Abstract
The effect of β-glucans 1,3/1,6 from Saccharomyces cerevisiae yeast at different inclusion percentages (0.0, 0.2, 0.4, 0.6, and 0.8%) in the diet for tropical gar (Atractosteus tropicus) larvae was evaluated on growth, digestive enzyme activity and, relative expression of the immune system genes. The bioassay started on the third day after hatching (DAH) and lasted 21 days, using a total of 1500 larvae of 0.055 ± 0.008 g and, a total length of 2.46 ± 0.26 cm. Larviculture was carried out in a recirculation system with 15 tanks of 70 L using a density of 100 organisms per experimental unit. No significant differences in larval growth were observed by the inclusion of β-glucans (p > 0.05). Digestive enzymes showed changes in lipase and trypsin activities, presenting higher values in fish fed 0.6% and 0.8% β-glucans diets compared to the other treatments (p < 0.05). Leucine-aminopeptidase, chymotrypsin, acid phosphatase, and alkaline phosphatase activity showed higher activities in larvae fed with a 0.4% β-glucan diet compared to the control group. The relative expression of intestinal membrane integrity (mucin 2) muc-2, (occludins) occ, (nucleotide-binding oligomerization domain) nod-2, and immune system lys (lysosome) genes showed over-expression in larvae fed the 0.4% β-glucan diet to the rest of the treatments (p < 0.05). The inclusion of β-glucans at 0.4–0.6% in diets for A. tropicus larvae could improve larviculture, as effects on the increase in the activity of several digestive enzymes and the expression of genes of the immune system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, there is a market focused on improving the health and survival of fish by adding various types of prebiotics to the formulations. In fish, the most commonly used prebiotics have been GOS (galactooligosaccharides), MOS (mannan oligosaccharides), FOS (fructooligosaccharides), scFOS (short-chain fructooligosaccharides), XOS (xylooligosaccharides), AXOS (arabinoxilooligosacáridos) and inulin, as well as their mixes (Guerreiro et al. 2017). In addition, depending on the sources of the prebiotics, we find different beneficial attributes, such as fat and sugar substitution, improved palatability through tongue lubrication, and even improved immune response (Singla and Chakkaravarthi 2017; Khangwal and Shukla 2019) . The polysaccharide β-glucan (1,3/1,6) is one of aquaculture’s most widely used immunostimulants (Miest et al. 2016). This prebiotic is classified as a dietary fiber with particularities to improve the innate immune response; at the cytokine level, it promotes better growth and tolerance to stress, improving survival rates (Wood 2007; Pizarro et al. 2014; Dos Santos-Voloski et al. 2019; Revina et al. 2020).
Thus, β-glucans are a valuable and functional ingredient with essential properties at the physiological level, one of the main characteristics of β-glucans from the yeast Saccharomyces cerevisiae is the immunostimulant functions (Wilson et al. 2015). The effects of β-glucans have been evaluated in fish such as Danio rerio inoculated with the pathogen Aeromonas hydrophila, where their effect decreased mortality (Rodríguez et al. 2009), by stimulating β-glucans proteins such as chemokines and cytokines, which are involved in the process of attraction and migration of leukocytes involved as antigens (Nunes et al. 2016). Likewise, the addition of β-glucans increases the expression of the IFN-γ (Interferon-gamma) gene, which plays an important role in the innate immune response (Xu et al. 2019). On the other hand, the muc-2 gene is a mucin releaser to allow the maintenance of the gastric system regarding its lubrication by creating a barrier of protection and permeability against pathogens (Pelaseyed et al. 2014; Xue et al. 2015). Also, the occludins are membrane proteins with a hermetic function at the cytoplasmic level and allow the cellular reorganization of actin, which enables movement functions, cell division, phagocytosis, and external activities (García & Jay 2006; Edelblum & Turner 2015; Kuo et al. 2019). The nod-2 gene (Nucleotide-binding Oligomerization Domain Protein) is part of the intracellular family, expressed in the epithelial region, specifically in Paneth cells involved in stem cell function, intestinal morphogenesis, and mainly for being antimicrobial agents in the microbiota region (Caruso et al. 2014; Gassler 2017). On the other hand, lysozyme is part of the community of proteins associated with the innate immune system, produced from neutrophils and macrophages, which act as receptors of the NOD family for the degradation of the bacterial cell wall (Ragland and Criss 2017; Kawai et al. 2018).
On another note, in southeastern Mexico, there are native fish with the potential for aquaculture. Among them, the tropical gar (Atractosteus tropicus) is one of the essential freshwater species in the State of Tabasco. This species inhabits freshwater bodies with abundant vegetation (Miller et al. 2005). It is a culturally valued specimen, has a high productive potential in the region, and is an important biological model (Márquez-Couturier et al. 2006; Méndez-Marin et al. 2012; Vázquez-Navarrete 2015). Most of the work has focused on nutrition to develop specific feeds, such as the determination of protein and lipid requirements, the design of micro-encapsulated diets, live food substitution, and digestive physiology, among others (Frías-Quintana et al. 2016; Sáenz de Rodrigáñez et al. 2018; Huerta-Ortiz et al. 2018; Jiménez-Martínez et al. 2020). Recently, we began to explore the evaluation of functional additives in diets for A. tropicus juvenile by including β-glucans (1,3/1,6), where the inclusion between 1.0 and 1.5% improves growth, the response of immune system genes and increases the activity of alkaline proteases, trypsin, leucine aminopeptidase, and α-amylase (Nieves-Rodríguez et al. 2018) . Also, FOS as another of the selected additives in aquaculture, improving enzymatic activity (acid proteases, chymotrypsin, and leucine aminopeptidase), the expression of intestinal barrier genes (occ, muc2, nod2, β-actin, ef1-a) and the modification of morphology in the intestinal barrier, from 5 g kg−1 for the juvenile stage (Sepúlveda-Quiroz et al. 2020). Therefore, due to the potentiating effects it has achieved for the species, some others, such as MOS, have been tested with favorable results in growth rates, digestive enzymatic activity, and immune markers (Nájera-Arzola et al. 2018). Accordingly, the aim of the present work was to evaluate the effects on growth, digestive enzyme activity, and the expression of membrane integrity and immune system genes on A. tropicus larvae by the inclusion of different concentrations of dietary β-glucans (1,3/1,6).
Materials and methods
Obtaining organisms
The experiment was carried out in the Laboratory of Physiology in Aquatic Resources (LAFIRA) from the División Académica de Ciencias Biológicas (DACBiol) at Universidad Juárez Autónoma de Tabasco (UJAT). The organisms for the experiment were obtained from a batch of A. tropicus broodstock available in the laboratory (one 3.8 kg female and five 1.5 kg males), spawning occurred by inducing it with the synthetic hormone GnRH (Syncrophorte) applied intramuscularly (using 35 μg·kg fish−1).
Experimental design
In this experiment, the effect of β-glucans at inclusion percentages of 0%, 0.2%, 0.4%, 0.6%, and 0.8% (control diet; diet 0.2, diet 0.4, diet 0.6 and diet 0.8, respectively) on A. tropicus larvae was evaluated. The bioassay was carried out for 21 days (24 DAH) from hatching (3 DAH), and a total of 1500 organisms (100 fish per experimental unit by triplicate) were used. Larval feeding was carried out five times a day with apparent satiety (8:00, 10:30, 12:30, 15:30, and 18:30 h), according to the feeding scheme proposed by Frías-Quintana et al. (2015). The experiment was carried out in a recirculation system connected to 15 tanks of 70 L with a constant flow of 10 L min−1 and a biological filter. Water quality was monitored every day with a HANNA multiparameter probe (HI9829-00041), measuring temperature (30.6 ± 0.14 °C), pH (7.1 ± 0.04) and dissolved oxygen (5.4 ± 0.34 mg L−1). In addition, the surplus feed was siphoned off at the end of the day, and every 48 h, the system was replaced with 20% water.
