Abstract
The purpose of this study was to explore the beneficial effects of sea buckthorn polysaccharide (SP) on lipid metabolism, liver, and intestinal health in zebrafish fed with high-fat diet (HFD). The zebrafish were fed with regular diet (RD), HFD, and HFD supplemented with 2 g/kg (HFD_2SP) and 4 g/kg (HFD_4SP) of SP, respectively. Growth, serum biochemistry, histopathology, expression of genes involved in lipid metabolism, inflammation, oxidative stress and tight junction, and changes in intestinal microbiota were detected. Results showed that adding 2 and 4 g/kg of SP in the HFD significantly improved the survival rate of zebrafish; reduced the levels of serum triglyceride (TG), total cholesterol (TC), aspartate aminotransferase (AST), and alanine transaminase (ALT); and alleviated the lipid accumulation in the liver of zebrafish. Furthermore, SP significantly enhanced the antioxidant capacity of liver and intestine by up-regulating the expression of Nrf2 and Cu/Zn-SOD and alleviated liver and intestinal inflammation induced by HFD through up-regulating the expression of TGF-β1 and suppressing the expression of P38MAPK, IL-8, and IL-1β. Especially, dietary SP normalized intestinal microbiota imbalance caused by HFD and inhibited the proliferation of harmful bacteria, i.e., Mycobacterium, but promoted the proliferation of intestinal beneficial bacteria, i.e., Cetobacterium. In summary, 2 and 4 g/kg of dietary SP significantly reduced lipid accumulation, alleviated inflammation and oxidative stress, and normalized the imbalance of intestinal microbiota induced by HFD and consequently improved the survival rate of zebrafish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term consumption of high-fat diet (HFD) contributes toward the development and progression of liver steatosis and related metabolic diseases (Begriche et al. 2006; Rolo et al. 2012). Moreover, evidence from mammal studies suggests that HFD induces intestinal dysbiosis, which ultimately promotes the development of systemic inflammation and obesity (Kangwan et al. 2021). Similarly, HFD also induced excessive lipid deposition in cultured fish and up-regulated the expressions of genes related to inflammatory cytokines, such as interleukin-8 (IL-8), IL-1β, and IL-6 in intestine or liver of cultured fish, which has become a major challenge for aquaculture in recent years (Liu et al. 2021, 2022).
Supplements such as prebiotics, probiotics, and polyphenols have been shown to reverse HFD-induced phenotypes and attenuate the severity of obesity and its associated metabolic complications. Polysaccharide is a kind of macromolecular compound, which generally participates in cell growth, metabolism, immunity, anti-oxidation, intestinal microflora homeostasis, and other life processes (Chen et al. 2018; Li et al. 2018, 2020; Sun et al. 2018; Wang et al. 2017, 2020; Zhong et al. 2020). Polysaccharide also exhibits function of inducing blood lipids, such as Gracilaria sulfate polysaccharide that can significantly reduce the serum TG and TC levels of mice fed with HFD (Huang et al. 2019) and Cichorium glandulosum polysaccharide that can reduce the fat accumulation in zebrafish fed with high-cholesterol diet (Li et al. 2018). It is worth noting that the chemical structure of polysaccharides is complex and changeable, so the biological activities of different polysaccharides may be quite different. Moreover, study even found that some polysaccharides had a negative effect on health (Zhang et al. 2019). Hence, it is necessary to study the activities of polysaccharides from different sources. Especially, the lipid-lowering mechanism of polysaccharides is still not completely understood.
Sea buckthorn (Hippophae rhamnoides Linn.) is widely distributed in temperate regions of Europe and Asia (Wang et al. 2021b), and it is rich in a variety of nutritional and medicinal components (Guo et al. 2020; Suryakumar and Gupta 2011; Zhang et al. 2017). Studies have shown that sea buckthorn polysaccharide (SP) has biological activities such as anti-oxidation, immunomodulation, and anti-inflammation (Wang et al. 2018; Wei et al. 2019; Zhao et al. 2020). It has been reported that sea buckthorn freeze-dried powder down-regulates lipogenic-related genes, including sterol-regulatory element binding protein-1c (SREBP-1c) and acetyl-CoA carboxylase (ACC), and up-regulates fatty acid oxidation related genes, such as hormone-sensitive triglyceride lipase (HSL) and carnitine palmitoyltransferase 1 (CPT-1), thus effectively reducing the lipid deposition in mice (Guo et al. 2020). However, research focusing on the effect of SP on lipid metabolism is not sufficient at present, and it is unclear whether SP can reduce lipid accumulation, inflammation, and oxidative stress induced by HFD through regulating intestinal microbiota.
In this study, zebrafish was used as an experimental animal model (Ka et al. 2020; Schlegel 2012). And the regulation mechanism of SP on lipid metabolism, liver, and intestinal health in zebrafish fed with HFD were explored by testing the growth, serum biochemistry, histopathology, expression of genes related to lipid metabolism, inflammation, oxidative stress and tight junction, and changes of intestinal microbiota, aiming to provide a reference for the prevention and treatment of HFD-induced diseases in fish.
Materials and methods
Experimental fishes and diets
Zebrafish (AB strains) were acquired from National Zebrafish Resource of China, Chinese Academy of Sciences. The second generation of zebrafish was used in this experiment. Experimental fishes were cultured in a circulating water system (Shanghai Haisheng Biological Experiment Equipment Co., Ltd., China).
Four kinds of diets were formulated, including regular diet (RD), high-fat diet (HFD), and HFD added with two levels (2 and 4 g/kg) of sea buckthorn polysaccharide (SP) (HFD_2SP and HFD_4SP). The lipid levels of RD and HFD were 54 and 142 g/kg (Table 1), according to the study of Zhang et al. (2019). The SP with a purity of 90% was isolated by hot-water extraction and purified by DEAE-cellulose ion-exchange chromatography, according to the method of Ni et al. (2013). SP levels were according to the results of our pre-experiment. The main ingredients were obtained in dry, ground form, and mixed thoroughly. The SP was dissolved in water before mixing with the other ingredients, based on the listed formulations. Two-millimeter granular feeds were created using a pelleting machine (F-26, SCUT industrial factory, Guangdong, China) and were air-dried and stored at − 20 °C until used. Pellets were crushed and sieved through a battery of sieves to obtain particles of 600–800 µm of diameter. The proximate compositions of the experimental diets were analyzed following standard methods (Latimer Junior, 2016).
