Abstract
The impact of water temperature on the physiology of Channa punctata (Bloch, 1793) was evaluated in the present study. Fish were acclimated at 25 ± 1 °C and then exposed at six different temperatures: 10, 15, 20, 25, 30, and 35 °C. C. punctata exposed at 10, 15, and 20 °C showed 30, 21, and 11% reduced food consumption, respectively compared to 25 °C. Significantly higher respiratory burst and myeloperoxidase activities were found in fish exposed at 20 and 25 °C after 12 h of exposure compared to other treatments. Nitric oxide synthase was significantly higher at 25 °C after 12 h and at 25 and 30 °C exposed fish after 7 days compared to others. The reduced glutathione level was significantly higher at 25 °C compared to other treatments after 7 days of exposure. The thiobarbituric acid reactive substances level was minimum at 25 °C. Significantly lower antioxidant enzymes, catalase, glutathione peroxidase, and glutathione S-transferase were found in gills of fish exposed at 25 °C compared to others in both samples. The highest antioxidant enzyme levels were found at 10 °C. Heat shock protein (Hsp) 70 levels were significantly lower in liver and muscle of fish exposed at 25 °C compared to other treatments. The Hsp level was significantly higher at 35 and 30 °C exposed fish compared to others after 12 h, and the level reduced after 7 days in these treatments. Thermal stress affects food consumption rate, immune system, antioxidant enzymes, and enzyme systems in fish. The elevated Hsp70 level serves as a biomarker of stress in C. punctata.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The abiotic environmental factor temperature plays a significant role in the life of poikilotherm organism fish. Temperature is considered as one of the major environmental factors in aquatic ecosystem as it influences the growth and survival rate of fish (Brander 1994; Killen 2014; Sharma et al. 2014). Exposure of fish to adverse environmental temperature deleteriously affects their biological functions that may cause a series of biochemical and physiological changes. Seasonal and diurnal fluctuations of water temperature influence individual fish throughout its life (Basu et al. 2002). Temperature beyond the optimal limit of a particular species has an adverse impact on fish health by modulating the metabolic rate, oxygen consumption, and the virulence of pathogens that in turn may cause a variety of pathophysiological disturbances (Gordon 2005; Dalvi et al. 2009). The food consumption rate and digestive enzyme activities are indicators of health status of fish and exposure of fish beyond their thermal tolerance limits affects these parameters (Chakrabarti and Sharma 2005). A shift in temperature range from the optimum changes the enzymatic activities. The food consumption rate and digestive enzyme activities in catla Catla catla and magur Clarias batrachus were reduced at < 25 °C (Singh et al. 2013; Sharma et al. 2014). Reduced food consumption rate makes the fish weak and prone to the diseases.
The rapid change of water temperature produces stress in fish and thereby affects the fish immune system (Mariana et al. 2019). The innate immune system is the fundamental defense mechanism in fish. Some of the immunological parameters, viz., respiratory burst activity, myeloperoxidase, and nitric oxide synthase, serve as bioindicators. The lysosomal protein myeloperoxidase is stored in the neutrophils, and it produces reactive oxygen species (ROS) like hypohalous acid (HOCl, HOBr, and HOI) and other toxic oxidants from hydrogen peroxide and halides. These oxidants oxidize organic molecules and have antimicrobial function (Kinkade Jr et al. 1983; Klebanoff 2005; Davies 2011). The nitric oxide synthase helps in the production of nitric oxide, the signaling molecule from L-arginine (Andrew and Mayer 1999). The inducible nitric oxide synthase also produces nitric oxide that shows a direct anti-microbial activity (Schairer et al. 2012).
The enhanced temperature results in higher oxygen consumption. Therefore, may up-regulate reactive oxygen species production as a side products of enhanced metabolism (Lushchak 2011). The imbalance between the production of oxidant and antioxidants components results into oxidative stress. Many of these compounds play an important role in metabolic pathways, essential for the defense system of the organism, and their byproducts show bactericidal activities (Billar and Takashi 2018). The antioxidant enzymes (viz., glutathione peroxidase, catalase, superoxide dismutase) stabilize and deactivate free radicals before they attack different components of cell (Blokhina et al. 2003; Noori 2012). Glutathione peroxidase protects the cells from free radicals, hydrogen, and lipid peroxides (Kohen and Nyska 2002), and glutathione S-transferases are crucial detoxification enzymes which mainly present in cytosol (Coles and Kadlubar 2003). Antioxidant enzymes and lipid peroxidation are the potential biomarkers of environmental temperature exposure in different organisms (Verlecar et al. 2007; Bocchetti et al. 2008). In the cell membrane, the reactive oxygen species causes lipid peroxidation and produces lipid hydroperoxide (LOOH) that further breaks down into aldehydes (malonaldehyde, isoprotans, hydrocarbons etc.). This affects the integrity of the membrane (Beevi et al. 2010). Heat shock proteins (Hsp), also known as stress proteins, are key markers to assess environmental stress (Iwama et al. 1999, 2004). Among various Hsps, Hsp70 is the most abundant and serves as an indicator of thermal stress (Nie et al. 2017; Saranyan et al. 2017). It plays a significant role in the tissue immune response (Fu et al. 2011).