Formulation and elaboration of experimental diets
Table 1 shows the formulation of the diets with the addition of the different percentages of β-glucans made from the base diet proposed by Frías-Quintana et al. (2015). Practical diets were prepared by the protocol proposed by Álvarez-González et al. (2001). Proximate analyses were determined according to the official methods of the Official Association of Analytical Chemistry (AOAC 1990). Ash was determined by heating the diet residue in a muffle at 550 °C for 24 h. Protein was determined by the Kjeldahl method using hydrochloric acid as a trapping medium and sodium hydroxide for quantification. Lipids were determined using the technique described by Bligh & Dyer (1959) and the nitrogen-free extract (NFE) described by Brett & Groves (1979).
Growth indexes
At the end of the bioassay, growth was evaluated using a digital scale (Ohaus HH120) for wet weight, and total length was measured with imageJ software (NIH, Bethesda, MD, USA). Likewise, the following zootechnical indexes were calculated according to the proposal of Tomás-Almenar et al. (2020).
Digestive enzyme activities
At the end of the experiment, three larvae per experimental unit were taken to determine the activity of digestive enzymes. The larvae were euthanized after a thermal shock (− 2 °C) and then dissected, the stomach and intestine were extracted. From the organs samples, the multi-enzyme extracts were prepared, for which the organs were homogenized in cold (4 °C) in a 1:10 weight/volume ratio in distilled water with an Ultra-Turrax homogenizer. The homogenized samples were centrifuged (12,000 g for 15 min at 4 °C), and the supernatant was separated into 60 μL aliquots in Eppendorf tubes to be frozen at − 80 °C until further use. The Bradford technique (1976) determined protein concentration using a bovine serum albumin curve as a standard. Acid protease activity was measured by the technique of Anson (1938), using hemoglobin (0.25%) as a substrate dissolved with buffer in 100 mmol L−1 glycine–HCl buffer at pH 2 and 37 °C. Alkaline protease activity by the technique described by Kunitz (1947) modified by Walter (1984) using casein (1%) as a substrate in 100 mmol L−1 tris–HCl buffer, 10 mmol L−1 CaCl2 (pH = 9.0) at 37 °C. In both techniques, 15 µl of extract, 15 min of reaction and reaction was stopped with 0.5 mL of 20% trichloroacetic. A unit of enzymatic activity was defined as 1 µg of tyrosine released by minute, a molar extinction coefficient (MEC) of 0.008 µM−1 cm−1 and reading at an absorbance of 280 nm were used. Trypsin activity by the technique of Erlanger et al. (1961) using 20 µL of enzyme extract in BAPNA (N-α-Benzoyl-DL-Arginine P-nitroanilide) 2 mmol L−1 substrate diluted in 50 mmol L−1 tris–HCL + CaCl2 buffer pH 8. Chymotrypsin activity was assayed by the technique described by Del Mar et al. (1979) using 15 µL of extract on SAPNA (Succinyl-(Ala)2-Pro-Phe-p-nitroanilide) substrate 1.25 mmol L−1 with 50 mmol L−1 tris–HCl buffer (pH 8.0) with a reaction time of 30 min. Leucine aminopeptidase activity was measured with the technique described by Maraux et al. (1973) using L-leucine-p-nitroanilide 1 mmol L−1 as substrate in sodium phosphate buffer 50 mmol L−1 at pH 7.8 and 37 °C for the techniques described above with 15 µl of extract with a reaction time 30 min. The measurement of these techniques was performed at an absorbance of 410 nm, using a molar extinction coefficient (MEC) of 9.64 µM−1 cm−1 for chymotrypsin and 0.0088 µM−1 cm−1 for trypsin and L-aminopeptidase.
Lipase activity was measured by the technique described by Versaw et al. (1989) using 10 µL of enzyme extract with β-naphthyl caprylate 100 mmol L−1 as substrate in tris–HCl buffer 50 mmol L−1 with sodium cholate 100 mmol L−1 at pH 7.5 at 37° C performed with an incubation time of 15 min, MEC of 0.02 µM−1 cm−1 and absorbance 540 nm was used. The α-amylase activity was calculated with the Somoyi-Nelson technique described by Robyt & Whelan (1968), using 15 µL of extract on starch (2%) as substrate in phosphate-sodium buffer 100 mmol L−1, NaCl 50 mmol L−1 at pH 7.5 with an incubation time of 30 min, an MEC 0.0034 µM−1 cm−1 and an absorbance of 600 nm. Finally, to measure acid and alkaline phosphatase activities, the technique of Bergmeyer et al. (1974) was applied using 2.04% 4-nitrophenylphosphate for acid phosphatases in citric acid and sodium citrate buffer (1:1 w/w) 0.1 mmol L−1 at pH 5.5, in glycine NaOH buffer 0.1 mmol L−1 at pH 10 for alkaline phosphatases, with an incubation time of 15 min, 10 µL of extract, an MEC 0.0185 µM−1 cm−1 and absorbance of 405 nm. The activities of all enzymes were calculated with the following equations: (1) Units per mL = (Δabs × final volume of the reaction (mL)) × (MEC × time (min) × enzymatic extract volume (mL)); (2) Units per mg of protein = Units per mL/mg of soluble protein.
Gene expression analysis
In the evaluation of the expression of genes involved in the barrier protection response nod-2 (Nucleotide-Binding and Oligomerization Domain), muc2 (Mucin2), and occ (Ocludin) and of the immune system lys (Lysosima), primers were designed from the transcriptome of A. tropicus (Martínez-Burguete et al. 2021) and ef1α (Elongation factor 1) and β-actin (Beta-Actin) were used as reference genes (Table 2), where the relative expression was calculated from the average of both reference genes compared to the control (Livak & Schmittgen 2001). For this purpose, three larvae (24 h of starvation) per experimental unit were sampled and euthanized with cold shock (− 2 °C) and preserved in RNA later (volume 1:10 volume:weight) for 24 h and then stored at ~ 80 °C until use. Total RNA was extracted with trizol reagent (Invitrogen®reagent code) according to the manufacturer’s instructions and total RNA was quantified spectrophotometrically (A260/280) using a nanodrop Jenway®. Subsequently, cDNA synthesis was performed from 1.5 µg of total RNA from the samples, under the specifications of the iScriptTM Reverse Transcription Supermix for RT-PCR kit (BIO-RAD®), using oligo dT and/or random primers in a final reaction volume of 20 µL per sample. The cDNA was stored at ~ 80 °C until further analysis. Quantification of relative gene expression was performed by qPCR analysis, using a CFX96 Touch™ Real-Time Thermal Cycler CFX96 (Bio-Rad) in a total volume reaction of 10 μL per sample, where 5 μL of SsoAdvancedTM Universal SYBR® Green Supermix (Bio-Rad), 4.5 μL cDNA, 0.5 μL of primers (10 μmol) were used. All reactions were performed using the following conditions: 1 cycle of 50 °C for 2 min, followed by 1 cycle 95 °C for 10 min, 40 cycles 95 °C for 15 s and 60 °C for 1 min, and ended with a melting curve under standard 60-cycle program conditions to confirm amplification of a single product in each reaction.
Statistical analysis
All data were analyzed using the normality test (Kolmogorov–Smirnov) and homoscedasticity (Levene). Growth data were analyzed by one-way analysis of variance (ANOVA), and the Tukey test was used as a post hoc test. Enzyme activity and gene expression analyses were performed using the Kruskal–Wallis nonparametric and Nemenyi test a posteriori. All analyses were performed in the Statistica 7 statistical program and a significance of p < 0.05 was used.
