Abstract
Understanding fish larval development is of a great interest for aquaculture production efficiency. Identifying possible indicators of fish larvae stress could improve the production and limit the mortality rate that larval stage is subjected to. Heat-shock proteins (HSPs) and heat-shock factors (HSFs) are well known as indicators of response to many kinds of stressor (e.g., environmental, morphological, or pathological changes). In this study, golden pompano larvae were raised at different temperatures (23 °C, 26 °C, and 29 °C), as well as three different diets (Artemia nauplii unenriched, Artemia nauplii enriched with Nannochloropsis sp., and Artemia nauplii enriched with Algamac 3080), and the expression of HSP60, HSP70, HSF1, HSP2, and GRP94 were monitored. While stress genes were widely expressed in the larval tissues, HSP60 and HSP70 were principally from the gills and heart; HSF1 principally from the muscle, brain, and heart; and GRP94 principally from the head kidney and spleen. Golden pompano larvae were found to be more sensitive to thermal changes at later larval stage, and 29 °C was showed to likely be the best condition for golden pompano larval development. Nannochloropsis sp.–enriched Artemia nauplii treatment was found to be the most appropriate feed type with moderate relative expressions of HSP60, HSP70, HSF1, HSF2, and GRP94.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During all their life stages, fish are exposed to changes in environmental conditions and constantly need to adjust to survive in their environment (e.g., predation, food availability, or seasonal and/or sudden fluctuations in water temperatures) (Barat et al. 2016). Likely to be exposed to stressful conditions due to their ectothermic properties, fish primarily undertake sophisticated mechanisms (i.e., physiological cascade of events: neuronal signaling, hormonal release, stored form of energy metabolizing, and liberation) in order to maintain their homeostasis (Schreck and Tort 2016). The reallocated energy, arising from the stress response, is of use for different processes such as hydromineral, immune, cardiovascular, or respiratory response.
Other physiological responses are also commonly initiated by a variety of stressors including heat, hypoxia, oxidative stressors, and pathogens. An adaptive response is then developed, called stress response or heat-shock response, to assist misfolded proteins in stressed cells (e.g., elevated temperatures cause cellular protein denaturation and aggregation) (Ooi and Prahlad 2017). The heat-shock response corresponds to a rapid production of highly conserved proteins, known as heat-shock proteins (HSPs) or protein chaperones (Nakai 2016). Expression of HSPs, induced and regulated mainly at the transcription level by heat-shock factor (HSF), facilitates many cellular processes including protein synthesis, protein folding, and suppress protein aggregation and inhibits protein denaturation following stress (Wang et al. 2016). This universal mechanism maintains protein homeostasis, protects cells from protein-damaging slander, and is an important biological defense system (Lindquist 1986). These coordinated changes, producing a cellular mRNA pool (transcriptome), are some of the quickest and more adaptable responses available to organisms subjected to environmental stress due to HSPs lack of introns (Buckley and Gracey 2006; Shi et al. 2015).
The golden pompano, Trachinotus ovatus (Linnaeus, 1758), widely distributed in Asia-Pacific regions and acclimated to temperatures between 25 and 32 °C (Niu et al. 2013), has become a fish of interest for commercial culture in recent years. Its fast growth, high flesh quality, and suitability for cage culture have made golden pompano an easy species to maintain in aquaculture (Ransangan et al. 2011). Nevertheless, fish from aquaculture can often suffer high temperature, poor water quality, and crowding environment, which can ultimately generate invasion of bacteria and viruses. These various environmental factors might impact the fish homeostasis, causing stress responses and leading fish to reallocate their energy to these adaptive responses. Optimum conditions for fish maintenance in aquaculture have been carefully studied and provided mainly to juvenile and adult stages (Guo et al. 2014; Ma et al. 2014; Tutman et al. 2004). Larval stage, on the contrary, has been underestimated and is now recognized to be an important bottleneck for growth rates and survival of specimens (Rønnestad et al. 2013). The marine teleost larval phase represents a follow-up of radical changes in behavior, metabolism, and morphology to reach the juvenile stage, in an extremely fast growth (Ma et al. 2014), governed by radical changes in gene expression and protein production. Used in previous studies as biomarkers of environmental stressors, heat-shock proteins could play a crucial role in understanding the overall development of such organisms (Buckley and Gracey 2006; Multhoff et al. 2015; Nie et al. 2017). These proteins are responsible for the maintenance of cellular homoeostasis, particularly in response to stressful conditions, and seem to be of a major influence in terms of good maturation and development of fish. Moreover, it is known that fish, depending on their developmental stages, can be more sensitive to stressors (Barton and Iwama 1991). These peaks of sensitivity occur during ontogenetic transitions such as hatching, exogenous feeding, smolting, or near the end of the reproduction (Schreck and Tort 2016).