Experimental process and sampling
After acclimatization, the healthy male zebrafish were selected for experiment. Before the start of the experiment, the fish fasted for 24 h. Two hundred twenty-eight of experimental fishes with an average weight of 0.47 g were randomly divided into 12 aquariums (15 × 20 × 30 cm) and fed with the four kinds of experimental diets respectively, with 3 replicates in each treatment group. In order to make zebrafish’s intake of SP consistent with the designed dose, each group of zebrafish was fed with the same amount of feed. Experimental fishes were fed three times a day with a feeding rate of 5% of the body weight (Gonzales et al. 2013; Lawrence et al. 2012). The water circulating pump was turned off when feeding the fish. Aquarium water was exchanged about 50%, daily. The water temperature (27 ± 1 °C) was kept by an automatic temperature control heater. During the experiment, the photoperiod is 12 h:12 h, the pH is 7.0–7.7, and the ammonia nitrogen concentration is less than 0.05 mg/L.
After 4-week feeding trial, the experimental fishes were anesthetized with MS-222 (70 mg/kg) aqueous solution. After weighing, blood was collected by 1 ml injection syringe, and serum samples were obtained by centrifugation (4 °C, 3000 r/min). Then, the experimental fishes were dissected on ice, and liver, intestine, and intestinal contents were separated. Each kind of samples from the experimental fishes in the same tanks was mixed. The separated serum and tissue samples were quickly frozen in liquid nitrogen and then transferred to a refrigerator at − 80 °C for storage until test.
Determination of samples
Histological analysis
The liver tissue isolated from zebrafish was fixed with 4% paraformaldehyde. The fixated samples were embedded in paraffin after dehydration by graded alcohol series and sectioned at 5–6 μm thickness using standard procedure. The sections were stained with hematoxylin and eosin (H&E, Jiancheng Institute of Biotechnology) and were observed and photographed under fluorescence microscope to analyze their histological characteristics.
Biochemical test
Biochemical indexes in serum (TC, TG, AST, and ALT) and the liver (TG, SOD, and MDA) were detected with commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The whole process is strictly operated according to the instructions of commercial kits. The detailed steps are presented in our previous study (Liu et al. 2021).
Analysis of intestinal microbiota
The total bacterial DNA of intestinal contents was extracted with the commercial kit (MoBio Laboratories Inc., Carlsbad, CA, USA) according to the manufacturer’s instructions. The content and integrity of extracted DNA was evaluated. And the DNA samples were stored at − 80 °C until used.
The 16S rRNA gene sequencing was performed using high variable region (V3 and V4) PCR amplification products. The primers 27F: 5′-AGRGTTYGATYMTGGCTCAG-3′ and 1492R: 5′-RGYTACCTTGTTACGACTT-3′, which were tagged with specific barcode per sample. After quantified by QuantiFluor™-ST (Promega, USA), the amplicon pools were prepared for libraries construction. SMRTbell libraries were prepared using the Pacific Biosciences SMRTbell™ Template Prep kit 1.0 (PacBio, USA) and sequenced on PacBio RS II (LC-Bio Technology Co., Ltd., Hangzhou, China).
Gene expression
The primer sequences used in this study are listed in Table 2. Total RNA was extracted with RNAiso Plus. The concentration and purity of the total RNA were determined with a spectrophotometer (Eppendorf, Inc., Hamburg, Germany). The first-strand cDNA was synthesized using PrimeScript™ RT reagent kit with gDNA Eraser (TaKaRa) depending on the manufacturer’s instruction. The amplification was carried out in a real-time PCR detection system (CFX96, Bio-Rad), and the condition was as follows: 95 °C for 30 s, then 40 cycles of 95 °C for 5 s, and 57–61 °C for 60 s. The amplification efficiency of each pair of primers ranged from 98 to 103%. The relative gene expression levels were calculated by 2−ΔΔCt method (Livak and Schmittgen 2001).
Statistical analysis
SPSS software was used to analyze the variance of the experimental data, and the experimental results were expressed by mean ± SD. Ducan’s method (Duncan, 1995) was used for multiple comparisons, and the significant difference level was P < 0.05.
The 16S rDNA sequencing results were analyzed by bio-informatics, including α diversity analysis, β diversity analysis, and significant difference analysis, and the significance level was P < 0.05.
Results
Effect of sea buckthorn polysaccharide (SP) on the growth and survival of zebrafish fed with HFD
The specific growth performance and survival rate (SR) of zebrafish are shown in Table 3. Compared with the regular diet (RD) group, the specific growth rate (SGR) and weight gain rate (WGR) of zebrafish in the HFD group significantly increased (P < 0.05), but there were no significant differences in SR of zebrafish between the HFD group and the RD group.
Compared with the HFD group, the SGR and WGR of zebrafish in the HFD_2SP group decreased significantly (P < 0.05), while the SR of zebrafish in the HFD_2SP group increased significantly (P < 0.05). The SGR and WGR of zebrafish in HFD_4SP group had no significant difference in comparison with the HFD group (P < 0.05). But the SR of zebrafish in the HFD_4SP group increased markedly compared with that in the HFD group (P < 0.05).
Effect of SP on biochemical indexes in serum and liver of zebrafish fed with HFD
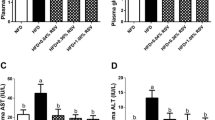
Serum AST, ALT, TG, and TC levels of zebrafish are shown in Table 4. Compared with the RD group, the serum AST and ALT levels of zebrafish in the HFD group significantly increased (P < 0.05), while the serum TG and TC levels of zebrafish in the HFD group had no significant differences in comparison with the RD group. Compared with the HFD group, serum AST, ALT, TG, and TC levels of zebrafish in the HFD_2SP and HFD_4SP groups significantly decreased (P < 0.05).
Levels of TG in the liver of zebrafish in each group are shown in Table 4. Compared with the RD group, TG levels in the liver of zebrafish in the HFD group increased significantly (P < 0.05). And an increasing trend in the liver index (HSI) of zebrafish in the HFD group compared with the RD group was also observed, even though there were no significant differences between these two groups (Table 4). Compared with the HFD group, the TG levels in the liver of zebrafish in HFD_2SP and HFD_4SP groups markedly declined (P < 0.05), and the HSI of zebrafish in the HFD_4SP group also markedly declined (P < 0.05).
Activities of SOD and levels of MDA in the liver of zebrafish in each group are shown in Table 4. Compared with the RD group, MDA levels in the liver of zebrafish in the HFD group increased significantly (P < 0.05), while SOD activities in the liver of zebrafish in the HFD group decreased significantly (P < 0.05). Compared with the HFD group, MDA levels in the liver of zebrafish in the HFD_2SP and HFD_4SP groups significantly decreased (P < 0.05), and SOD activity in the liver of zebrafish in the HFD_4SP group increased significantly (P < 0.05). The MDA levels in the liver of zebrafish in the HFD_4SP group were significantly lower than that in the HFD_2SP group (P < 0.05).