Results
Growth indexes
Table 3 shows the growth indexes A. tropicus larvae fed with the different inclusion percentages of β-glucans. A. tropicus larvae fed for 21 days with the different inclusion percentages of β-glucans showed no significant differences in growth (wet weight g and total length cm) with respect to larvae fed with control diet (p > 0.05). The other growth indexes also showed no significant differences compared to larvae fed with control treatment. Only in survival percentage of larvae fed with the control, 0.2% and 0.6% diets presented statistically significant higher values than larvae fed with the 0.4% and 0.8% β-glucan diets (p < 0.05).
Digestive enzyme activities
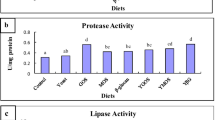
The activity of digestive enzymes in larvae fed with different percentages of β-glucan inclusion is shown in Table 4. The activity of acid and alkaline proteases was not modified by the inclusion of β-glucans (p > 0.05). On the other hand, the highest trypsin activity was detected in larvae fed with the 0.8% diet (608.67 ± 63.45 U mg protein−1) compared to larvae fed the control diet (189.13 ± 33 U mg protein−1; p < 0.05). The highest chymotrypsin (26.33 ± 3.32 U mg protein−1) and L-aminopeptidase (552.63 ± 38.06 U mg protein−1) activities were recorded in fish fed the 0.6% β-glucan diet relative to larvae fed the control diet (16.64 ± 1.05 U mg protein−1 and 214.64 ± 6.68 U mg protein−1, respectively; p < 0.05). On the other hand, the highest lipase activities were recorded in larvae fed the 0.6% and 0.8% β-glucan diet (3.56 ± 0.21 U mg protein−1 and 4.0 ± 0.46 U mg protein−1, respectively; p < 0.05). The highest acid and alkaline phosphatase activities were obtained in larvae fed the diet containing 0.4% β-glucans (0.71 ± 0.04 and 0.33 ± 0.04 U mg protein−1, respectively; p < 0.05). Finally, α-amylase activity did not show significant differences between treatments (p > 0.05).
Gene expression analysis
The relative expression of genes analyzed in A. tropicus larvae fed with different percentages of β-glucans is shown in Fig. 1. The expressions of muc-2 and occ showed the highest values for larvae fed with the inclusion of 0.4% β-glucans (14.75 ± 1.93 and 3.97 ± 0.30, respectively; p > 0.05; Fig. 1A and B). The highest lys expression (9.34 ± 0.39; p > 0.05) was detected for larvae fed with the inclusion of 0.4% β-glucans (Fig. 1C). The nod-2 gene had the highest expression (4.65 ± 1.05; p > 0.05) in larvae fed with the inclusion of 0.6% β-glucans (Fig. 1D).
Discussion
The use of β-glucans in A. tropicus larvae as a growth promoter did not show a direct effect; however, our results regarding SGR, FCR and survival are like those described by several authors when studying larviculture (Frías-Quintana et al. 2015; Palma-Cancino et al. 2019; Maytorena-Verdugo et al. 2022). In this regard, the study of Nieves-Rodríguez et al. (2018) with A. tropicus juveniles did not increased growth when using β-glucans as well as in other species, such as Oreochromis niloticus (Pilarski et al. 2017), Cyprinus carpio (Pionnier et al. 2013), and Morone chrysops x Morone saxatilis (Li et al. 2009). Low prebiotic concentrations typically will not directly increase growth (Dalmo and Bøgwald 2008) . Also, the metabolism of β-glucans to hydrolyze them from complex oligosaccharides to simple monosaccharides could only be performed by the action of some intestinal and pancreatic enzymes of the β-glucanase type mainly that are generally not present in carnivorous fish (Chen & Seviour 2007; González et al. 2011). Under this scenario, fish must have bacteria (microbiome) that can digest complex cell walls to simpler oligosaccharides, such as specific Bacteroides that use glycans as a substrate to be hydrolyzed by glycoside hydrolase (Temple et al. 2017). This capacity promotes the stability of the microbiome, thus linking the improvement of digestion and nutrient assimilation, as well as decreasing the damage caused by pathogens by improving the immune system (Ghanbari et al. 2015).
Considering the above-mentioned, β-glucans are polysaccharide chains and are structural components of the cell wall of various species such as yeast, cereals, fungi, bacteria, and some algae (Vetvicka et al. 2013). The types of bonds may vary according to the ownership of the sources, for example, the β-(1 → 3), (1 → 6)-glucan is specifically from yeast (Saccharomyces cerevisiae) and are activators of macrophage-mediated protective response, antioxidant effects, wound healing, and receptor-mediated immune cells (monocytes, lymphocytes, neutrophils, Langerhans cells) and non-immune cells (epithelial, endothelial and fibroblasts; Petravić-Tominac et al. 2010).
In addition, β-glucans from yeast form part of additives that promote beneficial effects in aquatic organisms (Dharsono et al. 2018). Thus, they have been evaluated in species such as Lutjanus peru by exposure of nanoparticles of 5 mg mL−1 of saline solution added to 100 mg of zinc hydrochloride; the results showed an improved cellular immune response, measuring levels of the enzyme superoxide dismutase, catalase, and peroxidase (Velazquez-Carriles et al. 2018). Likewise, its addition improved the viability of ZT4 cells (10 µg mL−1 for 24 h) that are targeted to glycoproteins to inhibit SVCV (carp virus) replication in a challenge tested with Danio rerio (Medina-Gali et al. 2018). Another effect when β-glucans are used was related as an agent against enteritis disorder in Oncorhynchus mykiss and Pagrus major at concentrations of 0.0%, 0.1%, and 0.2% over a 30-day period, which improved blood parameters, antioxidant capacity, stress tolerance, and growth rate performance (Ji et al. 2019; Shadrack et al. 2022). In crustaceans such as Litopenaeus vannamei (Álvarez-Sánchez et al. 2018), Portunus pelagicus (Anjugam et al. 2016), and Cherax tenuimanus (Sang and Fotedar 2010) its use has been successful with concentrations higher than 0.1% with beneficial results on the immune system.
One aspect to highlight is the increase in chymotrypsin-like and L-aminopeptidase activities, which showed the highest values for A. tropicus larvae fed with 0.4% β-glucans. This enhancement in digestive enzyme activities (proteases, lipases, α-amylase, and phosphatases) allows the larvae to take better advantage of the protein during the feeding changes; from the moment of the first feeding with live prey (Artemia sp. nauplii), the mixing of Artemia sp. biomass with the balanced feed and the weaning process of A. tropicus larvae, which present feeding habits typical of a carnivore in its early life stage (Frías-Quintana et al. 2015). In this aspect, the activity of digestive enzymes has been evaluated in several species, such as Lutjanus peru (Guzmán-Villanueva et al. 2014), Scophthalmus maximus (Miest et al. 2016) and Cyprinus carpio (Mohammadian et al. 2019). It should be noted that incremental effects of digestive enzyme activities are shown by including β-glucans, which is related to a more diverse microbial composition and improves gastric functions, absorption, intestinal digestion, and intestinal cell integrity (Dimitroglou et al. 2011; Santos et al. 2020). A study reported in Carassius auratus var. Pengze by Cao et al. (2019) shows increased lipase, trypsin, acid phosphatase, and alkaline phosphatase activities, indicating increased functionality of enterocytes (Dimitroglou et al. 2011). In addition, phosphatase activities are related to the transphosphorylation of phosphate mono esters and are considered essential for metabolic regulatory capacity, energy metabolism, and translation of cellular signaling pathways (Arias-Moscoso and Ezquerra-Brauer 2015).