Spotted snakehead Channa punctata, Bloch (Channidae family, Perciformes order) is an economically important freshwater, carnivore fish of South-East Asian countries (Talwar and Jhingran 1991; Froese and Pauly 2019). Usually, the fish is found in the derelict, swampy water bodies with vegetation. The air-breathing capacity makes the fish hardy and helps it to withstand low dissolve oxygen level of the water. In a natural water body, it breeds in the monsoon months and shows parental care. C. punctata is found around the temperature of 22–28 °C (Froese and Pauly 2019). The fish is marketed in a living condition; it has consumers’ preference for taste and nutritional and medicinal values (Haniffa et al. 2004). It is recommended in diet during convalescence (Marimuthu et al. 2009). So far, C. punctata is harvested from the natural water bodies; gradually, their number is decreasing due to habitat destruction and over fishing (Hossen et al. 2017). Therefore, development of proper husbandry is essential for the sustainable production and supply of this nutritious food fish. C. punctata is a good candidate for intensive culture as it tolerates a low dissolved oxygen level. Therefore, the impact of the abiotic factor, temperature on the food consumption rate, and immunological and stress biomarkers should be evaluated for the development of the proper culture technique of C. punctata. These biomarkers will help to understand the optimum culture conditions for this fish.
Materials and methods
Experimental conditions
Channa punctata (90 fish, length 18.2 ± 1.0 cm; wet weight 65.3 ± 5.42 g) were collected and transported from a local fish market and randomly distributed in glass aquaria (60 L each) maintained in the wet laboratory facility. The stocking density of fish was five fish aquarium-1. The fish were acclimated at 25 ± 1 °C for 21 days to avoid the transportation stress. Then, the fish were exposed at six different temperatures of 10, 15, 20, 25, 30, and 35 °C. In the present study, the 10–35 °C temperature was selected based on the range of temperature found in India in different months (Ahmad et al. 2014). As fish were acclimated at 25 °C prior to exposure, this was considered a control temperature. Three replicates were used for each temperature. The photoperiod was maintained as 12 h:12 h light and dark. Fish were fed with laboratory made pelleted feed at the rate of 5% of body weight throughout the experiment. The feed was composed of dry fish powder, wheat flour, cod liver oil, and vitamin-mineral premix. The protein and lipid contents of prepared feed were 42 and 10%, respectively.
Each aquarium (60 L) was connected with one filtration unit (Sera fil bioactive 130, Germany) and one cooling unit (Julabo F34, Julabo Labortechnik GmbH, Germany) or heating (Sera Aquarium Heater 300, Germany) unit for the maintenance of desirable temperature. A thermostatically controlled heater (Hicon, India) was used for the maintenance of water temperature at 35 °C. The water from fish culture aquarium was first circulated into the filtration unit, then to the cooling unit, and finally to the culture aquarium. The acclimation temperature was 25 °C, and the water temperature was either increased or decreased to achieve the assigned experimental temperature. The rate of temperature change was 1 °C per 12 h (i.e., 2 °C day-1). This required 2.5–7.5 days to reach the assigned temperature. The exact time of reaching the assigned temperature was recorded. The first sample was collected after 12 h and the second one after 7 days of reaching the assigned temperature. The total experimental exposure period was 7 days after reaching the assigned temperature. Water quality parameters, viz., temperature, pH, and dissolved oxygen, were monitored with HQ40d Multiparameter (Hach, USA) using a specific electrode. Dissolved oxygen level was maintained at 5.0 mg L-1 in all the treatment with the help of an aerator. The pH of water ranged from 7.7 - 8.0 during the study period.
The whole study was conducted following the guidelines of Institutional Animal Ethics Committee, IAEC (approved by Committee for the Purpose of Control and Supervision of Experiments on Animals, CPCSEA).
Survival rate and food consumption
Survival rate was monitored regularly. During each feeding, fish were allowed to feed for 1 h. Then unconsumed food was siphoned from individual aquarium and oven dried, and the weight was recorded. The difference between the mass of the food given and the remaining food in the aquarium after 1 h was considered as food consumption rate of individual fish and expressed as g fish-1.
Blood and tissue collection
Six fish (2 fish per aquarium × 3 replicates) per treatment (in each sampling) were collected after 12 h and 7 days of reaching the assigned temperature and anaesthetized with tricaine methanesulfonate, MS-222 (Sigma-Aldrich, USA) without showing any adverse effect on fish (Palić et al. 2006). Blood was collected from caudal vein of individual fish using 2 mL of disposable syringe (0.55 × 25 mm/24 × 1). Two different aliquots of blood were used for different analyses. The first aliquot was transferred to a micro-centrifuge tube (1.5 mL) coated with 2.7% ethylenediamine tetraacetic acid (EDTA) for respiratory burst activity estimation. The second aliquot was transferred to a micro-centrifuge tube (without EDTA), allowed to clot at room temperature for 1 h, stored at 4 °C, and centrifuged at 1500×g for 10 min. Then the serum was stored at − 20 °C and used for assays. Muscle, gills, and liver tissues were collected aseptically, frozen in liquid nitrogen, and stored at − 80 °C.
Biochemical assays
Respiratory burst activity
The respiratory burst activity was assayed using nitroblue tetrazolium, NBT (Anderson and Siwicki 1995). In 20 μL of blood sample, 20 μL of nitroblue tetrazolium (0.2%, Himedia, India) was added and kept at 25 °C for 30 min. In a separate tube, 25 μL of this mixture was taken, and dimethyl formamide (500 μL; Merck, India) was added to this mixture. This dissolved the formazan produced from nitroblue tetrazolium. The whole mixture was centrifuged at 2000×g for 5 min; the supernatant was collected, and the absorbance was recorded at 540 nm using a microplate reader (BioTek Synergy TM H1, USA).
Myeloperoxidase activity
The myeloperoxidase activity of serum was measured using a Hank’s balanced salt solution (HBSS) without Ca2+, Mg2+, and phenol red (Quade and Roth 1997). In an individual well of a 96-well microplate, 90 μL of HBSS (Himedia) and 10 μL of serum were added. Then, a 35 μL solution of 20-mM 3,3’,5,5’-tetramethylbenzidine hydrochloride and 5 mM H2O2 (Genei, India) were added into each well and incubated for 2 min, and 4 M sulfuric acid (35 μL) was added to stop the reaction. Optical density was measured at 450 nm.