HSFs have been suggested to play different roles in response to a variety of physiological and environmental stimuli, as well as development processes including gametogenesis, neurogenesis, and maintenance of sensory organs (Nakai 2016). The amount of HSF does not change with stress but its inactive form passes to a transcriptionally active state. The activated HSFs then bind to key promoter regions, called the heat-shock elements (HSEs) (Kim et al. 2015). This results in the transcription activation of HSPs gene and their fast expression, due to the absence of introns easing mRNA translation (Rabindran et al. 1993). HSF1, HSF2, HSF3, and HSF4 are four HSFs which have been characterized in vertebrates and named in order of discovery (Kim et al. 2015). In particular, HSF1 and HSF2 are jointly expressed either during physiological events or during stress. Some studies have shown that, in mammals, HSF1, the most studied HSF, has a role of classical stress-responsive factor that regulates the expression of HSPs (Pirkkala et al. 2001; Shabtay and Arad 2006). HSF2 was found to be more active under developmentally related conditions (Tateishi et al. 2009). Another difference between the two factors is that while HSF1 is expressed evenly, the levels of HSF2 fluctuate (Kim et al. 2015).
HSPs, expressed to act as molecular chaperones to assist misfolded proteins in stressed cells (Kim et al. 2015), range in size from 27 to 110 kDa and are categorized into several major families according to their molecular weight. Classified into five commonly used families: HSP100, HSP90, HSP70, HSP60, and small HSPs (Barat et al. 2016), HSPs are generally referred to as “stress proteins” in the literature (Iwama et al. 1998) and have functions including the stabilization of unfolded protein precursors before assembly, translocation of proteins into organelles, rearrangement of protein oligomers, dissolution of protein aggregates, and degradation of denatured proteins (Werner and Nagel 2010). The synthesis of HSPs increases in response to a variety of physical and chemical stressors, including temperature, salinity stress, metals, and some xenobiotics, and consequently, these proteins are considered good biomarkers of environmental stress (Giri et al. 2016). Known to be bind by both HSF1 and HSF2 (Barat et al. 2016), HSP70s are the most extensively studied family with highly conserved structures and are predominantly cytoplasmic proteins and present in many compartments of the cell. HSP70 is well known to its close relationship with the heat-stress resistance in marine animals (Cheng et al. 2007; Hong et al. 2015; Liu et al. 2004; Matranga et al. 2000; Wang et al. 2007). HSP60, a well-characterized chaperone, mainly localized in the mitochondrial matrix of eukaryotic cells, is a highly immunogenic molecule able to activate a large number of T cell types (Manos-Turvey et al. 2015). For example, Xu et al. (2011) have highlighted the importance of HSP60 in both immune and developmental processes and also suggested that the antiviral activity of HSP60 could be of a great interest in early larval stage studies (Xu et al. 2011).
Accumulation of misfolded proteins within the endoplasmic reticulum causes proteotoxic stress and results in the activation of the unfolded protein response from the glucose-regulated proteins (GRP) to maintain endoplasmic reticulum homeostasis (Zhu and Lee 2015). Member of the HSP90 family, a highly conserved and ubiquitous family of ATP-dependent molecular chaperones, the glucose-regulated protein, 94 kDa (GRP94), has been shown to be essential for the maturation of membrane-resident and secreted protein clients (Huck et al. 2017). GRP94 is one of the major protein that is involved in unfolded protein response and plays among other functions a crucial role in folding and exporting of Toll-like receptors (TLRs) and some other immune-relevant factors (Yang and Li 2005; Zhu and Lee 2015). TLRs receptors represent crucial sensors of pathogen-associated and danger-associated molecular patterns (Rebl et al. 2014).
The present study was conducted to evaluate the impact of change in temperature and nutrition on the expression of HSF1, HSF2, HSP60, HSP70, and GRP94 genes during the development of golden pompano larvae. To provide a comprehensive analysis of HSPs gene expression during larvae stage in relation to temperature and nutrition, its expression was firstly monitored during the 18 days of post hatching (DPH) at different temperatures (23, 26, and 29 °C). Then, gene expression at 28 DPH with three nutrient treatments was assessed. This study contributes to new knowledge on the importance of HSPs for the growth of fish larvae. Moreover, this helps to have a better understanding of fish larval development to improve the aquaculture production efficiency by identifying possible indicators of fish larvae growth.
Materials and methods
Ethics statement
The T. ovatus is not endangered or protected species, and there is no requirement for permission to perform experiments involving this species in China.