As for α-amylase activity, it does not show a significant difference with the inclusion of β-glucans. In this sense, Liranço et al. (2013) reported a decrease in activity in juveniles of Oreochromis niloticus since this enzyme degrades starches and glycogen from the cleavage of the α-glucosidic bond (Singh et al. 2012). Hence, the presence of β-glucan is a non-digestible carbohydrate, which has a different effect by reaching into the microbiota region and stimulating the secretion of short-chain fatty acids from bacterial fermentation (Wang et al. 2015). Regarding lipase in A. tropicus larvae, an increase in its activity was observed, which would be related to the greater capacity to break the ester bond of fatty acids and promote an improvement in lipid metabolism (Dawood et al. 2020). A trial with Pagellus acarne coincides that the implementation of prebiotics is related to greater availability of nutrients from the increase of digestive enzymatic activities, which promotes an increase in feed efficiency (Zaineldin et al. 2018). Furthermore, Sarao & Arora (2017) relate this effect to the modification of colonic bacteria and increased fermentation by microbial metabolism. In this same sense, it is mentioned that the use of prebiotics, such as β-glucans, can contribute to the increase of enzymatic activity of lipases, trypsin, proteases, and cellulases, this is due to the bacterial community by the production of extracellular enzymes as demonstrated in Lutjanus peru (Guzmán-Villanueva et al. 2014). However, the benefits of prebiotics may vary in each species, from stimulation of enzymatic activity, improvement of intestinal morphology, greater resistance to infections, increased growth, improved body composition, and a better balance of microbial communities (Merrifield et al. 2010).
On the other hand, β-glucans can stimulate humoral factors by recognizing damage-associated molecular patterns (DAMPs), which causes an energetic response against bacterial infections (Reis et al. 2021). Thus, an immunostimulant’s effects are to increase the protective response mechanisms, which reduces the effects of immune suppressors (stress, bacteria, contaminants, parasites, among others) through phagocytic activities, releasing nutrients, activating proteins and antioxidants (Kiron 2012). The first line of defense of the innate immune response is the mucus in fish, and it is known that in the case of β-glucans, they can increase the expression of mucins, which have the purpose of increasing mucus production (Anadón et al. 2019).
The muc-2 (Mucin-2) gene expresses a glycoprotein from epithelial cells, which creates a defense network from cell–matrix signaling and cytokines (Wang & El-Bahrawy 2014). Thus, by including 0.2 and 0.4% β-glucans in A. tropicus diets, the muc-2 expression increased significantly. In this aspect, Shelby et al. (2009) argue that in Oreochromis niloticus juvenile trial, the prolonged use of β-glucans can cause only a transitory effect, just as high doses reach to inhibit the immune effects, which response to the discrepancies to establish specific benefits in other teleosts. Similarly, the expression of occ (Occludin) and lys (Lysozyme) genes in A. tropicus larvae fed 0.4% β-glucan increases the expression of both genes. This increase in expressions upon additional β-glucan has been reported in other fish species, such as Cyprinus carpio, Oreochromis niloticus, and Danio rerio, as this prebiotic can stimulate immune activity with the action of lysozyme protein (Wang et al. 2017; Carballo et al. 2018; Nguyen et al. 2019). These proteins have a bactericidal effect, associated with the monocyte and macrophage system located in the mucus and lymphoid organs (kidney, gills, skin, thymus) and plasma, which is reflected by inhibition of the growth and invasion of pathogens (Dawood et al. 2015; Nayak et al. 2018). In teleosts, the claudin family is broad due to physiological plasticity; these molecules are found in higher amounts in epithelial tissues of the skin and gills, as these organs are exposed to water and are vulnerable to environmental changes (Kolosov et al. 2013). Unlike claudins, which are essential for macromolecular cohesion, the Occludin protein transcends in the assembly of such junctions, i.e., they maintain the stability of the intercellular barrier function of the plasma membrane, as it maintains its cohesion, which prevents cell diffusion and prevents the passage of harmful substances through intestinal cells in organisms (Campbell et al. 2017).
Relatedly, the nod-2 gene was overexpressed in A. tropicus larvae fed 0.6% β-glucans. The expression of nod-2 is known to synthesize a cytoplasmic sensor protein which has been previously reported in several teleost species associated with the gut microbiota (Azad et al. 2012). This molecule participates in the innate detection of potential pathogens, promotes the release of bacterial peptidoglycans, and regulates proinflammatory responses and intramacrophage survival (Zou et al. 2016). Likewise, its activity has been observed in enterocytes in Danio rerio, where its overexpression potentiated the recognition of inflammatory intestinal diseases (Salinas and Parra 2015) . Likewise, it has been demonstrated that β-glucans increase the immune and protective capacity against SVCV (Spring Viremia of Carp Virus) infection from the action of cytokines (Medina-Gali et al. 2018). Two trials conducted with Cyprinus carpio fed with β-glucans (6 mg per kg body weight) for 14 days and subsequently an intraperitoneal injection with Aeromonas salmonicida showed interesting results, where the highest level of cytokine expression was recorded after 6 h of sampling and subsequently reporting a decrease in gene transcript levels in intestine and kidney (Falco et al. 2012). The second assay showed peaks in the expression of C-reactive protein (CRP) at 6 h and 12 h for a subsequent decrease (Pionnier et al. 2013); both authors conclude that β-glucans promote resistance against bacterial infections. Similarly, in Pseudosciaena crocease, an experiment was conducted with diets supplemented with 0%, 0.09%, and 0.18% β-glucan for 4 weeks, where the 0.09% dose showed an increase in the phagocytic burst, respiratory and a lower mortality rate (Ai et al. 2007).
When comparing our results with that of Nieves-Rodríguez et al. (2018) in A. tropicus juveniles, similarities are observed by detecting an increase in the expression of immune response genes (il-10, tgf, muc2 and occ) with the intermediate supplementation of 1.5% of β-glucan, while the higher dose of 2% decreases the level of expression. Thus, overexpression in A. tropicus larvae was very clear at lower concentrations (0.4–0.6% of β-glucan) than with juveniles. However, physiological responses are not always clear since this depends on the dose and type of prebiotic administered, the developmental stage of the fish, and the infectious agent, among others, so that when the prebiotic is properly applied, an inflammatory response is enhanced as mentioned by Anderson (1992) , who indicate that if prebiotic doses are exceeded, it can cause immunosuppression due to interference in cytokines (Dung et al. 2021).
Conclusion
The inclusion of β-glucans in feed for A. tropicus larvae did not show a significant improvement in growth rates; however, an increase in lipase and trypsin activity was detected with the inclusion of 0.4% and 0.6% of β-glucans. Likewise, L-aminopeptidase, chymotrypsin, acid, and alkaline phosphatase activities increased with the addition of 0.4% of the prebiotic. The overexpression patterns of muc, lys, and occ may be associated with intestinal barrier maintenance, lubrication, and defense when using 0.2% and 0.4% of β-glucan, while the nod-2 gene increased its expression with supplementation of 0.6% of the prebiotic. Therefore, including doses between 0.4 and 0.6% of β-glucan in the diet for A. tropicus larvae improves physiological functions associated with the activity of digestive enzymes and the expression of genes associated with the intestinal barrier and immune system. Therefore, further studies are required to evaluate the microbiome and antioxidant capacity modification by means of stress tests and/or bacterial infections.
Data availability
The authors declare that the data supporting the findings of this study are available within the article.