Nitric oxide synthase
Nitric oxide synthase was assayed in muscle (Lee et al. 2003). In a 1-mL phosphate buffer (pH 7.4), a 100-mg tissue was homogenized and centrifuged at 10,500×g for 20 min at 4 °C. The supernatant (100 μL) was mixed with a 100 μL Griess reagent and incubated at 25 °C for 10 min. The Griess reagent was composed of 1% sulfanilamide, 0.1% N-(1-naphthyl) ethylenediamine, and 5% phosphoric acid (Himedia). The absorbance was recorded at 540 nm. The nitrite standard curve was prepared, and the concentration was expressed as mmol mg-1 tissue (wet weight basis).
Thiobarbituric acid reactive substances
In the muscle, thiobarbituric acid reactive substances (TBARS) was assayed (Ohkawa et al. 1979). In a 1.15% KCl solution (450 μL), the muscle (50 mg) was homogenized, and the sample was incubated for 1 h at 100 °C in acid medium (containing 0.45% sodium dodecyl sulfate and 0.6% thiobarbituric acid). After cooling, the sample was centrifuged at 825×g, and the absorbance of the supernatant was measured at 532 nm. The standard curve was prepared with 1,1,3,3-tetramethoxy propane (Himedia), and the concentration was expressed as μmol malondialdehyde mg-1 protein.
Reduced glutathione
Reduced glutathione (GSH) activity of gills was determined (Jollow et al. 1974). In a 10 mL phosphate buffer (0.1 M, pH 7.4), the gill tissue (1 g) was homogenized and centrifuged at 10,500×g for 20 min; the supernatant was treated as post-mitochondrial supernatant (PMS). The supernatant was precipitated with 4% sulfosalicylic acid (Himedia), kept at 4 °C for 1 h, and then centrifuged at 1500×g for 15 min at 4 °C. This supernatant was used for GSH assay. The assay mixture consisted of supernatant, 0.1 M phosphate buffer (pH 7.4), and 5-5-dithiobis-2-nitrobenzoic acid (DTNB, Himedia). Optical density was measured at 412 nm and expressed as μmol of DTNB g-1 tissue (wet weight basis).
Catalase
Catalase activity of gills was assayed using the method of Claiborne (1985). In a 10 μL of sample, 140 μL phosphate buffer (0.1 M, pH 7.4) was added and mixed well. The reaction was initiated with the addition of 50 μL of hydrogen peroxide (H2O2, 0.02 M). The change in absorbance was recorded at 240 nm. The activity was expressed as nmol of H2O2 consumed mg-1 protein min-1.
Glutathione peroxidase
The glutathione peroxidase (GPx) activity was estimated in gills (Mohandas et al. 1984). The composition of the reaction mixture was as follows: 140 μL phosphate buffer (0.1 M, pH 7.4), 10 μL EDTA (1 mM), 10 μL sodium azide (1 mM, CDH, India), 10 μL glutathione reductase (1 IU mL-1, Sigma, USA), 10 μL reduced glutathione (GSH, 1 mM, Sigma), 10 μL nicotinamide adenine dinucleotide phosphate reduced (NADPH, 0.2 mM, Himedia), 10 μL hydrogen peroxide (0.25 mM, Merck, Germany), and 10 μL sample. Oxidation of NADPH was recorded at 340 nm. The enzyme activity was calculated and expressed as nmol of NADPH oxidized mg-1 protein min-1 using a molar extinction coefficient of 6.22 × 103 M-1 cm-1.
Glutathione S-transferase
Glutathione S-transferase (GST) activity of gills was assayed (Habig et al. 1974). The composition of the reaction mixture was as follows: 30 μL supernatant, 147.5 μL phosphate buffer (0.1 M, pH 7.4), 2.5 μL 1-chloro-2-dinitrobenzene (CDNM, 0.60 mM, Sigma), and 20 μL reduced glutathione (10 mM, Himedia). Absorbance was monitored at 340 nm in kinetic mode with a BioTek microplate reader using Gen 5 software. The software helps to correct the path length automatically. Activity was expressed as nmol of CDNB mg-1 protein min-1. The molar extinction coefficient is 9.6 × 103 M-1 cm-1.
Heat shock protein 70
Fish Hsp70 ELISA kit (CUSABIO, Wuhan, China) was used for the estimation of heat shock protein (Hsp) 70 of muscle and liver. Tissue (100 mg) was rinsed with × 1 phosphate buffer saline (pH 7.4) and homogenized in 1 mL PBS using RQ-129/D (REMI, India) and stored overnight at − 20 °C. After two times of freeze-thawing (following the protocol of the manufacturer), the homogenate was centrifuged at 5000×g for 5 min at 4 °C. The supernatant was stored at − 80 °C for assay. Sample (50 μL) was added to the ELISA plate (96 wells plate) that was pre-coated with antibody. Conjugate (50 μL) was added to each well, except the blank; mixed well; and incubated at 37 °C for 60 min. Each well was aspirated and washed with 200 μL of washing buffer three times. A 50 μL of horseradish peroxidase-avidin (HRP-avidin) was added to each well, except the blank, and mixed properly and then incubated for 30 min at 37 °C. Aspiration was repeated for three times. Substrates A and B (each 50 μL, provided with the kit) were added to each well and incubated at 37 °C for 15 min. Stop solution (50 μL) was gently added to this and mixed properly. Absorbance was recorded at 450 nm, and concentration of Hsp70 was expressed (following the protocol of manufacturer) in pg mL-1(El-Ansary et al. 2012; Singh et al. 2015).
Total protein
The protein content of each tissue was measured using Folin Ciocalteus phenol reagent (Lowry et al. 1951). The optical density was measured at 750 nm. The standard was prepared with bovine serum albumin (BSA).