Larval rearing of golden pompano
This study stemmed from the same feeding trial as in our previous study (Ma et al. 2016). Fertilized eggs of golden pompano, after being transported from Lingshui, Hainan Province, to the Tropical Fisheries Research and Development Center, South China Sea Fisheries Research Institute (Chinese Academy of Fishery Science, Xincun Town), were hatched in 500-L fiberglass incubators at 26.5 °C with a hatching rate of 97.5 ± 1.5% (mean ± SD). On 2 DPH, larvae were stored into three 1000-L larval rearing tanks supplied with filtered seawater (5-μm pore size) from the bottom of each tank through upwelling. Larval rearing tanks were released with a daily exchange rate of 200% tank volume. To maintain dissolved oxygen close to saturation, two air stones were used in each tank. A light regime of 14 h of light and 10 h of dark was set up with a light intensity at 2400 lx. The salinity and water temperature were kept respectively at 33 ± 0.8‰ and 26.5 ± 1.0 °C throughout the experiment. Fish larvae were fed with rotifers (Brachionus rotundiformis) from 2 DPH to 10 DPH at a density of 10–20 ind/mL. Artemia nauplii were added into the rearing tank from 10 DPH until experiment was completed. Both rotifers and Artemia nauplii were enriched with docosahexaenoic acid (DHA) Protein Selco (INVE Aquaculture, Salt Lake City, USA) according to the manufacturer’s instructions. On 0, 1, 2, 3, 4, 5, 12, and 18 DPH, fish larvae were sampled (approximately 300 mg, wet weight) from rearing tanks in triplicates to study the ontogenetic expression of the heat-shock proteins. On 18 DPH, a total of 100 individuals were collected in triplicate, and examined and dissected under a stereo microscope for tissue expression analysis.
Response of HSP60, HSP70, HSF1, HSF2, and GRP94 genes to rearing temperature
At the time of arrival, all eggs were transferred into 500-L incubators and hatched at 26.5 °C. On 2 DPH, larvae with yolk sac were transferred to three different temperatures: 23, 26, and 29 °C with three replicates for each. The larvae were acclimatized at each desired temperature for 5 h, and then stocked in 500-L fiberglass tanks at a density of 60 fish L−1. Except for the rearing temperature, all the feeding protocols and rearing conditions were similar to the ones described in the previous section. Around 50 individuals were collected in triplicate on 12 DPH and 18 DPH to analyze the response of HSP60, HSP70, HSF1, HSF2, and GRP94 genes to rearing temperature.
Response of HSP60, HSP70, HSF1, HSF2, and GRP94 genes to nutrition manipulation
This present study is derived from the same feeding trial than the one in our previous study (Yang et al. 2015). The nutritional manipulation experiment was composed of three dietary treatments, each with three replicates. Larvae were fed with Artemia nauplii enriched differently with three diets. Artemia nauplii were (1) enriched with instant microalgal paste (Nannochloropsis sp., Qingdao Hong Bang Biological Technology Co., Ltd., Qingdao, China), (2) enriched with Algamac 3080® (Aquafauna, USA), and (3) without any enrichment as control. On 18 DPH, approximately 50 individuals were collected, in triplicate, for analysis of gene expression in relation to nutrition manipulations.
Total RNA extraction and reverse transcription
Total RNA was extracted using TRIzol (Invitrogen, USA). An electrophoresis on a formaldehyde-agarose gel (1.2%) was used to verify the RNA integrity. By absorbance at 260 nm, the RNA concentration was measured, and after an agarose gel electrophoresis, the purity was determined at the ratio of absorbance at 260 nm and 280 nm (260/280). RNA was reverse-transcribed to cDNA with oligo (dT) primers using a PrimeScript 1st strand cDNA synthesis kit (TaKaRa Biotechnology, Dalian Co., Ltd). The cDNA was used as a template in subsequent PCR.
Cloning of the gene cDNA and real-time PCR
Based on a test on golden pompano transcriptome sequences measured preliminary in our laboratory (Illumina HiSeq2000, annotated by NR, KOG, kegg, and Swissprot), the gene cloning primers were designed (Table 1) with Primer 5.0 (Premier Biosoft International, Palo Alto, CA, USA). The PCR reaction systems were as follows: 1 μL of golden pompano larval cDNA, 1 μL of gene-specific forward primer (F), 1 μL of gene-specific reverse primer (R), 0.5 μL of ExTaq, 5 μL of PCR buffer, 4 μL of dNTP mixture (2.5 μM), and 37.5 μL of ddH2O were mixed in a total volume of 50 μL. The PCR conditions included denaturation at 94 °C for 1 min, 35 cycles of 94 °C for 30 s, annealing temperature of each gene for 30 s, 72 °C for 4 min, followed by a 10-min extension at 72 °C. The PCR products were cloned into the PMD-19T vector (TAKARA, Japan) and sequenced.