Effect of SP on histopathological changes in the liver of zebrafish fed with HFD
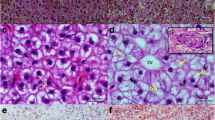
Histopathological changes in the liver of zebrafish in each group are shown in Fig. 1. In the RD group, hepatocytes of zebrafish are closely arranged, with clear boundaries. However, in the HFD group, hepatocytes of zebrafish are loosely arranged, with unclear boundaries. Particularly, in HFD_4SP group, hepatocytes of zebrafish are closely arranged, with clear boundaries.
Effects of SP on expression of genes related to lipid metabolism in the liver of zebrafish fed with HFD
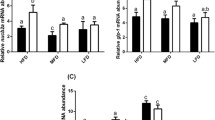
Changes in mRNA levels of lipid metabolism related genes in the liver of zebrafish of each group are shown in Table 5. Compared with the RD group, HFD depressed the expression of AMPKα1 and AMPKα2 in the liver, although significant difference was only observed in AMPKα1 (P < 0.05). Similarly, the mRNA levels of PPARα, ATGL, LPL, and CPT-1 in the liver of zebrafish fed with HFD also exhibited decreasing trends compared with those fed with RD, even though there were no significant differences observed (P > 0.05).
However, 4 g/kg of dietary SP significantly up-regulated the expression levels of AMPKα1, AMPKα2, PPARα, LPL, CPT-1, and ACC2 in the liver of zebrafish compared with those fed with HFD (P < 0.05). And the expression levels of AMPKα1, AMPKα2, ATGL, LPL, CPT-1, and ACC2 in the liver of zebrafish fed with HFD added with 2 g/kg of SP also showed an increasing trend compared with those fed with HFD, but there were no significant differences observed (P > 0.05).
Effects of SP on expression of genes involved in antioxidant defense and inflammation in the liver of zebrafish fed with HFD
Effects of SP on mRNA levels of genes related to antioxidant defense in the liver of zebrafish fed with HFD are shown in Table 6. Compared with the RD, HFD significantly depressed the expression of Nrf2 and Cu/Zn-SOD in the liver of zebrafish (P < 0.05). Similarly, the mRNA expression levels of CAT in the liver of zebrafish fed with HFD also exhibited decreasing trend compared with that fed with RD, even though there were no significant differences observed (P > 0.05). However, 2 g/kg of dietary SP significantly up-regulated the expression levels of Nrf2 and Cu/Zn-SOD in the liver of zebrafish compared with those fed with HFD (P < 0.05). Moreover, 4 g/kg of dietary SP significantly up-regulated the expression levels of Nrf2 and CAT in the liver of zebrafish compared with those fed with HFD (P < 0.05).
Effects of SP on expression of genes related to inflammation in the liver of zebrafish fed with HFD are shown in Table 7. Compared with the RD, HFD depressed the expression of IL-10 and TGF-β1 in the liver of zebrafish, although the significant difference was only observed in IL-10. But HFD significantly up-regulated the expression of P38 MAPK and IL-1β in the liver of zebrafish. However, 4 g/kg of dietary SP significantly up-regulated the expression levels of IL-10 and TGF-β1, but significantly depressed the expression levels of P38 MAPK, IL-8, and IL-1β in the liver of zebrafish compared with those fed with HFD (P < 0.05). And 2 g/kg of dietary SP significantly depressed the expression levels of P38 MAPK in the liver of zebrafish compared with those fed with HFD (P < 0.05). And the expression levels of IL-8 and IL-1β in the liver of zebrafish fed with 2 g/kg of dietary SP also showed decreasing trends compared with those fed with HFD, but there were no significant differences observed (P > 0.05).
Effects of SP on expression of genes involved in antioxidant defense, inflammation, and tight junction in intestine of zebrafish fed with HFD
Effects of SP on expression of genes involved in antioxidant defense in the intestine of zebrafish fed with HFD are shown in Table 6. The mRNA expression levels of Nrf2, Cu/Zn-SOD, and CAT in the intestine of zebrafish fed with HFD did not significantly change compared with that fed with RD (P > 0.05), but a decreasing trend of Nrf2 was observed in HFD group in comparison with RD. However, 2 g/kg of dietary SP significantly up-regulated the expression levels of Nrf2 and CAT in the intestine of zebrafish compared with that fed with HFD (P < 0.05). But 4 g/kg of dietary SP did not significantly up-regulate the expression levels of Nrf2, Cu/Zn-SOD, and CAT in the intestine of zebrafish compared with the zebrafish fed with HFD (P < 0.05).
Effects of SP on mNRA levels of genes related to inflammation in intestine of zebrafish fed with HFD are shown in Table 7. Compared with the RD, HFD up-regulated the expression of P38 MAPK, IL-8, and IL-1β in the intestine of zebrafish, although the significant difference was only observed in IL-8 and IL-1β. However, 2 or 4 g/kg of dietary SP significantly depressed the expression levels of P38 MAPK, IL-8, and IL-1β, except there was no significant difference observed in P38 MAPK of the HFD_4SP group (P > 0.05).
Effects of SP on expression of genes involved in tight junction of intestine of zebrafish fed with HFD are shown in Table 7. HFD did not significantly regulate the expression of Claudin-3c and Occludin in the intestine of zebrafish. However, 4 g/kg of dietary SP significantly up-regulated the expression levels of Occludin in the intestine of zebrafish (P < 0.05).
Effects of SP on intestinal microbiota of zebrafish fed with HFD
The Venn diagram result (Fig. 3a) showed that 192, 116, 222, and 132 unique OTUs were tested among the RD, HFD, HFD_2SP, and HFD_4SP groups. The alpha diversity of intestinal flora is reflected by indexes of Chao1, observed species, Shannon, and Simpson and are shown in Fig. 3 b, c, and d. There were no significant differences in Chao1, Shannon, and Simpson indexes among different groups. Beta diversity of intestinal flora is evaluated by principal coordinate analysis (PCoA) and is shown in Fig. 3e. PCoA analysis revealed that the composition of intestinal flora of zebrafish in the HFD group showed distinct differences with that in the RD group, while the composition of intestinal flora of zebrafish in the HFD_4SP group was slightly different from that in the RD group, but the composition of intestinal flora of zebrafish in the HFD_2SP group was markedly different from that in RD.
Species composition of intestinal flora of zebrafish in each group at phylum level is shown in Fig. 4a. The dominant flora in the intestine of zebrafish was Proteobacteria, Planctomycetes, and Actinobacteria. Compared with the RD group, the abundance of Proteobacteria, Planctomycetes, Actinobacteria, Chloroflexi, and Verrucomicrobia in the intestine of zebrafish in HFD group increased, while the abundance of Bacteroidetes, Firmicutes, and Acidobacteria decreased. Compared with HFD group, the abundance of Proteobacteria, Planctomycetes, and Verrucomicrobia in the intestine of zebrafish in HFD_2SP group decreased, while the abundance of Fusobacteria increased. Compared with the HFD group, the abundance of Proteobacteria, Chloroflexi, and Verrucomicrobia in the intestine of zebrafish in HFD_4SP group decreased, but the abundance of Actinobacteria increased.