Statistical analysis
Data were compiled as mean ± standard error. The regression analysis showed the relationship between the temperature and the food consumption rate of fish. The Shapiro-Wilk test (1965) showed the normality of different parameters (data). The multivariate test two-factor MANOVA was performed, and the Wilks’ Lambda row showed that the two-way MANOVA was statistically significant (P < 0.001). The parameters are significantly dependent on time and temperature (P < 0.001). Further analysis was performed to determine how the dependent variables differ among the different levels of temperature and time. The Tukey’s test (2013) showed the significant differences among the treatments. The SPSS software 26.0 (South Asia (P) Ltd., Bangalore, India) was used for analysis. Statistical significance was accepted at P < 0.05 level.
Results
Survival rate and food consumption
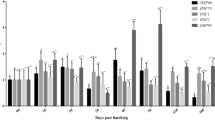
All fish survived in each culture unit. There was no mortality of fish. The results of food consumption rate are shown in Fig. 1. The food consumption rate showed direct relationship with water temperature. C. punctata consumed less food as the water temperature reduced from 25 °C. In the 10 °C treatment, as the temperature dropped to 15 °C, fish consumed 14.5% less food, and at 10 °C, 30% reduced food consumption was recorded. It required 7.5 days to reach 10 °C from the acclimation temperature 25 °C. The food consumption rate of C. punctata was recorded on day 8 (one day) of reaching the assigned temperature. A sixth-degree polynomial relationship (R2 = 0.81) was found between the water temperature and the food consumption rate of fish (Fig. 1a). In the 15 °C treatment, when temperature dropped to 20 °C, 10% less food consumption was recorded, and it was reduced 21% at 15 °C. On day 6, 15 °C was reached, and the food consumption rate was recorded for 4 days after reaching the assigned temperature. A fourth-degree polynomial relationship (R2 = 0.81) was found between the water temperature and the food consumption rate of fish (Fig. 1b). It required 2.5 days to reach 20 °C from 25 °C, and food consumption rate was recorded for consecutive 6 days after reaching the assigned temperature. In the 20 °C treatment, 11% reduced food consumption was recorded on the first day of reaching this temperature compared to the consumption rate at 25 °C. After 6 days of exposure, the food consumption rate further reduced to 24% compared to 25 °C treatment. Food consumption rate showed a sixth-degree polynomial relationship with the decreasing water temperature (R2 = 0.99) in this treatment (Fig. 1c). There was no significant difference in food consumption rate of fish exposed at 30 and 35 °C compared to the fish at 25 °C.
Effect of temperature on the food consumption rate of Channa punctata exposed at (a) 10, (b) 15, and (c) 20 °C temperatures. Water temperature gradually decreased from acclimation temperature of 25 to experimental temperatures 10, 15, and 20 °C. It required 2.5, 5, and 7.5 days to reach 10, 15, and 20 °C, respectively, from the acclimation temperature 25 °C. Food consumption rate was recorded for 6, 4, and 1 day(s) in 20, 15, and 10 °C treatments, respectively. Bar (means ± SEM) indicates the food consumption of fish at various temperatures (3 replicates)
Biochemical assays
Respiratory burst activity
Respiratory burst activity was significantly higher in fish exposed at 20 and 25 °C compared to other treatments after 12 h. After 7 days, highest activity was found at 25 °C and this treatment was followed by 15 and 30 °C. The lowest activity was found in fish exposed at 35 and 10 °C after 12 h and 7 days, respectively (Table 1).
Myeloperoxidase activity
Myeloperoxidase activity was significantly higher in fish exposed at 20 and 25 °C compared to other treatments after 12 h. There was no significant difference between these treatments. After 7 days of exposure, myeloperoxidase activity was significantly higher in fish exposed at 25–35 °C compared to fish exposed at 10–20 °C (Table 1).
Nitric oxide synthase
Nitric oxide synthase level was significantly higher in muscle of fish exposed at 25 °C after 12 h and 25 and 30 °C after 7 days of exposure compared to other treatments. Nitric oxide synthase level was minimum at 10 °C treatment in both samples (Table 1).
Thiobarbituric acid reactive substances
Thiobarbituric acid reactive substances (TBARS) level was significantly higher in fish exposed at 10 °C compared to other treatments after 12 h of exposure. After 7 days, TBARS level increased in fish exposed at 30 and 35 °C compared to the 12 h exposed fish of the same treatment. The TBARS level was minimum in fish cultured at 25 °C in both days of sampling (Table 1).
Reduced glutathione
The reduced glutathione (GSH) level was significantly higher in gills of fish exposed at 25 °C compared to other treatments after 12 h. A similar trend was found after 7 days of exposure. There was no significant difference in GSH level among fish exposed at 10, 15, and 35 °C after 12 h and 7 days of exposure (Table 1).
Catalase
In gills, significantly higher catalase activity was found at 10 °C compared to other treatments after 12 h of exposure. This group was followed by fish exposed at 35 °C. After 7 days, the catalase level increased significantly in 10–20 °C treatments compared to the activity found after 12 h of exposure in the respective treatment, whereas the catalase activity reduced in 30–35 °C treatments after 7 days compared to the 12 h exposed fish in the respective treatment. Catalase level was always minimum in fish exposed at 25 °C (Table 1).
Glutathione peroxidase
The effect of temperature change was recorded on glutathione peroxidase (GPx) level of gills. GPx level increased when the water temperature decreased from 25 °C to 20–10 °C after 12 h of exposure. It was interesting to record that GPx level was also higher in fish exposed at 30–35 °C compared to the fish exposed at 25 °C. Significantly lower GPx level was found in fish exposed at 25 °C in both samples. GPx level reduced significantly in all treatments after 7 days compared to the 12 h exposed fish in the same treatment, except the 20 °C treatment (Table 1).
