem Res 199:3073–3088. https://doi.org/10.1007/s12011-020-02416-0
Reda RM, El-Murr A, Abd Elhakim Y, El-Shahat W (2022) Aeromonas veronii detection in Egyptian fish farms with summer tilapia mortality outbreaks and the role of formic acid in limiting its spread. Aquac Res 53:940–956. https://doi.org/10.1111/are.15635
Rotruck JT, Pope AL, Ganther HE et al (1973) Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 179:588–590. https://doi.org/10.1126/science.179.4073.588
Saha M, Bandyopadhyay PK (2020) In vivo and in vitro antimicrobial activity of phytol, a diterpene molecule, isolated and characterized from Adhatoda vasica Nees. (Acanthaceae), to control severe bacterial disease of ornamental fish, Carassius auratus, caused by Bacillus licheniformis PKBMS16. Microb Pathog 141:103977. https://doi.org/10.1016/j.micpath.2020.103977
Sahreen A, Fatima K, Zainab T, Saifullah MK (2021) Changes in the level of oxidative stress markers in Indian catfish (Wallago attu) infected with Isoparorchis hypselobagri. Beni-Suef Univ J Basic Appl Sci 10:85. https://doi.org/10.1186/s43088-021-00174-z
Saljooghianpour M, Javaran TA (2013) Identification of phytochemical components of aloe plantlets by gas chromatography-mass spectrometry. Afr J Biotechnol 12:6876–6880. https://doi.org/10.4314/ajb.v12i49
Santos L, Ramos F (2018) Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int J Antimicrob Agents 52:135–143. https://doi.org/10.1016/j.ijantimicag.2018.03.010
Sattanathan G, Liu W-C, Padmapriya S et al (2023) Effects of Dietary Blend of Algae Extract Supplementation on Growth, Biochemical, Haemato-Immunological Response, and Immune Gene Expression in Labeo rohita with Aeromonas hydrophila Post-Challenges. Fishes 8:7. https://doi.org/10.3390/fishes8010007
Schieber M, Chandel NS (2014) ROS Function in Redox Signaling and Oxidative Stress. Curr Biol 24:R453–R462. https://doi.org/10.1016/j.cub.2014.03.034
Sharma A, Deo AD, Tandel Riteshkumar S et al (2010) Effect of Withania somnifera (L. Dunal) root as a feed additive on immunological parameters and disease resistance to Aeromonas hydrophila in Labeo rohita (Hamilton) fingerlings. Fish Shellfish Immunol 29:508–512. https://doi.org/10.1016/j.fsi.2010.05.005
Sheikhzadeh N, Tayefi-Nasrabadi H, Khani Oushani A, Najafi Enferadi MH (2012) Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 38:413–419. https://doi.org/10.1007/s10695-011-9519-7
Sidiq MJ, Jayaraj EG, Rathore SS et al (2023) Ameliorative role of dietary acidifier potassium formate on growth metrics, blood chemistry, gut health and well-being indices of rohu, Labeo rohita fingerlings. Fish Physiol Biochem 49:19–37. https://doi.org/10.1007/s10695-023-01171-y
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394. https://doi.org/10.1016/0003-2697(72)90132-7
Sridhar A, Guardiola FA, Krishnasamy Sekar R et al (2022) Comparative assessment of organic solvent extraction on non-specific immune defences of skin mucus from freshwater fish. Aquac Int 30:1121–1138. https://doi.org/10.1007/s10499-022-00847-1
Sridhar A, Krishnasamy Sekar R, Manikandan DB et al (2021a) Activity profile of innate immune-related enzymes and bactericidal of freshwater fish epidermal mucus extract at different pH. Environ Sci Pollut Res 28:33914–33926. https://doi.org/10.1007/s11356-020-11173-5
Sridhar A, Manikandan DB, Palaniyappan S et al (2021b) Correlation Between Three Freshwater Fish Skin Mucus Antiproliferative Effect and Its Elemental Composition Role in Bacterial Growth. Turk J Fish Aquat Sci 21:233–244. https://doi.org/10.4194/1303-2712-v21_5_03
Sutthi N, Panase A, Chitmanat C et al (2020) Effects of dietary leaf ethanolic extract of Apium graveolens L. on growth performance, serum biochemical indices, bacterial resistance and lysozyme activity in Labeo chrysophekadion (Bleeker, 1849). Aquac Rep 18:100551. https://doi.org/10.1016/j.aqrep.2020.100551
Talpur AD, Ikhwanuddin M (2013) Azadirachta indica (neem) leaf dietary effects on the immunity response and disease resistance of Asian seabass, Lates calcarifer challenged with Vibrio harveyi. Fish Shellfish Immunol 34:254–264. https://doi.org/10.1016/j.fsi.2012.11.003
Talpur AD, Munir MB, Mary A, Hashim R (2014) Dietary probiotics and prebiotics improved food acceptability, growth performance, haematology and immunological parameters and disease resistance against Aeromonas hydrophila in snakehead (Channa striata) fingerlings. Aquaculture 426–427:14–20. https://doi.org/10.1016/j.aquaculture.2014.01.013