Species composition of intestinal flora of zebrafish in each group at genus level is shown in Fig. 4b. Compared with the RD group, the abundance of Pseudomonas, Arenimonas, Sandaracinobacter, Ralstonia, Pirellula, and Mycobacterium in the intestine of zebrafish in the HFD group increased, while the abundance of Rhodobacter and Nitrospira decreased. Compared with the HFD group, the abundance of Arenimonas, Sandaracinobacter, and Mycobacterium in the intestine of zebrafish in the HFD_2SP and HFD_4SP groups decreased, but the abundance of Cetobacterium in the intestine of zebrafish in the HFD_2SP and HFD_4SP groups markedly increased.
Discussion
Dietary SP improved survival and alleviated lipid accumulation of zebrafish fed with HFD
Studies have been reported that appropriate increase of fat content in diet prompts the growth of fish, but excessive fat reduces the survival of fish (Liu et al. 2021; Dai et al. 2018; Landgraf et al. 2017). In the current study, HFD significantly prompted the growth of zebrafish in terms of SGR and WGR compared with the regular diet (RD). But a decrease trend in SR of zebrafish in the HFD group compared with the RD group was observed, even though there were no significant differences between these two groups. Interestingly, adding 2 g/kg of SP in the HFD significantly reduced the SGR and WGR of zebrafish to the normal level of the RD group, but adding 4 g/kg of SP in the HFD did not significantly reduce the SGR and WGR of zebrafish. However, the SR of zebrafish fed diet added with 2 and 4 g/kg of SP both increased significantly compared with that fed with the HFD, indicating that adding 2 and 4 g/kg of SP in the HFD can improve the survival of zebrafish. Similar effect has been found in golden pompano fed with konjac glucomannan (Li et al. 2021b).
Long-term feeding of HFD will lead to disorder of synthesis and secretion of TG in the liver and then affect the contents of TG and TC in serum (Ka et al. 2020; Liu et al. 2021). Therefore, the levels of liver TG and serum TG and TC are the main indexes to distinguish fatty liver in animals (Ka et al. 2020; Liu et al. 2021). In this experiment, TG level in the liver of zebrafish in the HFD group was significantly higher than that in the RD group. The result indicated that HFD caused lipid accumulation in the liver of zebrafish. Moreover, in the RD group, hepatocytes of zebrafish were closely arranged, with clear boundaries. However, in the HFD group, hepatocytes of zebrafish were loosely arranged, with unclear boundaries. Interestingly, adding 2 and 4 g/kg of SP in the HFD significantly reduced the TG levels in the liver and the TG and TC levels in the serum of zebrafish. These results indicated that dietary SP reduced lipid accumulation in the zebrafish caused by HFD. Similar results also have been reported in other polysaccharides, such as that pomelo fruitlet polysaccharide reduced lipid deposition in whole body and hepatocytes and ameliorated diet-induced nonalcoholic fatty liver disease in hybrid grouper (Zou et al., 2021), and Cichorium intybus polysaccharide significantly reduced the TC and TG of zebrafish and prevent liver steatosis (Li et al. 2018), although the mechanism has not been clearly explained.
To further explore the lipid reducing mechanism of SP, changes in mRNA levels of genes related to lipid metabolism in the liver of zebrafish were determined. AMPK is a key regulator of lipid metabolism in animals including fish (Herzig and Shaw 2018; Ran et al. 2021). Activation of AMPK activates CPT-1 and prompts fatty acid decomposition and down-regulates the expression of lipogenic genes, such as ACC1 and FAS (Herzig and Shaw 2018; Liu et al. 2021). Transcriptional effects of AMPK also participate in redirecting metabolism towards increased catabolism (Garcia and Shaw 2017; Zou et al. 2021). In this study, HFD depressed the expression of AMPKα1 in the liver of zebrafish compared with RD. And the mRNA expression levels of genes involved in lipid decomposition, such as PPARα, ATGL, LPL, and CPT-1 in the liver of zebrafish fed with the HFD, also exhibited decreasing trends compared with those fed with the RD. However, 4 g/kg of dietary SP significantly up-regulated the expression levels of AMPKα1, AMPKα2, PPARα, LPL, and CPT-1 in the liver of zebrafish compared with those fed with the HFD. And the expression levels of AMPKα1, AMPKα2, ATGL, LPL, and CPT-1 in the liver of zebrafish fed with HFD added with 2 g/kg of SP also showed increasing trends compared with those fed with HFD, even though there were no significant differences observed. Moreover, the correlation analysis result showed that the expression levels of AMPKα1, ATGL, and PPARα were negatively correlated (P < 0.05) with the liver TG levels (Fig. 2). Those results indicate that dietary SP can regulate the transcription levels of AMPKα and its downstream genes and consequently reduce the lipid accumulation caused by HFD in zebrafish. And this conclusion was supported by the study in grouper in which lipogenesis-related genes were down-regulated while lipolysis-related genes were up-regulated by pomelo fruitlet polysaccharide via AMPK (Zou et al. 2021). Interestingly, it has been reported that sea buckthorn freeze-dried powder down-regulates lipogenic related genes, including SREBP-1c, ACC, and SCD1, and up-regulates fatty acid oxidation related genes, such as HSL and CPT-1, thus effectively reducing the lipid deposition in mice (Guo et al. 2020). Hence, the SP may be the main component in sea buckthorn fruit to reduce lipid accumulation in zebrafish.
Heatmap of Spearman’s correlation between the biochemical index and expression levels of genes involved in lipid metabolism, inflammation, oxidative stress, and tight junction. The intensity of the colors represented the degree of association (red, positive correlation; blue, negative correlation). Significant correlations are marked by *P < 0.05; **P < 0.01
Dietary SP alleviated oxidative stress and inflammation in the liver of zebrafish induced by HFD
Long-term ingestion of HFD will not only cause excessive lipid accumulation in the body, but also cause liver damage (Li et al. 2021b; Zhang et al. 2019). Serum ALT and AST levels are important indicators for detecting liver function. When liver injury occurs, the contents of these two enzymes in serum will increase (Li et al. 2021b; Zhang et al. 2019). In this experiment, serum AST and ALT levels of zebrafish in the HFD group were significantly higher than those in the RD group, indicating the liver injury caused by HFD. However, adding 2 and 4 g/kg SP in HFD significantly reduced the AST and ALT levels in serum of zebrafish, which indicates that the SP can alleviate the liver injury caused by HFD in zebrafish. Zou et al. (2021) found that pomelo fruitlet polysaccharide significantly reduced ALT and AST levels in hepatocytes of hepatic steatosis model of grouper, which is similar to the results of this study.