Glutathione S-transferase
Significantly, the lower level of glutathione S-transferase (GST) was found in gills of fish exposed at 25 °C compared to other treatments in both samples. In gills of 10 °C exposed fish, 4- and 4.65-fold higher GST levels were found compared to control fish after 12 h and 7 days, respectively (Table 1).
Heat shock protein70
In the liver of C. punctata, Hsp70 level was significantly higher at 35 °C after 12 h of exposure. This group was followed by 30 °C treatment. Hsp70 was always minimum in fish exposed at 25 °C. After 7 days of exposure, Hsp70 level reduced significantly in all treatments compared to the 12 h exposed fish of same temperature (Fig. 2a). Like the liver, significantly higher level of Hsp70 was found in the muscle of fish exposed at 30 and 35 °C compared to the other treatments after 12 h of exposure. After 7 days, Hsp levels reduced in fish exposed at 30 and 35 °C compared to the 12 h exposed fish in the same treatment. Hsp70 level was minimum in fish exposed at 25 °C in both samples (Fig. 2b).
Discussion
Water temperature is an important factor that influences the physiology and survival rate of the aquatic species. Lowering of temperature from the acclimation temperature caused mortality of catla and magur (Singh et al. 2013; Sharma et al. 2014). This was very interesting that there was no mortality of C. punctata at 10 °C. In the present study, reduced food consumption was recorded with decreasing water temperature. The earlier study with catla and magur also showed that food consumption rates were affected at temperature below 25 °C (Ahmad et al. 2014; Sharma et al. 2017). Like catla and magur, food consumption rate of spotted snakehead was also unaffected at temperatures 30–35 °C. The low temperature has a significant negative impact on fish appetite, growth, and activity (Brown et al. 1989; Clark et al. 1995; Bendiksen et al. 2002). Fish are exposed at low temperature during winter that results into reduced food consumption and poor fish growth. Water temperature below 25 °C affected the food consumption rate and digestive enzyme activities in fish, and these parameters served as indicators of stress in fish (Ahmad et al. 2014).
In C. punctata, the impact of temperature change on the immune system was documented. A significant reduction in respiratory burst, myeloperoxidase, and nitric oxide synthase levels were observed in spotted snakehead exposed at < 25 °C, except similar levels of respiratory burst and myeloperoxidase were found in fish exposed at 20 and 25 °C after 12 h of exposure. These parameters are indicators of compromised immune system of fish. The respiratory burst activity is an important oxygen-dependent pathogen-killing mechanism in phagocytic cells, like monocytes, macrophages, and neutrophils. In fish, non-specific immune activity plays a significant role to give protection against pathogens (Haugland et al. 2012). Myeloperoxidase is released from cytoplasmic granules of neutrophils and monocytes. It reacts with the H2O2 produced in respiratory burst to form a complex which can oxidize a variety of substances. The hypochlorous acid, the product of this reaction, is cytotoxic, and it helps neutrophil to kill bacteria and other pathogens (Klebanoff 1999). The reduced values of respiratory burst activity and myeloperoxidase at 10–20 °C after 7 days compared to 12 h exposed C. punctata showed the long-term effect of temperature mediated stress in fish. A decreased level of nitric oxide synthase was observed with the lowering of water temperature from the acclimation temperature, 25 °C. A similar result was reported in catla exposed at low temperature (Sharma et al. 2017). After 7 days of exposure, the elevated level of nitric oxide synthase was recorded compared to the 12 h exposed fish at 10–20 °C.
The exposure of C. punctata at 10–20 °C for 12 h caused a significant oxidation in the muscle as TBARS level was higher in these treatments compared to 25–30 °C treatments. The increased lipid peroxidation and disturbed antioxidant enzymes resulted in additional stress in spotted snakehead at low temperature. Significantly higher lipid peroxidation was found in spotted snakehead exposed at 32 °C compared to the control group kept at 20 °C (Kaur et al. 2005). A 2.7-fold increase in lipid peroxidation was observed in juvenile European seabass Dicentrarchus labrax during post-thermal stress (Vinagre et al. 2012). Like 10 °C exposed C. punctata, significantly higher TBARS levels were also found at 30–35 °C exposed fish (compared to 25 °C) after 7 days of exposure. The exposure of bald notothen Pagothenia borchgrevinki at 4 °C for 3 weeks caused higher levels of lipid peroxides (Almroth et al. 2015). This showed the impact of long duration exposure in the muscle.
GSH level was affected in gills of C. punctata exposed at low and high temperatures compared to the control group. A similar result was also observed in the same species exposed at 32 °C (Kaur et al. 2005). In temperature stress, probably thiols content of the cells is modulated to overcome the effect of reactive oxygen species. Therefore, they are the first to be used in cellular defense against oxidative stress (Lushchak and Bagnyukova 2006a, 2006b; Bagnyukova et al. 2007). In gills of spotted snakehead, antioxidant enzymes catalase, GPx, and GST levels were enhanced when the water temperature dropped from 25 to 20–10 °C and also increased from 25 to 30–35 °C. An increase in ambient temperature caused higher antioxidant enzyme activities. This may be related to the higher activity of glutathione-dependent enzymes, especially GPx and GST. These enzymes played an important role in antioxidant defenses against oxidative stress (Lushchak and Bagnyukova 2006a, 2006b; Bagnyukova et al. 2006, 2007). GPx catalyzes the reduction of H2O2 using reduced glutathione and provides protection to the animal cells against oxidative damage (Kohen and Nyska 2002). Higher antioxidant enzyme was found in European seabass exposed at different temperatures (Vinagre et al. 2012). The exposure of bald notothen P. borchgrevinki at 4 °C for 12 h showed enhanced levels of antioxidant enzymes, viz., glutathione reductase, glutathione peroxidase, and glutathione S-transferase levels (Almroth et al. 2015). After 7 days, catalase levels in gills of C. punctata showed different responses compared to the 12 h exposed fish in the same treatment. Catalase level increased at 10–20 °C, whereas the level reduced at 25–35 °C. In the present study, increased levels of antioxidant enzymes were also found at low temperature. The cold temperatures are accompanied by an elevated physical dissolution of oxygen and may enhance the availability of oxygen to ROS-generating processes at low temperatures (Grim et al. 2010). The two-factor MANOVA shows that the temperature has a statistically significant effect on all parameters (P < 0.001); the sampling time, i.e., first sampling (after 12 h of reaching the assigned temperature) and second sampling (after 7 days), also shows a significant impact on the parameters, except for the dependent variables myeloperoxidase, TBARS, and GST (Table 1). Further, significant interaction has been found between temperature and time for all parameters, except nitric oxide synthase.