The level of heat-shock protein gene expression in golden pompano larvae was analyzed by quantitative real-time PCR. Gene-specific primer pairs for the heat-shock protein gene (Table 1) were amplified in LightCycler480 II (Roche, Switzerland). EF-1α was used as the internal reference and amplified. The cycling conditions for heat-shock protein genes and EF-1α were as follows: 1 min at 95 °C, followed by 40 cycles 95 °C for 15 s, and 60 °C for 1 min. Dissociation curves were used to guarantee that only one single PCR product was amplified in each gene reaction. For each test, three replicates were performed. The relative quantification (RQ) was calculated using ΔΔCT (comparative threshold cycle) method ΔCT = CT of target gene − CT of EF-1α, ΔΔCT = ΔCT of any sample − ΔCT of calibrator sample. The efficiencies of the primers (E) were E H-FABP = 0.1003.
Statistical analysis
The data were expressed as mean ± SD, and compared with one-way ANOVA (PASW Statistics 18.0, Chicago, SPSS Inc.). Tukey’s test was used for multiple range comparisons with the level of significant difference set at P < 0.05. All data were tested for normality, homogeneity, and independence to satisfy the assumptions of ANOVA.
Results
Ontogenetic expression of HSP60, HSP70, HSF1, HSF2, and GRP94 genes
The relative expression of HSP60 gene in golden pompano larvae increased from post hatching until 2 DPH, with a drastic fall on 3 DPH. The highest level of expression was on 4 DPH (Fig. 1). Afterwards, the expression of HSP60 reduced significantly, and reached the lowest level on 18 DPH when fish were completely in exogenous feeding. The relative expression of HSP70 gene in golden pompano larvae reached the highest level on 1 DPH (Fig. 1). Afterwards, the expression of HSP70 reduced significantly, and remained low until 18 DPH. The expression of HSF1 gene increased steadily until 2 DPH then significantly declined and stabilized from 3 DPH to 18 DPH (Fig. 1). The expression of HSF2 gene was the highest from hatching until 2 DPH. The expression of HSF2 dropped slightly between 3 DPH and 5 DPH, then significantly at 12 DPH, staying constantly low until 18 DPH. Finally, the expression of GRP94 gene has peaked on 1 DPH and dropped until 3 DPH. It then significantly increased on 4 and 5 DPH, followed by a drop until 18 DPH. The highest level of expression was on 4 and 5 DPH.
Ontogenetic expression of HSP60, HSP70, HSF1, HSF2, and GRP94 in golden pompano larvae. The analyses were carried out by qRT-PCR and analysis results were calculated by 2-ΔΔCt method. Each bar represents the mean ± SD (n = 3). Different lowercase letters indicate statistically significant differences (P < 0.05)
Tissue expression of HSP60, HSP70, HSF1, HSF2, and GRP94 genes
On 18 DPH, the highest expression level of HSP60 was observed in the heart of larval golden pompano, followed by the gill, brain, lung, and liver (Fig. 2). The expression of HSP60 could be detected in every tissue sampled but were lowest in the eye and head kidney. The highest expression level of HSP70 was observed mainly in the gill of larvae, followed by the heart, spleen, and intestine (Fig. 2). The expression of HSP70 was especially lower in the kidney, compared to that observed in the other tissues. HSF1 expression was significantly higher in the muscles, heart, and brain of larval golden pompano (Fig. 2), and lowest in the kidney, compared to that observed in other tissues. HSF2 was very highly expressed in the liver, but was also highly variable between replicates, as shown by the error bar. Apart from the very high expression in the liver, HSF2 was also significantly expressed in the brain, muscle, stomach, intestine, and heart. Finally, the expression of GRP94 was significantly higher in the head kidney and spleen.