References
Ai Q, Mai K, Zhang L, Tan B, Zhang W, Xu W, Li H (2007) Effects of dietary β-1, 3 glucans on innate immune response of large yellow croaker, Pseudosciaena crocea. Fish & Shellfish Immunol 22:394–402. https://doi.org/10.1016/j.fsi.2006.06.011
Álvarez-González CA, Civera-Cerecedo R, Ortiz-Galindo JL, Dumas S, Moreno-Legorreta M, Grayeb-Del AT (2001) Effect of dietary protein level on growth and body composition of juvenile spotted sand bass, (Paralabrax maculatofasciatus), fed practical diets. Aquac 194:151–159. https://doi.org/10.1016/S0044-8486(00)00512-3
Álvarez-Sánchez AR, Nolasco-Soria H, Peña-Rodríguez A, Mejía-Ruíz H (2018) In vitro digestibility of Yarrowia lipolytica yeast and growth performance in whiteleg shrimp Litopenaeus vannamei. Turk. J.F. Aquat Sci 18:395–404. https://doi.org/10.4194/1303-2712-v18_3_05
Anadón A, Ares I, Martínez-Larrañaga MR, Martínez MA (2019) Prebiotics and probiotics in feed and animal health. Nutrac Vet Medic 261–285. https://doi.org/10.1007/978-3-030-04624-8_19
Anderson DP (1992) Immunostimulants, adjuvants, and vaccine carriers in fish: applications to aquaculture. Ann Rev Fish Dis 2:281–307. https://doi.org/10.1016/0959-8030(92)90067-8
Anjugam M, Iswarya A, Vaseeharan B (2016) Multifunctional role of β-1, 3 glucan binding protein purified from the hemocytes of blue swimmer crab Portunus pelagicus and in vitro antibacterial activity of its reaction product. Fish Shellfish Immunol 48:196–205. https://doi.org/10.1016/j.fsi.2015.11.023
Anson ML (1938) The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J Gen Physiol 22:79–89. https://doi.org/10.1085/jgp.22.1.79
AOAC (1990) Official Methods of Analysis of the Association of Official Analytical Chemists, 15th edn. Washington, DC, USA., Association of Official Analytical Chemists
Arias-Moscoso JL, Ezquerra-Brauer JM (2015) Shrimp alkaline phosphatase: structure, characteristics, and functions. Biotecnia. 17(1):47–54. https://doi.org/10.18633/bt.v17i1.19
Azad AK, Sadee W, Schlesinger LS (2012) Innate immune gene polymorphisms in tuberculosis. Infect Immun 80:3343–3359. https://doi.org/10.1128/IAI.00443-12
Bergmeyer HU, Bernt E, Lachenicht R (1974) Alkaline phosphatase in serum determination with automatic analysers. In Methods enzymatic anal. 864–868. Academic Press. https://doi.org/10.1016/B978-0-12-091302-2.50069-4
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917. https://doi.org/10.1139/o59-099
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brett JR, Groves TDD (1979) Physiological energetics. In Bioenergetics and Growth. Eds Hoar WS, Randall DJ, Brett JR. Fish. Physiol. 8(6):1–786. https://doi.org/10.1016/S1546-5098(08)60029-1
Campbell HK, Maiers JL, De Mali KA (2017) Interplay between tight junctions & adherens junctions. Exp Cell Res 358(1):39–44. https://doi.org/10.1016/j.yexcr.2017.03.061
Cao H, Yu R, Zhang Y, Hu B, Jian S, Wen C, Yang G (2019) Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze). Aquac 508:106–112. https://doi.org/10.1016/j.aquaculture.2019.04.064
Carballo C, Chronopoulou EG, Letsiou S, Maya C, Labrou NE, Infante C, Manchado M (2018) Antioxidant capacity and immunomodulatory effects of a chrysolaminarin-enriched extract in Senegalese sole. Fish & Shellfish Immunol 82:1–8. https://doi.org/10.1016/j.fsi.2018.07.052
Caruso R, Warner N, Inohara N, Núñez G (2014) NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 41:898–908. https://doi.org/10.1016/j.immuni.2014.12.010
Chen J, Seviour R (2007) Medicinal importance of fungal beta-(1→3), (1→6)-glucans. Mycol Res 111(6):635–652. https://doi.org/10.1016/j.mycres.2007.02.011
Dalmo RA, Bøgwald J (2008) ß-glucans as conductors of immune symphonies. Fish & Shellfish Immunol 25(4):384–396. https://doi.org/10.1016/j.fsi.2008.04.008
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S, El Basuini MF, Hossain MS, Wei H (2015) Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream. Pagrus Major Aquac Nutri 23(1):148–159. https://doi.org/10.1111/anu.12376
Dawood MAO, Magouz FI, Salem MFI (2020) Synergetic effects of Lactobacillus plantarum and β-Glucan on digestive enzyme activity, intestinal morphology, growth, fatty acid, and glucose-related gene expression of genetically improved farmed tilapia. Probiotics & Antimicro. Prot 12(2):389–399. https://doi.org/10.1007/s12602-019-09552-7
DelMar EG, Largman C, Broderick JW, Geokas MC (1979) A sensitive new substrate for chymotrypsin. Anal Biochem 99:316–320. https://doi.org/10.1016/S0003-2697(79)80013-5
Dharsono T, Rudnicka K, Wilhelm M, Schoen C (2018) Effects of yeast (1,3)-(1,6)-beta-glucan on severity of upper respiratory tract infections: a double-blind, randomized, placebo-controlled study in healthy subjects.". J Am Coll of Nutrit 38(1):40–50. https://doi.org/10.1080/07315724.2018.1478339
Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, Daniels C, Davies SJ (2011) Microbial manipulations to improve fish health and production – a Mediterranean perspective. Fish & Shellfish Immunol 30(1):1–16. https://doi.org/10.1016/j.fsi.2010.08.009
dos Reis IC, Fierro-Castro C, Gonçalves GS, Moromizato BS, Tort L, Biller JD (2021) β- glucan mimics tissue damage signaling and generates a compensation between the head kidney and spleen to activate acquired immunity in vaccinated tilapia (Oreochromis niloticus). Fish and Shellfish Immunol 117:179–187. https://doi.org/10.1016/j.fsi.2021.08.003
Dos Santos-Voloski AP, de Figueiredo-Soveral L, Dazzi CC, Sutili F, Frandoloso R, Kreutz LC (2019) β-Glucan improves wound healing in silver catfish (Rhamdia quelen). Fish & Shellfish Immunol 93:575–579. https://doi.org/10.1016/j.fsi.2019.08.010
Dung NT, Trong TD, Vu NT, Binh NT, Minh TTL, Luan LQ (2021) Radiation synthesis of selenium nanoparticles capped with β-Glucan and its immunostimulant activity in cytoxan-induced immunosuppressed Mice. Nanomaterials 11(9):24–39. https://doi.org/10.3390/nano11092439
Edelblum KL, Turner JR (2015) Epithelial cells: structure, transport, and barrier function. In Mucosal Immunology. Eds. Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN. Academic Press. 187–210. https://doi.org/10.1016/B978-0-12-415847-4.00012-4
Erlanger BF, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95(2):271–278. https://doi.org/10.1016/0003-9861(61)90145-X
Falco A, Frost P, Miest J, Pionnier N, Irnazarow I, Hoole D (2012) Reduced inflammatory response to Aeromonas salmonicida infection in common carp (Cyprinus carpio L.) fed with β-glucan supplements. Fish & Shellfish Immunol 32:1051–1057. https://doi.org/10.1016/j.fsi.2012.02.028
Frías-Quintana CA, Márquez-Couturier G, Alvarez-González CA, Tovar-Ramírez D, Nolasco-Soria H, Galaviz-Espinosa MA, Gisbert E (2015) Development of digestive tract and enzyme activities during the early ontogeny of the tropical gar Atractosteus tropicus. Fish Physiol and Biochem 41(5):1075–1091. https://doi.org/10.1007/s10695-015-0070-9
Frías-Quintana CA, Domínguez-Lorenzo J, Álvarez-González CA, Tovar-Ramírez D, Martínez-García R (2016) Using cornstarch in microparticulate diets for larvicultured tropical gar (Atractosteus tropicus). Fish Physiol and Biochem 42(2):517–528. https://doi.org/10.1007/s10695-015-0156-4
García E, Jay D (2006) Platelet filamin: a cytoskeletal protein involved in cell signal integration and function. Arch. Cardio de Méx. 76(S4):67–75. https://www.medigraphic.com/pdfs/archi/ac-2006/acs064g.pdf (Access 27 December 2022)
Gassler N (2017) Paneth cells in intestinal physiology and pathophysiology. W J Gastrointestinal Pathophysiol 8(4):150. https://doi.org/10.4291/wjgp.v8.i4.150
Ghanbari M, Kneifel W, Domig KJ (2015) A new view of the fish gut microbiome: advances from next-generation sequencing. Aquac 448:464–475. https://doi.org/10.1016/j.aquaculture.2015.06.033
González I, Infante D, Peteira B, Martínez B, Arias Y, González N, Miranda I (2011) Caracterización bioquímica de aislamientos de Trichoderma spp. promisorios como agentes de control biológico. II. Expresión de actividad glucanasa. Rev. Protec. Veg. 26(1):23–29. http://scielo.sld.cu/pdf/rpv/v26n1/rpv04111.pdf (Access 27 December 2022).