The damage of liver (increased ALT and AST levels) may be related to oxidative stress induced by HFD (Kowalczyk et al., 2021). Therefore, in this study, we detected the activity of major antioxidant enzymes, i.e., SOD, expression levels of antioxidant related genes, and contents of product of lipid peroxidation, i.e., MDA in the liver of zebrafish. HFD caused a significant decrease in SOD activity and expression levels of antioxidant related genes, including Nrf2 and Cu/Zn-SOD in the liver of zebrafish, and led to the significant increase in MDA content in the liver, indicating that HFD induced oxidative damage in the liver. This may be the important reason for the increase of ALT and AST levels in serum of zebrafish fed with HFD. Interestingly, adding 2 and 4 g/kg SP in HFD significantly up-regulated the expression levels of Nrf2 and decreased the MDA content in the liver of zebrafish. Moreover, the correlation analysis result showed that the AST and ALT levels in serum were negatively correlated (P < 0.05) with the expression levels of Nrf2 and CAT in the liver (Fig. 2). These results indicate that SP can protect the liver of zebrafish from oxidative damage induced by HFD through up-regulating antioxidant related genes. This positive effect of polysaccharide was also reported by Zhang et al. (2021) in which Lycii fructus polysaccharide increased SOD activity and reduced MDA levels and reversed the abnormal oxidative stress through inhibition of mitochondrial-mediated apoptotic pathway in zebrafish.
Furthermore, it is widely accepted that inflammation is a key factor that causes liver damage, and HFD results in inflammation (Liu et al. 2022; Arias-Jayo et al. 2018). And anti-inflammatory effects of natural polysaccharides also have been reported in previous studies (Hou et al. 2020; Zou et al. 2021; Mohammadi et al. 2022). Hence, in this study, the expression of genes involved in inflammation in the liver of zebrafish were further detected. Compared with the RD, HFD significantly depressed the expression of anti-inflammatory factors, i.e., IL-10 in the liver of zebrafish, but up-regulated the expression of proinflammatory factors, such as P38 MAPK and IL-1β in the liver of zebrafish, which indicate the increased inflammation in the liver of zebrafish induced by HFD. However, 4 g/kg of dietary SP significantly up-regulated the expression levels of IL-10 and TGF-β1, but significantly depressed the expression levels of P38 MAPK, IL-8, and IL-1β in the liver of zebrafish compared with those fed with HFD. And 2 g/kg of dietary SP significantly depressed the expression levels of P38 MAPK in the liver of zebrafish compared with those fed with HFD. These results indicate that dietary SP can alleviate inflammation in the liver of zebrafish induced by HFD. And this positive effect was supported by study of Zou et al. (2021) in which pomelo fruitlet polysaccharide ameliorated high lipid-induced in inflammatory responses in hybrid grouper. In addition, the liver TG levels were positively correlated (P < 0.05) with the expression levels of a proinflammatory factor, i.e., P38 MAPK in the liver of zebrafish (Fig. 2). Moreover, the expression levels of a proinflammatory factor, i.e., IL-1β in the liver of zebrafish, were positively correlated (P < 0.05) with the serum AST. But the expression levels of anti-inflammatory factors including IL-10 and TGF-β1in the liver of zebrafish were negatively correlated (P < 0.05) with the serum AST (Fig. 2). Those results indicate that SP can depress inflammatory response and consequently alleviate liver injury induced by HFD in zebrafish. Oxidative stress induced by excessive lipid accumulation in tissues of fish fed HFD induces inflammatory responses (Xie et al. 2020). Hence, the suppressed oxidative stress by SP may be the important reason for the reduced inflammatory responses in the liver of zebrafish.
Dietary SP alleviates oxidative stress and inflammation in intestine of zebrafish induced by HFD
It has been reported that 0.01% Astragalus polysaccharide improved the genes expression of SOD, GST and GPx in zebrafish intestine (Li et al. 2021a). In this study, the mRNA expression levels of Nrf2, Cu/Zn-SOD, and CAT in the intestine of zebrafish fed with HFD did not significantly change compared with that fed with RD. However, 2 g/kg of dietary SP significantly up-regulated the expression levels of Nrf2 and CAT in the intestine of zebrafish compared with the zebrafish fed with the HFD, and 4 g/kg of dietary SP also promoted transcription of Nrf2 and CAT in the intestine of zebrafish, although it did not reach a significant level, indicating dietary SP can protect intestine from the oxidative stress by up-regulating antioxidant related genes.
Furthermore, studies have shown that prolonged exposure of HFD can perturb immune homeostasis, inducing intestinal inflammation in mammal and fish (Devkota et al. 2012; Liu et al., 2022). As such, we further determined the gene expression of inflammatory factors in the intestine. The results revealed that compared with the RD, HFD significantly up-regulated the expression of IL-8 and IL-1β in the intestine of zebrafish. However, 2 or 4 g/kg of dietary SP significantly depressed the expression levels of IL-8 and IL-1β in the intestine of zebrafish, indicating that dietary SP can alleviate inflammation in intestine of zebrafish caused by HFD. It has been reported that SP alleviated LPS-induced inflammation in IPEC-J2 cells by inhibiting the MAPK/NF-κB signaling pathway (Zhao et al. 2020). Hence, the way of SP alleviates intestinal inflammation needs to be further tested.
Besides, gut dysbiosis caused by HFD might subsequently aggravate the destruction of the mucus barrier and increase epithelial permeability of the small intestine (Kangwan et al. 2021). In this study, HFD did not significantly regulate the expression of Claudin-3c and Occludin in the intestine of zebrafish, which needs further verification. However, 4 g/kg of dietary SP significantly up-regulated the expression levels of Occludin in the intestine of zebrafish, indicating that 4 g/kg of dietary SP might improve intestinal health by decreasing epithelial permeability. Similarly, it has been reported that 0.01% Astragalus polysaccharide up-regulated the expression of Occludin1 in intestine of zebrafish (Li et al. 2021a). But its mechanism needs further exploration.
Dietary SP normalizes intestinal microbiota imbalance that induced by HFD in zebrafish
Intestinal microbiota is closely related to the nutrition, metabolism, and immunity of the host (Arias-Jayo et al. 2018; Liu et al. 2022). And dietary lipid levels affect the composition and quantity of intestinal flora (Bisanz et al. 2019). In this study, the dominant flora in intestine of zebrafish are Proteobacteria, Planctomycetes, and Actinobacteria, which are differed from mammals (Bisanz et al. 2019; Tian et al. 2021). This is similar to other studies on grass carp (Liu et al. 2022), but the composition and abundance of intestinal flora of different fish are still different, which may be related to the experimental environment, fish species, and feeding habits.