Expressions of HSP60, HSP70, HSF1, HSF2, and GRP94 in golden pompano larvae after 18 DPH in different tissues. The analysis were carried out by qRT-PCR, and analysis results were calculated by 2-ΔΔCt method. Each Bar represent the mean ± SD (n = 3). Different lowercase letters indicate statistically significant differences (P < 0.05)
Response of HSP60, HSP70, HSF1, HSF2, and GRP94 genes to water temperature
The expression of HSP60 was significantly affected by the rearing temperature (P < 0.05, Fig. 3a). On both 12 and 18 DPH, the highest expressions of HSP60 was observed when fish were cultured at 23 °C, then decreased with a raise in temperature. The lowest expression was observed in fish cultured at 29 °C (P < 0.05) with an expression level reduced to half compared to that at 23 °C. The expression of HSP70 was different to that of HSP60. At 12 DPH, the highest expression of HSP70 was observed when fish were cultured at 23 °C and 26 °C. At 29 °C, the expression was significantly lower compared to that at 26 °C (P < 0.05, Fig. 3b), but not different to 23 °C due to a higher variability at 23 °C. On 18 DPH, the highest expression of HSP70 was observed in fish cultured at 23 °C and the lowest at 29 °C. In this study, the expression of HSF1 was not significantly affected by the rearing temperature on 12 DPH (Fig. 3c). However, on 18 DPH, significantly higher expression of HSF1 was observed in fish cultured at 26 °C, while the expression observed in fish cultured at 23 °C and 29 °C was relatively similar. Similar to HSF1, the expression of HSF2 was not significantly affected by the rearing temperature on 12 DPH (Fig. 3d). However, at 18 DPH, a significantly higher expression of HSF2 was observed in fish cultured at 23 °C compared to 29 °C, while expression of the gene at 26 °C was not significant to either 23 °C or 29 °C. Finally, the expression of GRP94 was only significantly higher in fish at 18 DPH and cultured at 23 °C (Fig. 3e).
a–e Responses of HSP60, HSP70, HSF1, HSF2, and GRP94 to water temperature in golden pompano larvae. The analyses were carried out by qRT-PCR, and analysis results were calculated by 2-ΔΔCt method. Each bar represents the mean ± SD (n = 3). Different lowercase letters indicate statistically significant differences (P < 0.05)
Response of HSP60, HSP70, HSF1, HSF2, and GRP94 genes to nutrition manipulation
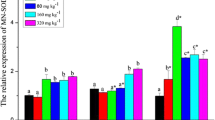
The expression levels of HSP60 and HSP70 were both significantly affected by the nutrition manipulation (P < 0.05, Fig. 4a, b). The highest expressions of HSP60 and HSP70 were observed in fish fed with unenriched food compared to the two other treatments (Nannochloropsis sp.–enriched Artemia nauplii and Algamac 3080–enriched Artemia nauplii). The lowest level of expression was reached with fish fed with Algamac 3080–enriched Artemia nauplii.
a–e Responses of HSP60, HSP70, HSF1, HSF2, and GRP94 to nutrient manipulations in golden pompano larvae. The analyses were carried out by qRT-PCR, and analysis results were calculated by 2-ΔΔCt method. Each bar represent the mean ± SD (n = 3). Different lowercase letters indicate statistically significant differences (P < 0.05)
On the contrary, the expression level of HSF1, significantly affected by the nutrition manipulation (P < 0.05, Fig. 4c), was the lowest with unenriched food treatment. The highest expression of HSF1 was observed in fish fed with Algamac 3080–enriched Artemia nauplii. Expression of HSF1 with fish fed with Nannochloropsis sp.–enriched Artemia nauplii was not significantly different from the one with fish fed with unenriched food treatment. Due to a higher variability of HSF1’s expression with the treatment of Nannochloropsis sp.–enriched Artemia nauplii, its expression level was not significantly different from the two other treatments. Similar to HSF1, the expression level of HSF2 was significantly higher for both fish fed with Algamac 3080–enriched Artemia nauplii and Nannochloropsis sp. compared to fish fed with the unenriched treatment (P < 0.05, Fig. 4d). At least, in comparison to the unenriched feeding treatment, the expression level of GRP94 was significantly higher in fish fed with Algamac 3080–enriched Artemia nauplii and lower with fish fed with Nannochloropsis sp.–enriched Artemia nauplii (P < 0.05, Fig. 4e).
Expressions of HSP60, HSP70, HSF1, HSF2, and GRP94 in normally and abnormally developed golden pompano larvae
The levels of expression of HSP60, HSP70, HSF1, HSF2, and GRP94 were relatively similar regarding normally developed fish (Fig. 5). Concerning abnormally developed fish, the levels of expression of HSP60, HSF1, HSF2, and GRP94, particularly, were significantly higher than compared to normally developed fish. HSF1 and GRP94 were both at the same relative level of expression, then HSF2’s expression was higher but subjected to higher variability and finally, HSP60’s expression was a bit lower in comparison. On the opposite, the expression level of HSP70 was significantly lower with abnormally developed golden pompano larvae than with normally developed ones.
Expressions of HSP60, HSP70, HSF1, HSF2, and GRP94 in normally and abnormally developed golden pompano larvae on 18 DPH. The analyses were carried out by qRT-PCR, and analysis results were calculated by 2-ΔΔCt method. Every vertical bars were shown as mean ± SD (n = 3). +/+ indicates significant up regulation, and −/− indicates significant downregulation (P < 0.05)
Discussion
The stress response to heat has been studied in different organs of fish, and probably every tissue responds to this stressor (Das et al. 2015). The present study cloned and analyzed the expression of stress protein genes during the early development of golden pompano.