Guerreiro I, Oliva-Teles A, Enes P (2017) Prebiotics as functional ingredients: focus on Mediterranean fish aquaculture. Rev in Aquac 10(4):800–832. https://doi.org/10.1111/raq.12201
Guzmán-Villanueva LT, Ascencio-Valle F, Macías-Rodríguez ME (2014) Effects of dietary β-1,3/1,6-glucan on the antioxidant and digestive enzyme activities of Pacific red snapper (Lutjanus peru) after exposure to lipopolysaccharides. Fish Physiol Biochem 40(3):827–837. https://doi.org/10.1007/s10695-013-9889-0
Huerta-Ortiz M, Álvarez-González CA, Civera-Cerecedo R, Martínez-García R, Camarillo-Coop S, Goytortúa-Bores E, Pérez-Morales A (2018) Optimum level of dietary lipids for growth, chemical composition, and apparent digestibility of lipids for Atractosteus tropicus. Lat Am J Aquatic Res 46(5):1073–1082. https://doi.org/10.3856/vol46-issue5-fulltext-19
Ji L, Fu S, Ji R, Li X, Liu Y (2019) β-glucan mitigated trinitrobenzene sulfonic acid-induced enteritis in the rainbow trout (Oncorhynchus mykiss). Aquac 513:734–393. https://doi.org/10.1016/j.aquaculture.2019.734393
Jiménez-Martínez LD, Tovar-Ramírez D, Álvarez-González CA, Peña-Marín E, Camarillo-Coop S, Martínez-García R, Concha-Frías B (2020) Assessment of dietary lipid sources in tropical gar, Atractosteus tropicus larvae: growth parameters and intermediary lipogenic gene expression. Aquac Res 51(7):2629–2640. https://doi.org/10.1111/are.14603
Kawai Y, Mickiewicz K, Errington J (2018) Lysozyme counteracts β-lactam antibiotics by promoting the emergence of L-form bacteria. Cell 172(5):1038–1049. https://doi.org/10.1016/j.cell.2018.01.021
Khangwal I, Shukla P (2019) Potential prebiotics and their transmission mechanisms: recent approaches. J Food Drug Anal 27(3):649–656. https://doi.org/10.1016/j.jfda.2019.02.003
Kiron V (2012) Fish immune system and its nutritional modulation for preventive health care. Anim Feed Sci and Technol 173(1–2):111–133. https://doi.org/10.1016/j.anifeedsci.2011.12.015
Kolosov D, Bui P, Chasiotis H, Kelly SP (2013) Claudins in Teleost Fishes. Tissue Barriers 1(3):e25-391. https://doi.org/10.4161/tisb.25391
Kunitz M (1947) Crystalline soybean trypsin inhibitor: II General Properties. J G Physiol 30(4):291–310. https://doi.org/10.1085/jgp.30.4.291
Kuo WT, Shen L, Zuo L, Shashikanth N, Ong MLD, Wu L, Turner JR (2019) Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression. Gastroenterol 157(5):1323–1337. https://doi.org/10.1053/j.gastro.2019.07.058
Li P, Wen Q, Gatlin DM (2009) Dose-dependent influences of dietary β-1,3-glucan on innate immunity and disease resistance in hybrid striped bass. Morone chrysops x Morone saxatilis. Aquac Res 40(14):1578–1584. https://doi.org/10.1111/j.1365-2109.2009.02257.x
Liranço ADDS, Ciarlini PC, Moraes G, Camargo ALS, Romagosa E (2013) Mannanoligosaccharide (mos) and β-glucan (β-glu) in dietary supplementation for Nile tilapia juveniles dept in cages. Pan-American J. Aquatic. Sci. 8(2):112–125. https://panamjas.org/pdf_artigos/PANAMJAS_8(2)_112-125.pdf (Access 27 December 2022).
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) Methods. San Diego 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Maraux S, Louvard D, Baratti J (1973) The aminopeptidase from hog-intestinal brush border. Biochem Biophys Acta 321:282–295. https://doi.org/10.1016/0005-2744(73)90083-1
Márquez-Couturier G, Alvarez-González CA, Contreras-Sánchez WM, Hernández-Vidal U, Hernández-Franyutti AA, Mendoza-Alfaro RE, Aguilera-González C, García-Galano T, Civera-Cerecedo R, Goytortua-Bores E (2006) Avances en la Alimentación y Nutrición del Pejelagarto Atractosteus Tropicus. En: Editores: L. Elizabeth Cruz Suárez, Denis Ricque Marie, Mireya Tapia Salazar, Martha G. Nieto López, David A. Villarreal Cavazos, Ana C. Puello Cruz y Armando García Ortega. Avances en Nutrición Acuícola VIII .VIII Simposium Internacional de Nutrición Acuícola. 15 - 17 Noviembre. Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, México. ISBN 970–694–333–5. https://nutricionacuicola.uanl.mx/index.php/acu/article/view/181 (Access 27 December 2022)
Martínez-Burguete T, Peña-Marin ES, García-Gasca A, Alvarez-González CA, Llera-Herrera R (2021) Nutrigenomic marker discovery by de novo transcriptomic sequencing during early development of the tropical gar (Atractosteus tropicus). Aquac Res 52(8):3829–3842. https://doi.org/10.1111/are.15228
Maytorena-Verdugo CI, Peña-Marín ES, Alvarez-Villagómez CS, Pérez-Jiménez GM, Sepúlveda-Quiroz CA, Alvarez-González CA (2022) Inclusion of mannan-oligosaccharides in diets for tropical gar Atractosteus tropicus larvae: effects on growth, digestive enzymes, and expression of intestinal barrier genes. Fishes. 7(3)-127. https://doi.org/10.3390/fishes7030127
Medina-Gali RM, del Mar O-V, Mercado L, Novoa B, Coll J, Perez L (2018) Beta-glucan enhances the response to SVCV infection in zebrafish. Develop Comparative Immunolo 84:307–314. https://doi.org/10.1016/j.dci.2018.02.019
Méndez-Marin O, Hernández-Franyutti A, Álvarez-González CA, Contreras-Sánchez WM, Uribe-Aranzábal M (2012) Histología del ciclo reproductor de hembras del pejelagarto Atractosteus tropicus (Lepisosteiformes: Lepisosteidae) en Tabasco, México. Rev. Biol. Trop. 60(4):1857–1871. https://revistas.ucr.ac.cr/index.php/rbt/article/view/2186/53198 (Access 27 December 2022).