Furthermore, HFD induces intestinal dysbiosis encompassing changes in composition balance and massive redistribution with bacteria (Arias-Jayo et al. 2018; Liu et al. 2022). And Cistanche deserticola polysaccharide can increase the beneficial flora and regulate the diversity of intestinal flora (Fu et al. 2020). In this study, there were no significant differences in Chao1, Simpson, and Shannon indexes of intestinal flora in zebrafish in each group, which indicated that HFD and HFD added with SP did not change α diversity of intestinal flora in zebrafish. This is similar to the study in which the consumption of HFD did not affect the total number of bacterial copies and α diversity of zebrafish larvae (Arias-Jayo et al. 2018). However, the β diversity of intestinal flora in zebrafish was regulated by the HFD and dietary SP. PCoA analysis showed that the species composition of intestinal flora of zebrafish in the HFD group showed distinct differences with that in the RD group. Similar results have been reported both in zebrafish and grass carp (Arias-Jayo et al. 2018; Liu et al. 2022).
Moreover, studies have shown that dietary supplements can alter the lipid metabolism and inflammation through intestinal microbiota. The sea buckthorn freeze-dried powder alleviated HFD-induced obesity by regulating the gut microbiota in mice (Guo et al. 2020). And Astragalus polysaccharides modulate intestinal microbiota in grass carp (Ctenopharyngodon idellus) (Shi et al. 2021). In this study, the composition of intestinal microbiota of zebrafish in HFD_2SP and HFD_4SP groups was markedly different from the HFD group, indicating that dietary SP normalized intestinal microbiota imbalance that was induced by HFD in zebrafish (Figs. 3a and 4). And the normalized intestinal microbiota by dietary SP might contribute to the regulation of lipid metabolism and inflammation in zebrafish fed with HFD.
Particularly, Mycobacterium has been reported as a pathogenic bacteria, which causes mycobacteriosis in a wide range of animals including fish (Machida et al. 2021; Mugetti et al. 2020). In this study, HFD increased the abundance of Mycobacterium in the intestine of zebrafish, indicating that HFD stimulates the proliferation of harmful bacteria in zebrafish. The correlation analysis showed that the abundance of Mycobacterium was negatively correlated with the gene expression levels of triglyceride hydrolase, i.e., ATGL in the liver, and proinflammatory factor, i.e., TGF-β1 in intestine, indicating Mycobacterium might be a harmful bacteria to inhibit fat decomposition and promote intestinal inflammation (Fig. 5 a and b). However, 2 and 4 g/kg of dietary SP reduced the abundance of Mycobacterium in the intestine of zebrafish, indicating that dietary SP might inhibit the proliferation of harmful bacteria and consequently reduce the lipid accumulation and inflammation induced by HFD in zebrafish.
Heatmap of Spearman’s correlation between the abundance of gut microbiota (at the genus level) and a the biochemical index, expression levels of genes involved in lipid metabolism, and b the expression levels of genes involved in inflammation, oxidative stress, and tight junction. The intensity of the colors represented the degree of association (red, positive correlation; blue, negative correlation). Significant correlations are marked by *P < 0.05; **P < 0.01
To our surprise, the abundance of Cetobacterium which has been reported as a beneficial bacteria in fish (Xie et al. 2021; Wang et al. 2021a) markedly increased in the intestine of zebrafish in HFD_2SP and HFD_4SP groups compared with the HFD group. Moreover, the correlation analysis showed that the abundance of Cetobacterium was negatively correlated with serum TG, TC, and ALT levels, and the expression level of P38MAPK in the liver, but positively correlated with the expression level of CAT in intestine (Fig. 5 a and b), indicating that Cetobacterium are beneficial bacteria to reduce the lipid deposition and alleviate HFD induced liver inflammation in fish. This conclusion also can be supported by Xie et al. (2021) in which Cetobacterium somerae fermentation product can improve gut and liver health of common carp and decrease lipid deposition in the liver. In addition, study has reported that exogenous Cetobacterium somerae could increase the level of intestinal short chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, which has been reported as a medium in intestine-liver axis to regulate liver metabolism (Albillos et al. 2020; Ringseis et al. 2020). Hence, the increased Cetobacterium by dietary SP also might prompt the production of SCFAs in the intestine of zebrafish and participate in metabolic and immunity regulation of zebrafish fed with HFD, which is worth further exploration.
Conclusion
This study explored the beneficial effects of dietary SP on lipid metabolism, liver, and intestinal health in zebrafish fed with HFD. And the results revealed that 2 or 4 g/kg SP significantly reduced lipid accumulation, alleviated inflammation and oxidative stress induced by HFD in zebrafish, and consequently improved the survival rate of zebrafish. These positive effects might be owing to the normalization of SP on the imbalance of intestinal microbiota caused by HFD through inhibiting the proliferation of harmful bacteria and stimulating the proliferation of beneficial bacteria.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Albillos A, De Gottardi A, Rescigno M (2020) The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol 72(3):558–577. https://doi.org/10.1016/j.jhep.2019.10.003
Arias-Jayo N, Abecia L, Alonso-Sáez L, Ramirez-Garcia A, Rodriguez A, Pardo MA (2018) High-fat diet consumption induces microbiota dysbiosis and intestinal inflammation in zebrafish. Microb Ecol 76(4):1089–1101. https://doi.org/10.1007/s00248-018-1198-9
Begriche K, Igoudjil A, Pessayre D, Fromenty B (2006) Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion 6(1):1–28. https://doi.org/10.1016/j.mito.2005.10.004
Bisanz JE, Upadhyay V, Turnbaugh JA, Ly K, Turnbaugh PJ (2019) Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe 26(2):265-272.e264. https://doi.org/10.1016/j.chom.2019.06.013
Chen M, Lu B, Li Y, Wang Y, Zheng H, Zhong D, Xie Z (2018) Metabolomics insights into the modulatory effects of long-term compound polysaccharide intake in high-fat diet-induced obese rats. Nutr Metab 15(1):8. https://doi.org/10.1186/s12986-018-0246-2