Tan X, Sun Z, Chen S et al (2017) Effects of dietary dandelion extracts on growth performance, body composition, plasma biochemical parameters, immune responses and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol 66:198–206. https://doi.org/10.1016/j.fsi.2017.05.028
Teoh C-Y, Loo E-V (2022) Potential of Safmannan as a feed additive for juvenile African catfish (Clarias gariepinus): Growth, feed utilization efficiency, serum lysozyme activity, and total viable bacterial count in the gut. J Appl Aquac 0:1–13. https://doi.org/10.1080/10454438.2022.2034702
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 446:88–96. https://doi.org/10.1016/j.aquaculture.2015.03.014
Veerabadhran M, Manivel N, Sarvalingam B et al (2023) State-of-the-art review on the ecotoxicology, health hazards, and economic loss of the impact of microcystins and their ultrastructural cellular changes. Aquat Toxicol 256:106417. https://doi.org/10.1016/j.aquatox.2023.106417
Vicente IST, Fleuri LF, Carvalho PLPF et al (2019) Orange peel fragment improves antioxidant capacity and haematological profile of Nile tilapia subjected to heat/dissolved oxygen-induced stress. Aquac Res 50:80–92. https://doi.org/10.1111/are.13870
Vignesh S, Krishnaveni G, Walter Devaa JC et al (2022) Experimental challenge of the freshwater fish pathogen Aeromonas hydrophila Ah17 and its effect on snakehead murrel Channa striata. Aquac Int 30:1221–1238. https://doi.org/10.1007/s10499-022-00856-0
Vinosha M, Palanisamy S, Anjali R et al (2020) Sulfated galactan from Halymenia dilatata enhance the antioxidant properties and prevents Aeromonas hydrophila infection in tilapia fish: In vitro and in vivo study. Int J Biol Macromol 158:569–579. https://doi.org/10.1016/j.ijbiomac.2020.04.212
Wang E, Chen X, Wang K et al (2016) Plant polysaccharides used as immunostimulants enhance innate immune response and disease resistance against Aeromonas hydrophila infection in fish. Fish Shellfish Immunol 59:196–202. https://doi.org/10.1016/j.fsi.2016.10.039
Wang W, Cao Y, Li J et al (2023) The impact of osmotic stresses on the biofilm formation, immunodetection, and morphology of Aeromonas hydrophila. Microbiol Res 269:127301. https://doi.org/10.1016/j.micres.2023.127301
Watts JEM, Schreier HJ, Lanska L, Hale MS (2017) The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources. Sinks and Solutions Mar Drugs 15:158. https://doi.org/10.3390/md15060158
Wen X, Chu P, Xu J et al (2021) Combined effects of low temperature and salinity on the immune response, antioxidant capacity and lipid metabolism in the pufferfish (Takifugu fasciatus). Aquaculture 531:735866. https://doi.org/10.1016/j.aquaculture.2020.735866
Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5:51–66. https://doi.org/10.1038/nprot.2009.197
Wu Y, Gong Q, Fang H et al (2013) Effect of Sophora flavescens on non-specific immune response of tilapia (GIFT Oreochromis niloticus) and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol 34:220–227. https://doi.org/10.1016/j.fsi.2012.10.020
Xue M, Xiao Z, Li Y et al (2022) Isolation, Identification and Characteristics of Aeromonas caviae from Diseased Largemouth Bass (Micropterus salmoides). Fishes 7:119. https://doi.org/10.3390/fishes7030119
Yang J, Lin Y, Wei Z et al (2023) Edwardsiella ictaluri Almost Completely Occupies the Gut Microbiota of Fish Suffering from Enteric Septicemia of Catfish (Esc). Fishes 8:30. https://doi.org/10.3390/fishes8010030
Yazarlu O, Iranshahi M, Kashani HRK et al (2021) Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol Res 174:105841. https://doi.org/10.1016/j.phrs.2021.105841
Yeganeh S, Adel M, Nosratimovafagh A, Dawood MAO (2021) The Effect of Lactococcus lactis subsp. lactis PTCC 1403 on the Growth Performance, Digestive Enzymes Activity, Antioxidative Status, Immune Response, and Disease Resistance of Rainbow Trout (Oncorhynchus mykiss). Probiotics Antimicrob Proteins 13:1723–1733. https://doi.org/10.1007/s12602-021-09787-3
Yonar ME, Mişe Yonar S, İspir Ü, Ural MŞ (2019) Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish Shellfish Immunol 89:83–90. https://doi.org/10.1016/j.fsi.2019.03.038