Heat shock proteins are another protective mechanism of cellular system that protect cell against heat and cold shock and environmental contaminants. Various studies suggested that cellular stress response modulates the Hsps and enhances the survival and health of the stressed fish (Wang et al. 2007; Liu et al. 2017; Sales et al. 2019). Higher level of Hsp70 was found in liver and muscle of C. punctata exposed at 30–35 °C compared to the other treatments after 12 h of exposure. An acute and chronic temperature exposure of sea bream Spondyliosoma cantharus resulted into increased Hsp70 expression in fish exposed at a high temperature (32 °C) compared to fish maintained at 25 °C (Deane and Woo 2005). Increased levels of Hsp70 were observed in Florida pompano Trachinotus carolinus and spotted sea bass Lateolabrax maculates in acute thermal stress (Cardoso et al. 2015; Shin et al. 2018). In the present study, the increased Hsp70 level might be linked with an antioxidant enzyme. In C. punctata, elevated levels of Hsp70 and catalase were found at 30–35 °C after 12 h of exposure. After 7 days, the expressions of Hsp70 were lower in the liver and muscle, and also the catalase level reduced compared to 12 h exposed fish at 30 and 35 °C. The increased levels of Hsp70 and catalase were found in mud crab Scylla paramamosain exposed at 35 °C (Liu et al. 2018). The elevated level of Hsp70 lasted for a few hours (Somero 2002). Tissue-specific expression of Hsp70 was also recorded in the present study as the higher level of Hsp70 was found in the liver compared to the muscle of fish exposed at the same treatment. In western painted turtle Chrysemys picta, around 3- and 4-fold higher expressions of Hsp 73 were found in the brain and liver compared to the skeletal muscle (Scott et al. 2003). In thermal stress, tissue-specific regulation of HSF1 was found in zebrafish Danio rerio (Rabergh et al. 2000). The study showed the presence of two zHSF1 mRNA forms that were expressed in a tissue-specific fashion in heat stress.
Conclusions
In Channa punctata, temperature below 25 °C affected food consumption rate, immune system, antioxidant enzymes, enzyme systems, and Hsp70 and caused lipid peroxidation. Temperature above 25 °C affected antioxidant enzymes and enzyme system and resulted into elevated Hsp70 level and lipid peroxidation of tissues. These information will help to develop suitable culture strategy for this economically important food fish. The present study also confirms the suitability of the studied parameters as efficient thermal biomarkers for fish.
Data availability
All data related to this study are included in the manuscript.
References
Ahmad T, Singh SP, Khangembam BK, Sharma JG, Chakrabarti (2014) Food consumption and digestive enzyme activity of Clarias batrachus exposed to various temperatures. Aquac Nutr 20:265–272
Almroth BC, Asker N, Wassmur B, Rosengren A, Jutfelt F, Gräns A et al (2015) Warmer water temperature results in oxidative damage in an Antarctic fish, the bald notothen. J Exp Mar Biol Ecol 468:130–137
Anderson DP, Siwicki AK (1995) Basic haematology and serology for fish health programs. In: Shariff M, Arthur JR, Subasinghe RP (eds) Diseases in Asian aquaculture II, Manila. Fish Health Section, Asian Fisheries Society, Manila, Philippines, pp 185–202
Andrew PJ, Mayer B (1999) Enzymatic function of nitric oxide synthases. Cardiovasc Res 43:521–531
Bagnyukova TV, Chahrak OI, Lushchak VI (2006) Coordinated response of goldfish antioxidant defenses to environmental stress. Aquat Toxicol 78:325–331
Bagnyukova TV, Lushchak OV, Storey KB, Lushchak VI (2007) Oxidative stress and antioxidant defense responses by goldfish tissues to acute change of temperature from 3 to 23 °C. J Therm Biol 32:227–334
Basu N, Todgham AE, Ackerman PA, Bibeau MR, Nakano K, Schulte PM, Iwama GK (2002) Heat shock protein genes and their functional significance in fish. Gene 295:173–183
Beevi SS, Narasu ML, Gowda BB (2010) Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Food Hum Nutr 65:8–17
Bendiksen EÅ, Jobling M, Arnesen AM (2002) Feed intake of Atlantic salmon parr Salmo salar L. in relation to temperature and feed composition. Aquac Res 33:525–532
Billar JD, Takashi LS (2018) Oxidative stress and fish immune system: phagocytosis and leukocyte respiratory burst activity. An Acad Bras Cienc 90:3403–3414
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bocchetti R, Lamberti CV, Pisanelli B, Razzetti EM, Maggi C, Catalano B, Sesta G, Martuccio G, Gabellini M, Regoli F (2008) Seasonal variations of exposure biomarkers, oxidative stress responses and cell damage in the clams, Tapes philippinarum, and mussels, Mytilus galloprovincialis, from Adriatic Sea. Mar Environ Res 66:24–26
Brander KM (1994) Patterns of distribution, spawning, and growth in North Atlantic cod: the utility of inter-regional comparisons. ICES Mar Sci Symp 198:406–413
Brown JA, Pepin P, Methven DA, Somerton DC (1989) The feeding, growth and behaviour of juvenile cod, Gadus morhua L, in cold environments. J Fish Biol 35:373–380
Cardoso CM, Sartorio PV, Machado AS, Vignardi CP, Rojas DC, Passos MJ, Rocha AJ, Van Ngan P, Gomes V (2015) Hsp70 and p53 expressions and behavior of juvenile pompano, Trachinotus carolinus (Perciformes, Carangidae), at controlled temperature increase. J Exp Mar Biol Ecol 470:34–42