Dai YJ, Jiang GZ, Yuan XY, Liu WB (2018) High-fat-diet-induced inflammation depresses the appetite of blunt snout bream (Megalobrama amblycephala) through the transcriptional regulation of leptin/mammalian target of rapamycin. Br J Nutr 120(12):1422–1431. https://doi.org/10.1017/S000711451800288X
Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Chang EB (2012) Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487(7405). https://doi.org/10.1038/nature11225
Duncan DB (1955) Multiple range and multiple F-tests. Biometrics 11:1–42. https://doi.org/10.2307/3001478.10.1089/zeb.2013.0891
Fu Z, Han L, Zhang P, Mao H, Zhang H, Wang Y, Liu E (2020) Cistanche polysaccharides enhance echinacoside absorption in vivo and affect the gut microbiota. Int J Biol Macromol 149:732–740. https://doi.org/10.1016/j.ijbiomac.2020.01.216
Garcia D, Shaw RJ (2017) AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell 66(6):789–800. https://doi.org/10.1016/j.molcel.2017.05.032
Gonzales JM Jr, Law SHW (2013) Feed and feeding regime affect growth rate and gonadosomatic index of adult zebrafish (Danio rerio). Zebrafish 10(4):532–540. https://doi.org/10.1089/zeb.2013.0891
Guo C, Han L, Li M, Yu L (2020) Seabuckthorn (Hippophaë rhamnoides) freeze-dried powder protects against high-fat diet-induced obesity, lipid metabolism disorders by modulating the gut microbiota of mice. Nutrients 12(1):265. https://doi.org/10.3390/nu12010265
Herzig S, Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19(2):121–135. https://doi.org/10.1038/nrm.2017.95
Hou C, Chen L, Yang L, Ji X (2020) An insight into anti-inflammatory effects of natural polysaccharides. Int J Biol Macromol 153:248–255. https://doi.org/10.1016/j.ijbiomac.2020.02.315
Huang S, Pang D, Li X, You L, Zhao Z, Cheung PCK, Liu D (2019) A sulfated polysaccharide from Gracilaria lemaneiformis regulates cholesterol and bile acid metabolism in high-fat diet mice. Food Funct 10(6):3224–3236. https://doi.org/10.1039/C9FO00263D
Ka J, Pak B, Han O, Lee S, Jin SW (2020) Comparison of transcriptomic changes between zebrafish and mice upon high fat diet reveals evolutionary convergence in lipid metabolism. Biochem Biophys Res Commun 530(4):638–643. https://doi.org/10.1016/j.bbrc.2020.07.042
Kangwan N, Pratchayasakul W, Kongkaew A, Pintha K, Chattipakorn N, Chattipakorn SC (2021) Perilla seed oil alleviates gut dysbiosis, intestinal inflammation and metabolic disturbance in obese-insulin-resistant rats. Nutrients 13(9):3141. https://doi.org/10.3390/nu13093141
Kowalczyk P, Zaczek Z, Osowska S, Sobocki J (2021) AST and ALT correlate with oxidative stress in hpn patients. Clinical Nutrition ESPEN 46:655–656
Landgraf K, Schuster S, Meusel A, Garten A, Riemer T, Schleinitz D, Körner A (2017) Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol 17(1):4. https://doi.org/10.1186/s12899-017-0031-x
Latimer Junior GW (2016) Official methods of analysis of AOAC International. AOAC International, Rockville
Lawrence C, Best J, James A, Maloney K (2012) The effects of feeding frequency on growth and reproduction in zebrafish (Danio rerio). Aquaculture 368:103–108. https://doi.org/10.1016/j.aquaculture.2012.09.022
Li L, Su Y, Feng Y, Hong R (2020) A comparison study on digestion, anti-inflammatory and functional properties of polysaccharides from four Auricularia species. Int J Biol Macromol 154:1074–1081. https://doi.org/10.1016/j.ijbiomac.2020.02.324
Li M, Ma J, Ahmad O, Cao Y, Wang B, He Q, Shang J (2018) Lipid-modulate activity of Cichorium glandulosum Boiss et Huet polysaccharide in nonalcoholic fatty liver disease larval zebrafish model. J Pharmacol Sci 138(4):257–262. https://doi.org/10.1016/j.jphs.2018.09.012
Li Y, Ran C, Wei K, Xie Y, Xie M, Zhou W, Zhou Z (2021a) The effect of Astragalus polysaccharide on growth, gut and liver health, and anti-viral immunity of zebrafish. Aquaculture 540:736677. https://doi.org/10.1016/j.aquaculture.2021.736677
Li Y, Liang S, Shao Y, Li Y, Chen C, You C, Wang S (2021b) Impacts of dietary konjac glucomannan supplementation on growth, antioxidant capacity, hepatic lipid metabolism and inflammatory response in golden pompano (Trachinotus ovatus) fed a high fat diet. Aquaculture 545:737113. https://doi.org/10.1016/j.aquaculture.2021.737113
Liu G, Yu H, Wang C, Li P, Liu S, Zhang X, Ji H (2021) Nano-selenium supplements in high-fat diets relieve hepatopancreas injury and improve survival of grass carp Ctenopharyngodon Idella by reducing lipid deposition. Aquaculture 538:736580. https://doi.org/10.1016/j.aquaculture.2021.736580
Liu S, Yu H, Li P, Wang C, Liu G, Zhang X, Ji H (2022) Dietary nano-selenium alleviated intestinal damage of juvenile grass carp (Ctenopharyngodon idella) induced by high-fat diet: Insight from intestinal morphology, tight junction, inflammation, anti-oxidization and intestinal microbiota. Animal Nutrition 8(1):235–248. https://doi.org/10.1016/j.aninu.2021.07.001
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Machida Y, Tang BCC, Yamada M, Sato S, Nakajima K, Matoyama H, Kato G (2021) Mycobacteriosis in cultured koi carp Cyprinus carpio caused by Mycobacterium paragordonae and two Mycolicibacterium spp. Aquaculture 539:736656. https://doi.org/10.1016/j.aquaculture.2021.736656
Mohammadi G, Karimi AA, Hafezieh M, Dawood MA, Abo-Al-Ela HG (2022) Pistachio hull polysaccharide protects Nile tilapia against LPS-induced excessive inflammatory responses and oxidative stress, possibly via TLR2 and Nrf2 signaling pathways. Fish Shellfish Immunol 121:276–284. https://doi.org/10.1016/j.fsi.2021.12.042
Mugetti D, Varello K, Gustinelli A, Pastorino P, Menconi V, Florio D, Prearo M (2020) Mycobacterium pseudoshottsii in Mediterranean fish farms: new trouble for European aquaculture? Pathogens 9(8):610. https://doi.org/10.3390/pathogens9080610
Ni W, Gao T, Wang H, Du Y, Li J, Li C, Bi H (2013) Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J Ethnopharmacol 150(2):529–535. https://doi.org/10.1016/j.jep.2013.08.055
Ran C, Xie M, Li J, Xie Y, Ding Q, Li Y, Zhou Z (2021) Dietary nucleotides alleviate hepatic lipid deposition via exogenous AMP-mediated AMPK activation in zebrafish. J Nutr 151(10):2986–2996. https://doi.org/10.1093/jn/nxab232
Ringseis R, Gessner DK, Eder K (2020) The gut–liver axis in the control of energy metabolism and food intake in animals. Annual Review of Animal Biosciences 8(1):295–319. https://doi.org/10.1146/annurev-animal-021419-083852