HSP70 and HSP60 are both required in mitochondria for the translocation of proteins into the organelle and for their assembly into functional complexes. Protein, maintained unfolded by HSP70, enters the organelle to be transferred by HSP60 that then promotes the folding and assembly of these proteins into oligomeric structures (Parsell and Lindquist 1993). Our results showed presence of HSP60 expression principally in the heart and gill of golden pompano. HSP60 was previously observed in the gill, liver, spleen, or head kidney with lower response in cardiac tissue and was also found to play a significant role in dealing with heat-induced damage in the liver with greater expression in the liver than in other tissues (Shi et al. 2015). Similarly, HSP70 expression was greatest in the gill then the heart of golden pompano on 18 DPH. Strong rises in HSP70 levels have been previously reported in the gills in response to a variety of stressors (Das et al. 2015). The gills are likely to be the first organs to response to stress due to their direct exposure to the external environment by their function of water filtration. HSP60 and HSP70, closely related in term of activities, are unsurprisingly found in a similar relative order in term of proportion in the different tissues experimented on. Presence of HSPs in those tissues may point out their relative sensitivity to heat-induced cell damage in golden pompano larvae. HSF1 was expressed in every tissue sampled with a higher expression in the muscle, brain, and heart. Those results are in accordance with previous studies where HSF1 expression was mainly detected in the brain tissue of zebrafish (Yeh et al. 2006) and in the brain, kidney, heart, and gill of goldfish (Kim et al. 2015). HSF1 and HSF2 were both more expressed in the first DPHs which sustained with the fact that HSF activity permits the transcription of HSPs in the early larval stages.
During our experiment, GRP94 expression was considerably higher in the head kidney and spleen than in other tissues. The head kidney, a unique teleost organ, is made up of cytokine-producing lymphoid cells and endocrine cells secreting cortisol, catecholamine, and thyroid hormones (Geven and Klaren 2016). On the other hand, the spleen plays an important role in innate and adaptive immunity, erythrocyte turnover, and hemoglobin recycling (Papetti et al. 2015). Knowing GRP94 implication in immune responses (Rebl et al. 2014), our results concerning its tissue expression were then congruent with the fact that the head kidney and spleen are two of the most important immune organs. HSP60 expression varied during our study between hatching and 18 DPH. The lowest level of expression took place at the beginning of the exogenous phase when golden pompano digestive system undergoes an important structural and functional development to adapt to exogenous feeding, before the depletion of yolk sac reserves, while the highest levels were found just before and just after the transformation on 3 DPH, which correspond to major changes resulting in the development of the larval alimentary canal (Govoni et al. 1986). This transformation is well known to involve a big change in allocation of energy (Govoni et al. 1986), which could explain why HSP60 expression level decreases so quickly on 3 DPH during our study. HSP60, known to play a part in both immune and developmental processes, and also to have an antiviral activity (Xu et al. 2011), may have an increase in activity from hatching until the transformation corresponding to the development of the larval alimentary canal. After the HSP60 expression level decrease at the transformation, its sudden rise again could potentially be caused by the first exogenous feeding resulting in an antiviral activity. HSP70 expression, on the other hand, fluctuated from 1 DPH to 18 DPH. With HSP70 known to have a part in heat response (Hong et al. 2015), the peak in HSP70 expression early after hatching may be related to the adaptation of golden pompano larvae to the surrounding water temperature.
While HSF1 expression increased from hatching to a peak on 2 DPH, HSF2 expression started to a peak directly from hatching and slowly decreased until 18 DPH. This difference in relative expression of HSFs could be explained by the fact that HSF1, a typical stress-responsive factor, regulates the expression of HSPs, when HSF2 is somewhat more active under developmentally related conditions (Kim et al. 2015). Finally, the peak of GRP94 expression happened on the first days of exogenous feed (4 DPH and 5 DPH), which correlated with an increase of external intakes resulting in a potential rise in pathogenic agents in the organism. GRP94 seems to play an important part in the building of a larval immune system.