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bøgwald J, Ringø E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquac 302(1–2):1–18. https://doi.org/10.1016/j.aquaculture.2010.02.007
Miest JJ, Arndt C, Adamek M, Steinhagen D, Reusch TBH (2016) Dietary β-glucan (MacroGard®) enhances survival of first feeding turbot (Scophthalmus maximus) larvae by altering immunity, metabolism, and microbiota. Fish Shellfish Immunol 48:94–104. https://doi.org/10.1016/j.fsi.2015.11.013
Miller RR, Minckley WL, Norris SM (2005) Freshwater fishes of Mexico. University of Chicago. Association with the Museum of Zool. Univ. of Michigan. No. QL 629. M54
Mohammadian T, Nasirpour M, Tabandeh MR, Mesbah M (2019) Synbiotic effects of β-glucan, mannan oligosaccharide and Lactobacillus casei on growth performance, intestine enzymes activities, immune-hematological parameters, and immune-related gene expression in Common carp, Cyprinus carpio: an experimental infection with Aeromonas hydrophila. Aquac 511:634–197. https://doi.org/10.1016/j.aquaculture.2019.06.011
Nájera-Arzola IC, Álvarez-González CA, Frías-Quintana CA, Peña E, Martínez-García R, Camarillo-Coop S, Gisbert E (2018) Evaluación de oligosacáridos de manano (MOS) en dietas balanceadas para juveniles de pejelagarto (Atractosteus tropicus). Hidrobiológica 28(3):239–246. https://doi.org/10.24275/uam/izt/dcbs/hidro/2018v28n3/pena
Nayak S, Khozin-Goldberg I, Cohen G, Zilberg D (2018) Dietary supplementation with ω6 LC-PUFA-rich algae modulates zebrafish immune function and improves resistance to Streptococcal Infection. Frontiers in Immunol 9:1960. https://doi.org/10.3389/fimmu.2018.01960
Nguyen TM, Mandiki SNM, Tran TNT, Larondelle Y, Mellery J, Mignolet E, Kestemont P (2019) Growth performance and immune status in common carp Cyprinus carpio as affected by vegetable oil-based diets supplemented with β-glucan. Fish and Shellfish Immunol 92:288–299. https://doi.org/10.1016/j.fsi.2019.06.011
Nieves-Rodríguez KN, Álvarez-González CA, Peña-Marín E, Vega-Villasante F, Martínez-García R, Camarillo-Coop S, Tovar-Ramírez D, Guzmán-Villanueva L (2018) Effect of-glucans in diets on growth, survival, digestive enzyme activity, and immune system and intestinal barrier gene expression for tropical gar (Atractosteus tropicus) juveniles. Fishes Villahermosa Tabasco 33(3):27. https://doi.org/10.3390/fishes3030027
Nunes AC, Sena MM, Garcia SB, Pereira APL, Trugilo KP, Watanabe MAE, De Oliveira KB (2016) Análise da deleção 32 do receptor de quimiocina CCR5 em descendentes asiáticos em Maringá-Paraná. Biosaúde, 15(1):12–21. http://rdu.unicesumar.edu.br/handle/123456789/4672 (Access 27 December 2022)
Palma-Cancino DJ, Martínez-García R, Álvarez-González CA, Camarillo-Coop S, Peña-Marín ES (2019) Evaluation of feeding strategies in tropical gar (Atractosteus tropicus Gill) larvae growth, survival and cannibalism. Ecosys Rec Agropecuario 6(17):273–281. https://doi.org/10.19136/era.a6n17.2092
Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GMH, Schütte A, Hansson GC (2014) The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 260(1):8–20. https://doi.org/10.1111/imr.12182
Petravić-Tominac V, Zechner-Krpan V, Grba S, Srečec S, Panjkota-Krbavčić I, Vidović L (2010) Biological effects of yeast β-glucans. Agricul. Conspectus Sci 75(4):149–158. https://hrcak.srce.hr/file/98743 (Access 27 December 2022)
Pilarski F, Ferreira de Oliveira CA, Darpossolo de Souza FPB, Zanuzzo FS (2017) Different β-glucans improve growth performance and bacterial resistance in Nile tilapia. Fish and Shellfish Immunol 70:25–29. https://doi.org/10.1016/j.fsi.2017.06.059
Pionnier N, Falco A, Miest J, Frost P, Irnazarow I, Shrive A, Hoole D (2013) Dietary β-glucan stimulate complement and C-reactive protein acute phase responses in common carp (Cyprinus carpio) during an Aeromonas salmonicida infection. Fish & Shellfish Immunol 34(1):819–831. https://doi.org/10.1016/j.fsi.2012.12.017
Pizarro S, Ronco AM, Gotteland M (2014) Betaglucanos: ¿Qué tipos existen y cuáles son sus beneficios en la salud? Revista Chilena De Nutrición 41(4):4339–4446. https://doi.org/10.4067/S0717-75182014000400014
Ragland SA, Criss, AK (2017) From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLoS pathogens 13(9):e-100 6512. https://doi.org/10.1371/journal.ppat.1006512
Rai AK, Saikia P, Mech B (2013) Histochemical localization of alkaline phosphatase activity during cutaneous wound healing in a catfish under acid stress. Int J Sci Res Publ 3:1–9. https://www.ijsrp.org/research-paper-0813/ijsrp-p20112.pdf (Access 27 December 2022).