Rolo AP, Teodoro JS, Palmeira CM (2012) Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radical Biol Med 52(1):59–69. https://doi.org/10.1016/j.freeradbiomed.2011.10.003
Schlegel A (2012) Studying non-alcoholic fatty liver disease with zebrafish: a confluence of optics, genetics, and physiology. Cell Mol Life Sci 69(23):3953–3961. https://doi.org/10.1007/s00018-012-1037-y
Shi F, Lu Z, Yang M, Li F, Zhan F, Zhao L, Qin Z (2021) Astragalus polysaccharides mediate the immune response and intestinal microbiota in grass carp (Ctenopharyngodon idellus). Aquaculture 534:736205. https://doi.org/10.1016/j.aquaculture.2020.736205
Sun X, Duan M, Liu Y, Luo T, Ma N, Song S, Ai C (2018) The beneficial effects of Gracilaria lemaneiformis polysaccharides on obesity and the gut microbiota in high fat diet-fed mice. Journal of Functional Foods 46:48–56. https://doi.org/10.1016/j.jff.2018.04.041
Suryakumar G, Gupta A (2011) Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L). J Ethnopharmacol 138(2):268–278. https://doi.org/10.1016/j.jep.2011.09.024
Tian B, Zhao J, Zhang M, Chen Z, Ma Q, Liu H, Li J (2021) Lycium ruthenicum anthocyanins attenuate high-fat diet-induced colonic barrier dysfunction and inflammation in mice by modulating the gut microbiota. Mol Nutr Food Res 65(8):2000745. https://doi.org/10.1002/mnfr.202000745
Wang A, Zhang Z, Ding Q, Yang Y, Bindelle J, Ran C, Zhou Z (2021a) Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 13(1):1–15. https://doi.org/10.1080/19490976.2021.1900996
Wang K, Xu Z, Liao X (2021b) Bioactive compounds, health benefits and functional food products of sea buckthorn: a review. Critical Reviews in Food Science and Nutrition: 1-22https://doi.org/10.1080/10408398.2021b.1905605
Wang L, Zhan H, Wu G, Zeng Y (2020) Effect of operational strategies on the rapid start-up of nitrogen removal aerobic granular system with dewatered sludge as inoculant. Biores Technol 315:123816. https://doi.org/10.1016/j.biortech.2020.123816
Wang S, Li Q, Zang Y, Zhao Y, Liu N, Wang Y, Mei Q (2017) Apple polysaccharide inhibits microbial dysbiosis and chronic inflammation and modulates gut permeability in HFD-fed rats. Int J Biol Macromol 99:282–292. https://doi.org/10.1016/j.ijbiomac.2017.02.074
Wang X, Liu J, Zhang X, Zhao S, Zou K, Xie J, Wang Y (2018) Seabuckthorn berry polysaccharide extracts protect against acetaminophen induced hepatotoxicity in mice via activating the Nrf-2/HO-1-SOD-2 signaling pathway. Phytomedicine 38:90–97. https://doi.org/10.1016/j.phymed.2017.11.007
Wei E, Yang R, Zhao H, Wang P, Zhao S, Zhai W, Zhou H (2019) Microwave-assisted extraction releases the antioxidant polysaccharides from seabuckthorn (Hippophae rhamnoides L.) berries. Int J Biol Macromol 123:280–290. https://doi.org/10.1016/j.ijbiomac.2018.11.074
Xie J, Liao S, Wang R, He X, Fang H, Zhuang Z, Niu J (2020) Molecular cloning, functional characterization and expression analysis of p65 subunit of golden pompano (Trachinotus ovatus) and response to high fat diet and LPS administration. Aquaculture 514:734508. https://doi.org/10.1016/j.aquaculture.2019.734508
Xie M, Zhou W, Xie Y, Li Y, Zhang Z, Yang Y, Zhou Z (2021) Effects of Cetobacterium somerae fermentation product on gut and liver health of common carp (Cyprinus carpio) fed diet supplemented with ultra-micro ground mixed plant proteins. Aquaculture 543:736943. https://doi.org/10.1016/j.aquaculture.2021.736943
Zhang W, Zhang X, Zou K, Xie J, Zhao S, Liu J, Wang Y (2017) Seabuckthorn berry polysaccharide protects against carbon tetrachloride-induced hepatotoxicity in mice via anti-oxidative and anti-inflammatory activities. Food Funct 8(9):3130–3138. https://doi.org/10.1039/C7FO00399D
Zhang Z, Ran C, Ding QW, Hl L, Xie MX, Yl Y, Zhou ZG (2019) Ability of prebiotic polysaccharides to activate a HIF1α-antimicrobial peptide axis determines liver injury risk in zebrafish. Communications Biology 2(1):274. https://doi.org/10.1038/s42003-019-0526-z
Zhang F, Zhang X, Gu Y, Wang M, Guo S, Liu J, ... & Duan JA (2021) Hepatoprotection of Lycii fructus polysaccharide against oxidative stress in hepatocytes and larval zebrafish. Oxidative medicine and cellular longevity 2021: 3923625https://doi.org/10.1155/2021/3923625
Zhao L, Li M, Sun K, Su S, Geng T, Sun H (2020) Hippophae rhamnoides polysaccharides protect IPEC-J2 cells from LPS-induced inflammation, apoptosis and barrier dysfunction in vitro via inhibiting TLR4/NF-κB signaling pathway. Int J Biol Macromol 155:1202–1215. https://doi.org/10.1016/j.ijbiomac.2019.11.088
Zhong R, Wan X, Wang D, Zhao C, Liu D, Gao L, Cao H (2020) Polysaccharides from marine Enteromorpha: structure and function. Trends Food Sci Technol 99:11–20. https://doi.org/10.1016/j.tifs.2020.02.030
Zou C, Fang Y, Lin N, Liu H (2021) Polysaccharide extract from pomelo fruitlet ameliorates diet-induced nonalcoholic fatty liver disease in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Fish Shellfish Immunol 119:114–127. https://doi.org/10.1016/j.fsi.2021.09.034
Funding
This research was funded by the National Key Research and Development Program of China (Project No. 2019YFD0901002), Northwest A & F University Young Talent Training Program (2452018030), Postdoctoral Science Foundation of China (2016M600821), and Key Research and Development Project of Shaanxi Province (2018NY-008).
Author information
Authors and Affiliations
Contributions
Ying Lan, Cheng Zhang, Jinding Zhang, and Haibo Yu contributed to the study conception and design. Material preparation, data collection and analysis were performed by Chi Wang and Pengju Li. The first draft of the manuscript was written by Ying Lan, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The experiment procedures were conducted in compliance with the Guidelines for Animal Research which was approved by Animal Care Committee of Northwest A&F University (Yang ling, China).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, Y., Wang, C., Zhang, C. et al. Dietary sea buckthorn polysaccharide reduced lipid accumulation, alleviated inflammation and oxidative stress, and normalized imbalance of intestinal microbiota that was induced by high-fat diet in zebrafish Danio rerio. Fish Physiol Biochem 48, 1717–1735 (2022). https://doi.org/10.1007/s10695-022-01105-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-022-01105-0