Fluctuation in temperature is a crucial factor of vital rate regulation during early fish life and can greatly impact enzyme activity, metabolism, growth, and development of fish (Sun et al. 2016). Metabolic rate of ectothermic animals is dependent strongly on the surrounding temperature (Korajoki and Vornanen 2014). During our experimental process, golden pompano larvae that were accustomed to an aquatic temperature of 26.5 °C ± 1.0 °C were exposed to temperatures of 23 °C, 26 °C, and 29 °C on 12DPH and 18 DPH. Levels of expression of HSP60 have been shown to be on 12 or 18 DPH similarly decreasing with temperature elevation. Yet Xu et al. (2011) pointed out that low level of expression of HSP60 at early larval stages may reflect the normal development of fish fry (Xu et al. 2011). The expression of HSP70 was the lowest on both 12 and 18 DPH at 29 °C while the stress response was similarly higher for 23 °C and 26 °C. Moreover, Wang et al. (2007) reported that mild or moderate heat shock resulted in elevated HSP70 levels in the heart tissue of Cyprinus carpio (Wang et al. 2007). In light of those facts, a lower relative expression of HSP60 and HSP70 could likely show better larvae development. Here, the most appropriate temperature to limit the larval stress and have the lowest HSP60 and HSP70 activity was 29 °C.
While on 12 DPH, fluctuations in temperature did not impact HSF1, HSF2, and GRP94 expression level; on 18 DPH, HSF1 was highly expressed at 26 °C, HSF2 at 23 °C and 26 °C, and GRP94 at 23 °C. Those results may suggest that HSFs and GRP94 are more sensitive to stress at later larval stage. Here, lower larval stress response was observed at 23 °C and 29 °C in relation to HSF1 expression, at 29 °C in relation HSF2 expression, and at 26 °C and 29 °C in relation to GRP94 expression. Overall, it would seem that 29 °C may provide the best conditions for larval development in golden pompano.
HSPs have been found to have value as markers of stress in nutritional studies in fish (Roberts et al. 2010). In particular, Urán et al. (2009) showed that variations of enterocytes at the earliest stages of a pathogenesis, caused by the nutritional treatment, concurred with a release of HSPs (Urán et al. 2009).
This study assessed the influence of three feed types on stress protein expression. Larval stage is site of a well-defined feeding behavior to control the quantity and type of food ingested. Precise knowledge of the larval nutritional requirements would contribute to improving feeding efficiency and fish quality. Indeed, larval feeding success depends on development of physiological functions of the digestive system, unlike in adult fish (Hekimoglu et al. 2014).
The expressions of HSP60 and HSP70 in fish larvae on 18 DPH were similarly affected by nutrient manipulation (Fig. 4). Artemia nauplii enriched with Algamac 3080® can enhance fish growth and reduce malformation. Fish fed Nannochloropsis sp.–enriched Artemia nauplii achieved higher survival but higher jaw malformation. Algamac 3080® or Nannochloropsis sp. supplement levels are important factors of deformed fish (Ma et al. 2018), and malformation may be stressful to HSPs genes expression. The expression level was lowest when Algamac 3080–enriched Artemia nauplii were used, suggesting that HSP60 and HSP70 were sensitive to nutrient enhancement. Previous studies have shown that higher levels of HSP70 were correlated with higher levels of granulocytes and lower lymphocyte levels in the blood (Sagstad et al. 2007), and that food deprivation enhanced HSP70 protein expression in fish larvae (Cara et al. 2005). Feeding of Algamac 3080–enriched Artemia nauplii is then likely the most suitable feeding regime than of Nannochloropsis sp.–enriched Artemia nauplii treatment according to the level of HSP60 and HSP70 expressions.
On the contrary, HSF1 and HSF2 responded better to unenriched Artemia nauplii treatment compared to enriched ones (Fig. 4). Soybean-fed fish subjected to nutritional diseases (e.g., inflammation of the distal intestine) showed gene expression changes considered as anti-inflammatory responses (Skugor et al. 2011). Our experiment revealed that GRP94 expression was lowest on fish fed with instant Nannochloropsis sp.–enriched Artemia nauplii treatment (Fig. 4). This treatment may suggest less immune response when Nannochloropsis sp.–enriched Artemia nauplii treatment is used compared to the other two treatments.
Using Nannochloropsis sp.–enriched Artemia nauplii treatment, responses of HSP60 and HSP70 were slightly higher than the lowest expressed with Algamac 3080–enriched Artemia nauplii. And responses of HSF1 and HSF2 were slightly higher than the lowest expressed with unenriched treatment. Overall, when Nannochloropsis sp.–enriched Artemia nauplii was used, the stress response was relatively mild and alike with every stress proteins (Fig. 4).
Abnormally developed golden pompano larvae showed a higher level of expression of HSP60, HSF1, HSF2, and GRP94 than normally developed ones. This may suggest that abnormally developed larvae are likely to be subject to immune and developmental stress or viral activity.
The level of expression of HSP70 was, on the contrary, diminished in abnormally developed golden pompano larvae. Lower expression of HSP70 could be related to the fact that fish developmental stress response was to be expected with abnormally developed fish while HSP70 is more heat-associated. Nonetheless, Werner et al. (2006) found significantly higher HSP70 levels in abnormally developed larvae than in normally developed larvae (Werner et al. 2007).