Yuan X-Y, Liu M-Y, Cheng H-H et al (2019) Replacing fish meal with cottonseed meal protein hydrolysate affects amino acid metabolism via AMPK/SIRT1 and TOR signaling pathway of Megalobrama amblycephala. Aquaculture 510:225–233. https://doi.org/10.1016/j.aquaculture.2019.05.056
Zahran E, El Sebaei MG, Awadin W et al (2020) Withania somnifera dietary supplementation improves lipid profile, intestinal histomorphology in healthy Nile tilapia (Oreochromis niloticus), and modulates cytokines response to Streptococcus infection. Fish Shellfish Immunol 106:133–141. https://doi.org/10.1016/j.fsi.2020.07.056
Zanuzzo FS, Sabioni RE, Montoya LNF et al (2017) Aloe vera enhances the innate immune response of pacu (Piaractus mesopotamicus) after transport stress and combined heat killed Aeromonas hydrophila infection. Fish Shellfish Immunol 65:198–205. https://doi.org/10.1016/j.fsi.2017.04.013
Zeng S, Duan Y, Li X et al (2023) Effects of Cryptocaryon irritans infection on the histopathology, oxidative stress, immune response, and intestinal microbiota in the orange-spotted grouper Epinephelus coioides. Fish Shellfish Immunol 133:108562. https://doi.org/10.1016/j.fsi.2023.108562
Zhang W, Belton B, Edwards P et al (2022) Aquaculture will continue to depend more on land than sea. Nature 603:E2–E4. https://doi.org/10.1038/s41586-021-04331-3
Zhang X, Sun Z, Cai J et al (2020) Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Fish Shellfish Immunol 102:430–439. https://doi.org/10.1016/j.fsi.2020.04.051
Zhao X, Wang X, Li H et al (2023) Comparative Expression Profiling Reveals the Regulatory Effects of Dietary Mannan Oligosaccharides on the Intestinal Immune Response of Juvenile Megalobrama amblycephala against Aeromonas hydrophila Infection. Int J Mol Sci 24:2207. https://doi.org/10.3390/ijms24032207
Zhou C, Lin H, Ge X et al (2015) The Effects of dietary soybean isoflavones on growth, innate immune responses, hepatic antioxidant abilities and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol 43:158–166. https://doi.org/10.1016/j.fsi.2014.12.014
Zhou P, Huang H, Lu J et al (2021) The mutated Bacillus amyloliquefaciens strain shows high resistance to Aeromonas hydrophila and Aeromonas veronii in grass carp. Microbiol Res 250:126801. https://doi.org/10.1016/j.micres.2021.126801
Zhu W, Su J (2022) Immune functions of phagocytic blood cells in teleost. Rev Aquac 14:630–646. https://doi.org/10.1111/raq.12616
Acknowledgements
The first author Sivagaami Palaniyappan is grateful to “RUSA, 2.0-Biological Sciences, Bharathidasan University” for providing Project Fellowship (Ref. No. 02BDU/RUSA 2.0/TRP/BS/Date:22/04/2021). The authors are thankful to UGC-SAP-DRS-II (F.3–9/2013[SAP-II], Department of Science and Technology-Fund for Improvement of Science and Technology Infrastructure (DST-FIST) Level-I (stage-II) (Ref. No. SR/FST/LSI-647/2015(C) Date.11.08.2016) and Department of Science and Technology Promotion of University Research and Scientific Excellence (DST PURSE Phase—II) (Ref. No. SR/PURSE PHASE 2/16(G) /& 16(C) Date. 21.02.2017) of the Department of Animal Science, Bharathidasan University for the instrumentation facility.
Funding
This research work was supported by Rashtriya Uchchatar Shiksha Abhiyan (RUSA) – 2.0. Biological Sciences. Bharathidasan University, Tiruchirappalli, Tamil Nadu, India.
Author information
Authors and Affiliations
Contributions
S. P.: Methodology, Investigation, Formal analysis, Data curation, Writing – original draft. A. S.: Resources, Formal analysis, Writing– review & editing. Z. A. K. & G. T.: review & editing. T. R.: conceptualization, project administration, supervision, validation, visualization, writing – review and editing.
Corresponding author
Ethics declarations
Ethical approval
The animal study was reviewed and approved by Institutional Animal Ethical Committee (IACE) Department of Animal Science, Bharathidasan University, Tiruchirappalli – 620 024. Ref. No: BDU/IAEC/P19/2021.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Palaniyappan, S., Sridhar, A., Kari, Z.A. et al. Potentials of Aloe barbadensis inclusion in fish feeds on resilience to Aeromonas hydrophila infection in freshwater fish Labeo rohita. Fish Physiol Biochem 49, 1435–1459 (2023). https://doi.org/10.1007/s10695-023-01266-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01266-6