Chakrabarti R, Sharma JG (2005) Digestive physiology of fish larvae during ontogenic development: a brief overview. Indian J Anim Sci 75:1337–1347
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC Handbook of methods in oxygen radical research. CRC Press Inc, Boca Raton, pp 283–284
Clark DS, Brown JA, Goddard SJ, Moir J (1995) Activity and feeding behaviour of Atlantic cod (Gadus morhua) in sea pens. Aquaculture 131:49–57
Coles BF, Kadlubar FF (2003) Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors 17:115–130
Dalvi RS, Pal AK, Tiwari LR, Das T, Baruah K (2009) Thermal tolerance and oxygen consumption rates of the catfish Horabagrus brachysoma (Günther) acclimated to different temperatures. Aquaculture 295:116–119
Davies MJ (2011) Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr 48:8–19
Deane EE, Woo N (2005) Expression studies on glucose-6-phosphate dehydrogenase in sea bream: effects of growth hormone, somatostatin, salinity and temperature. J Exp Zool A 303:676–688
El-Ansary AK, Bacha AB, Kotb M (2012) Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation 9:74 http://www.jneuroinflammation.com/content/9/1/74
Froese R, Pauly D (2019) Fish base. World Wide Web electronic publication. Available at: http://www.fishbase.org (12/2019) (accessed on July 20, 2020)
Fu D, Chen J, Zhang Y, Yu Z (2011) Cloning and expression of a heat shock protein (HSP) 90 gene in the haemocytes of Crassostrea hongkongensis under osmotic stress and bacterial challenge. Fish Shellfish Immunol 31:118–125
Gordon CJ (2005) Temperature and toxicology: an integrative, comparative, and environmental approach. CRC Press, Boca Raton
Grim JM, Miles DRB, Crockett EL (2010) Temperature acclimation alters oxidative capacities and composition of membrane lipids without influencing activities of enzymatic antioxidants or susceptibility to lipid peroxidation in fish muscle. J Exp Biol 213:445–452
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Haniffa MA, Marimuthu K, Nagarajan M, Arokiaraj AJ, Kumar D (2004) Breeding behaviour and parental care of the induced breed spotted murrel Channa punctata under captivity. Curr Sci 86:1375–1376
Haugland GT, Jordal AEO, Wergeland HI (2012) Characterization of small, mononuclear blood cells from salmon having high phagocytic capacity and ability to differentiate into dendritic like cells. PLoS One 7:e49260
Haynes W (2013) Tukey’s Test. In: Dubitzky W, Wolkenhauer O, Cho KH, Yokota H (eds) Encyclopedia of systems biology. Springer, New York. https://doi.org/10.1007/978-1-4419-9863-7_1212
Hossen MA, Paul AK, Hossain MDY, Ohtomi J, Sabbir W, Rahman O, Jasmin J, Khan MDN, Islam MDAR, Khatun D, Kamruzzaman SK (2017) Estimation of biometric indices for snakehead Channa punctata (Bloch, 1793) through multi-model inferences. JJBS 12:197–202
Iwama GK, Vijayan MM, Forsyth RB, Ackerman PA (1999) Heat shock proteins and physiological stress in fish. Am Zool 39:901–909
Iwama GK, Afonso LO, Todgham A, Ackerman P, Nakano K (2004) Are hsps suitable for indicating stressed states in fish? J Exp Biol 207:15–19
Jollow DJ, Mitchell JR, Zampaglione NA, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Kaur M, Atif F, Ali M, Rehman H, Raisuddin S (2005) Heat stress-induced alterations of antioxidants in the freshwater fish Channa punctata Bloch. J Fish Biol 67:1653–1665
Killen SS (2014) Growth trajectory influences temperature preference in fish through an effect on metabolic rate. J Anim Ecol 83:1513–1522
Kinkade JM Jr, Pember SO, Barnes KC, Shapira R, Spitznagel JK, Martin LE (1983) Differential distribution of distinct forms of myeloperoxidase in different azurophilic granule subpopulations from human neutrophils. Biochem Biophys Res Commun 114:296–303
Klebanoff SJ (1999) Myeloperoxidase. Proc Assoc Am Physicians 111:383–389
Klebanoff SJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–625
Kohen R, Nyska A (2002) Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650
Lee DU, Kang YJ, Park MK, Lee YS, Seo HG, Kim TS, Kim CH, Chang KC (2003) Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-α, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci 73:1401–1412
Liu Y, Ma D, Zhao C, Xiao Z, Xu S, Xiao Y, Wang Y, Liu Q, Li J (2017) The expression pattern of hsp70 plays a critical role in thermal tolerance of marine demersal fish: multilevel responses of Paralichthys olivaceus and its hybrids (P. olivaceus♀ × P. dentatus♂) to chronic and acute heat stress. Mar Environ Res 129:386–395
Liu Z-M, Zhu X-L, Lu J, Cai W-J, Ye Y-P, Yao-Ping Lv Y-P (2018) Effect of high temperature stress on heat shock protein expression and antioxidant enzyme activity of two morphs of the mud crab Scylla paramamosain. Comp Biochem Physiol A 223:10–17
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Lushchak VI, Bagnyukova TV (2006a) Temperature increase results in oxidative stress in goldfish tissues. 1. Indices of oxidative stress. Comp Biochem Physiol C Toxicol Pharmacol 143:30–35
Lushchak VI, Bagnyukova TV (2006b) Temperature increase results in oxidative stress in goldfish tissues. 2. Antioxidant and associated enzymes. Comp Biochem Physiol C Toxicol Pharmacol 143:36–41