Revina O, Avsejenko J, Revins V, Sargautis D, Cīrule D, Valdovska A (2020) Effect of dietary supplementation with β-glucan on growth performance and skin-mucus microbiota of sea trout (Salmo trutta). Fisheries & Aquatic Life 28(3):155–165. https://doi.org/10.2478/aopf-2020-0019
Robyt JF, Whelan W (1968) Amylases starch and its derivates; Readlet, J.A., Ed, Chapman and Hall: London, UK. 430–476
Rodríguez I, Chamorro R, Novoa B, Figueras A (2009) β-Glucan administration enhances disease resistance and some innate immune responses in zebrafish (Danio rerio). Fish & Shellfish Immunol 27(2):369–373. https://doi.org/10.1016/j.fsi.2009.02.007
Sáenz de Rodrigáñez M, Aguilar-Tellez FV, Alarcón-López FJ, Pedrosa-Islas R, Peña-Marín ES, Martínez-García R, Álvarez-González CA (2018) Alimentos microencapsulados para el cultivo de larvas de pejelagarto (Atractosteus tropicus). Revista de Biología Tropical 66(3):1298–1313. https://doi.org/10.15517/rbt.v66i3.31727
Salinas I, Parra D (2015) Fish mucosal immunity: intestine. Mucosal Health in Aquac. 135–170. https://doi.org/10.1016/B978-0-12-417186-2.00006-6
Sang HM, Fotedar R (2010) Effects of dietary β – 1,3 – glucan on the growth, survival, physiological and immune response of marron, Cherax tenuimanus (Smith, 1912). Fish Shellfish Immunol 28(5–6):957–960. https://doi.org/10.1016/j.fsi.2010.01.020
Santos W, Costa L, López-Olmeda J, Costa N, Santos F, Oliveira C, Ribeiro P (2020) Dietary protein modulates digestive enzyme activities and gene expression in red tilapia juveniles. Animal 14(9):1802–1810. https://doi.org/10.1017/S1751731120000543
Sarao LK, Arora M (2017) Probiotics, prebiotics, and microencapsulation: a review. Critical Rev Food Sci Nutrition 57(2):344–371. https://doi.org/10.1080/10408398.2014.887055
Sepúlveda-Quiroz CA, Peña-Marín ES, Pérez-Morales A, Martínez-García R, Alvarez-Villagomez CS, Maytorena-Verdugo CI, Camarillo-Coop S, Vissio PG, Pérez-Sirkin D, Tovar-Ramírez D, Galaviz M, Álvarez-González CA (2020) Fructooligosaccharide supplementation in diets for tropical gar (Atractosteus tropicus) juvenile: effects on morphophysiology and intestinal barrier function. Aquac Res 52(1):37–50. https://doi.org/10.1111/are.14867
Shadrack RS, Manabu I, Yokoyama S, Koshio S, Miguel VA, Yukun Z, El Basuini MF (2022) Specific importance of low-level dietary supplementation of yeast strain in red sea bream. Annals of Animal Sci 22:1073–1085. https://doi.org/10.2478/aoas-2022-0012
Shelby RA, Lim C, Yildirim-Aksoy M, Welker TL, Klesius PH (2009) Effects of yeast oligosaccharide diet supplements on growth and disease resistance in juvenile nile tilapia. Oreochromis Niloticus J Appl Aquac 21(1):61–71. https://doi.org/10.1080/10454430802694728
Singh M, Kim S, Liu SX (2012) Effect of purified oat β-Glucan on fermentation of set-style yogurt mix. J Food Sci 77(8):E195–E201. https://doi.org/10.1111/j.1750-3841.2012.02828.x
Singla V, Chakkaravarthi S (2017) Applications of prebiotics in food industry: a review. Food Sci Technol Internat 23(8):649–667. https://doi.org/10.1177/1082013217721769
Temple MJ, Cuskin F, Baslé A, Hickey N, Speciale G, Williams SJ, Lowe EC (2017) A Bacteroidetes locus dedicated to fungal 1, 6-β-glucan degradation: unique substrate conformation drives specificity of the key endo-1, 6-β-glucanase. J Biol Chem 292(25):10639–10650. https://doi.org/10.1074/jbc.M117.787606
Tomás-Almenar C, Toledo-Solís FJ, Larrán AM, de Mercado E, Alarcán FJ, Rico D, Martín-Diana AB, Fernández I (2020) Effects and safe inclusion of narbonne vetch (Vicia narbonensis) in rainbow trout (Oncorhynchus mykiss) diets: towards a more sustainable aquaculture. Anim 10(11):1–19. https://doi.org/10.3390/ani10112175
Vázquez-Navarrete CJ (2015) Empoderamiento de las organizaciones sociales en el cultivo de pejelagarto (Atractosteus tropicus) en el sureste de México. Agro Productividad 8(3). https://mail.revista-agroproductividad.org/index.php/agroproductividad/%20article/view/659 (Acess 27 December 2022)
Velazquez-Carriles C, Macias-Rodríguez ME, Carbajal-Arizaga GG, Silva-Jara J, Angulo C, Reyes-Becerril M (2018) Immobilizing yeast β-glucan on zinc-layered hydroxide nanoparticle improves innate immune response in fish leukocytes. Fish & Shellfish Immunol 82:504–513. https://doi.org/10.1016/j.fsi.2018.08.055
Versaw WK, Cuppett SL, Winters DD, Williams LE (1989) An improved colorimetric assay for bacterial lipase in nonfat dry milk. J Food Sci 54(6):1557–1558. https://doi.org/10.1111/j.1365-2621.1989.tb05159.x
Vetvicka V, Vannucci L, Sima P (2013) The effects of β - glucan on fish immunity. North American J Med Sci 5(10):580–588. https://doi.org/10.4103/1947-2714.120792
Villanueva LTG (2014) Efecto del β-glucano 1,3/1,6 sobre la respuesta inmune, la actividad enzimática digestiva y la expresión de genes de Lutjanus peru y Sparus aurata. http://cibnor.repositorioinstitucional.mx/jspui/handle/1001/89 (Access 27 December 2022)
Walter HE (1984) Proteases, and their inhibitors. Method with haemoglobin, casein and azocoll as substrate. Methods of enzymatic análisis 270–277
Wang J, El-Bahrawy M (2014) Expression profile of mucins (MUC1, MUC2, MUC5AC, and MUC6) in ovarian mucinous tumours: changes in expression from benign to malignant tumours. Histopathol 66(4):529–535. https://doi.org/10.1111/his.12578
Wang X, Huang M, Yang F, Sun H, Zhou X, Guo Y, Zhang M (2015) Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohyd Polym 125:232–240. https://doi.org/10.1016/j.carbpol.2015.02.040
Wang P, Jiang C, Liu S, Cui P, Zhang Y, Zhang S (2017) Trans-generational enhancement of C-type lysozyme level in eggs of zebrafish by dietary β-glucan. Dev Comparative Immunol 74:25–31. https://doi.org/10.1016/j.dci.2017.04.006
Wilson W, Lowman D, Antony SP, Puthumana J, Singh IB, Philip R (2015) Immune gene expression profile of Penaeus monodon in response to marine yeast glucan application and white spot syndrome virus challenge. Fish & Shellfish Immunol 43(2):346–356. https://doi.org/10.1016/j.fsi.2014.12.032
Wood PJ (2007) Cereal β-glucans in diet and health. J Cereal Sci 46(3):230–238. https://doi.org/10.1016/j.jcs.2007.06.012
Xu H, Jiang Y, Xu X, Su X, Liu Y, Ma Y, Cao X (2019) Inducible degradation of lncRNA Sros1 promotes IFN-γ-mediated activation of innate immune responses by stabilizing Stat1 mRNA. Nature Immunol 20(12):1621–1630. https://doi.org/10.1038/s41590-019-0542-7
Xue C, Xi B, Ren M, Dong J, Xie J, Xu P (2015) Molecular cloning, tissue expression of gene Muc2 in blunt snout bream Megalobrama amblycephala and regulation after re-feeding. Chinese J Oceanol Limnol 33(2):291–298. https://doi.org/10.1007/s00343-015-4047-4
Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Bakr A, El-Keredy AM, Dawood MAO, Dossou S, Wang W, Yukun Z (2018) Bacillus subtilis as probiotic candidate for red sea bream: growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol 79:303–312. https://doi.org/10.1016/j.fsi.2018.05.035
Zou PF, Chang MX, Li Y, Xue NN, Li JH, Chen SN, Nie P (2016) NOD2 in zebrafish functions as antibacterial and also antiviral responses via NF-kβ, and also MDA5. RIG-I and MAVS Fish Shellfish Immunol 55:173–185. https://doi.org/10.1016/j.fsi.2016.05.031
Funding
This research was funded by the National Council on Science and Technology (CONACYT) in Mexico, grant number CB-2016–01-282765. This study was carried out with the collaboration of Red CYTED LARVAplus (117RT0521). L.A.C-R. acknowledges the National Council on Science and Technology (CONACYT, Mexico) for the master fellowship No. 1078719.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.A.C-R., F.J.T-S., and C.A.A-G.; methodology, S.A.F-G., C.S.A-V., R.G-Z.; formal analysis, L.A.C-R., F.J.T-S., S.C–C., R.M-G.; writing—original draft preparation: L.A.C-R.; writing—review and editing, all authors of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Animals were handled in compliance with the Norma Oficial Mexicana NOM-062-ZOO-1999 from Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, the Mexican standards for good welfare practices of laboratory animals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cigarroa-Ruiz, L.A., Toledo-Solís, F.J., Frías-Gómez, S.A. et al. Addition of β-glucans in diets for tropical gar (Atractosteus tropicus) larvae: effects on growth, digestive enzymes and gene expression of intestinal epithelial integrity and immune system. Fish Physiol Biochem 49, 613–626 (2023). https://doi.org/10.1007/s10695-023-01207-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01207-3