Mariana S, Alfons, Gamal B (2019) Impact of heat stress on the immune response of fishes. J Sur Fish Sci 5:149–159
Marimuthu K, Arokiaraj J, Haniffa MA (2009) Effect of diet quality on seed production of the spotted snakehead Channa punctatus (Bloch). Am-Eurasian J Sustain Agric 3:344–347
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ (1984) Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic nephropathy. Biochem Pharmacol 33:1801–1807
Nie H, Liu L, Huo Z, Chen P, Ding J, Yang F, Yan X (2017) The HSP70 gene expression responses to thermal and salinity stress in wild and cultivated Manila clam Ruditapes philippinarum. Aquaculture 470:149–156
Noori S (2012) An overview of oxidative stress and antioxidant defensive system. Sci Rep 1:1–9
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Palić D, Herolt DM, Andreasen CB (2006) Anesthetic efficacy of tricaine methanesulfonate, metomidate and eugenol: effects on plasma cortisol concentration and neutrophil function in fathead minnows (Pimephales promelas Rafinesque, 1820). Aquaculture 254:675–685
Quade MJ, Roth JA (1997) A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol 58:239–248
Rabergh CMI, Airaksinen S, Soitamo A, Bjorklund HV, Johansson T, Nikinmaa M, Sistonen L (2000) Tissue-specific expression of zebrafish (Danio rerio) heat shock factor 1 mRNAS in response to heat stress. J Exp Biol 203:1817–1824
Sales CF, Lemos FS, Morais RD, Thomé RG, Santos HB, Pinheiro AP, Bazzoli N, Rizzo E (2019) Thermal stress induces heat shock protein 70 and apoptosis during embryo development in a Neotropical freshwater fish. Reprod Fertil Dev 31:547–556. https://doi.org/10.1071/RD18217
Saranyan PV, Ross NW, Benfey TJ (2017) Erythrocyte heat shock protein responses to chronic (in vivo) and acute (in vitro) temperature challenge in diploid and triploid salmonids. Comp Biochem Physiol A 206:95–104
Schairer DO, Chouake J, Nosanchuk JD, Friedman JM (2012) The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 3:271–279
Scott MA, Locke M, Buck LT (2003) Tissue-specific expression of inducible and constitutive Hsp70 isoforms in the western painted turtle. J Exp Biol 206:303–311
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality. Biometrika 52:591–611
Sharma JG, Singh SP, Mittal P, Chakrabarti R (2014) Impact of temperature gradient on the Indian major carp Catla catla larvae. Proc Natl Acad Sci India Sect B Biol Sci 86:269–273
Sharma JG, Singh SP, Chakrabarti R (2017) Effect of temperature on digestive physiology, immune-modulatory parameters, and expression level of Hsp and LDH genes in Catla catla (Hamilton, 1822). Aquaculture 479:134–141
Shin MK, Park HR, Yeo WJ, Han KN (2018) Effects of thermal stress on the mRNA expression of SOD, HSP90, and HSP70 in the spotted sea bass (Lateolabrax maculatus). Ocean Sci J 53:43–52
Singh SP, Sharma JG, Ahmad T, Chakrabarti R (2013) Effect of water temperature on the physiological responses of Asian catfish Clarias batrachus (Linnaeus 1758). Asian Fish Sci 26:26–38
Singh KM, Sharma JG, Chakrabarti R (2015) Simulation study of natural UV-B radiation on Catla catla and its impact on physiology, oxidative stress, Hsp70 and DNA fragmentation. Photochem Photobiol 149:156–163
Somero GN (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr Comp Biol 42:780–789
Talwar PK, Jhingran AG (1991) Inland fishes of India and adjacent countries. vol 1. A.A. Balkema, Rotterdam, p 541
Verlecar XN, Jena KB, Chainy GB (2007) Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem Biol Interact 167:219–226
Vinagre C, Madeira D, Narciso L, Cabral HN, Diniz M (2012) Effect of temperature on oxidative stress in fish: lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol Indic 23:274–279
Wang J, Wei Y, Li X, Cao H, Xu M, Dai J (2007) The identification of heat shock protein genes in goldfish (Carassius auratus) and their expression in a complex environment in Gaobeidian Lake, Beijing, China. Comp Biochem Physiol C Toxicol Pharmacol 145:350–362
Acknowledgments
Authors are thankful to Prof. P. Mittal, Satyawati College, University of Delhi, for his significant contribution in the statistical analysis and interpretation.
Funding
This study has been carried out with the financial support from Indian Council of Agricultural Research, ICAR, New Delhi (NFBSFARA project, AS-2001/2010-11).
Author information
Authors and Affiliations
Contributions
RC and JGS designed the study. TA, SPS, JGS, and RC cultured the fish and analyzed samples. RC and JGS prepared and revised the manuscript. RC, SPS, and TA prepared the tables and graphs. RC performed statistical analysis.
Corresponding author
Ethics declarations
The guidelines of Animal Ethics Committee (IAEC), Department of Zoology, University of Delhi, Delhi, India, was followed for the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, S.P., Ahmad, T., Sharma, J. et al. Effect of temperature on food consumption, immune system, antioxidant enzymes, and heat shock protein 70 of Channa punctata (Bloch, 1793). Fish Physiol Biochem 47, 79–91 (2021). https://doi.org/10.1007/s10695-020-00896-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00896-4