Our results, showing increase in stress protein, particularly regarding the immune response, correlate with abnormal larval development. This finding may corroborate with previous studies showing that abnormally developed larvae are likely to be subject to difficulties concerning their ability to interact with their environment and are frequently subject to high mortality rates (Sfakianakis et al. 2015).
In conclusion, HSPs are essential key indicators of fish larva stress-related response. Fish larva size at hatching, yolk absorption, growth, feeding, and digestion in early stages are greatly influenced by environmental temperature. Based on our experiment, golden pompano larvae were found to be more sensitive to thermal changes at later larval stage. Diminution in stress was also noticed at 29 °C, which shows that it may likely be the best condition for golden pompano larval development. Expression of stress protein was not equally spread with change in nutrition treatment. Nonetheless Nannochloropsis sp.–enriched Artemia nauplii treatment was the most appropriate feed type, specifically due to the moderate relative expression of HSP60, HSP70, HSF1, HSF2, and GRP94. Hence, it is essential to understand and highlight the optimum conditions of growth for larva stages because larvae undergo a pattern of changes associated to differences in digestive and development requirements, increasing the demand of energy and changing energy allocation.
References
Barat A, Sahoo PK, Kumar R, Goel C, Singh AK (2016) Transcriptional response to heat shock in liver of snow trout (Schizothorax richardsonii)—a vulnerable Himalayan Cyprinid fish. Funct Integr Genomics 16:203–213
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1:3–26
Buckley BA, Gracey AY, Somero GN (2006) The cellular response to heat stress in the goby Gillichthys mirabilis: a cDNA microarray and protein-level analysis. J Exp Biol 209:2660–2677
Cara JB, Aluru N, Moyano FJ, Vijayan MM (2005) Food-deprivation induces HSP70 and HSP90 protein expression in larval gilthead sea bream and rainbow trout. Comp Biochem Physiol B Biochem Mol Biol 142:426–431
Cheng P, Liu X, Zhang G, He J (2007) Cloning and expression analysis of a HSP70 gene from Pacific abalone (Haliotis discus hannai). Fish Shellfish Immunol 22:77–87
Das P, Gupta A, Manna SK (2015) Heat shock protein 70 expression in different tissues of Cirrhinus mrigala (Ham.) following heat stress. Aquac Res 36:525–529
Geven EJ, Klaren PH (2016) The teleost head kidney: Integrating thyroid and immune signalling. Dev Comp Immunol 66:73–83
Giri SS, Sen SS, Jun JW, Sukumaran V, Park SC (2016) Immunotoxicological effects of cadmium on Labeo rohita, with emphasis on the expression of HSP genes. Fish Shellfish Immunol 54:164–171
Govoni JJ, Boehlert GW, Watanabe Y (1986) The physiology of digestion in fish larvae. Environ Biol Fish 16:59–77
Guo H, Ma Z, Jiang S, Zhang D, Zhang N, Li Y (2014) Length-weight relationship of oval pompano, Trachinotus ovatus (Linnaeus 1758) (Pisces: Carangidae) cultured in open sea floating sea cages in South China Sea. Indian J Fish 61:93–95
Hekimoglu MA, Süzer C, Kop A, Saka S, Fırat K (2014) Comparison of ontogenic development and digestive enzymes in ornamental goldfish (Carassius auratus auratus L.) larvae fed with decapsulated cysts and nauplii of artemia Pakistan. J Zool 46:669–676
Hong J, Mao Y, Niu S, Sun T, Su Y (2015) Molecular characterization and expression of HSP70, HSF and HSBP genes in Octopus vulgaris during thermal stress 海洋学报(英文版). 34:62–72
Huck JD, Que NL, Hong F, Li Z, Gewirth DT (2017) Structural and functional analysis of GRP94 in the closed state reveals an essential role for the pre-N domain and a potential client-binding site. Cell Rep 20:2800–2809
Iwama GK, Thomas PT, Forsyth RB, Vijayan MM (1998) Heat shock protein expression in fish. Rev Fish Biol Fish 8:35–56
Kim SS, Chang Z, Park JS (2015) Identification, tissue distribution and characterization of two heat shock factors (HSFs) in goldfish (Carassius auratus ). Fish Shellfish Immunol 43:375–386
Korajoki H, Vornanen M (2014) Species- and chamber-specific responses of 12 kDa FK506-binding protein to temperature in fish heart. Fish Physiol Biochem 40:539–549
Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191
Liu J, Yang WJ, Zhu XJ, Karounarenier NK, Rao RK (2004) Molecular cloning and expression of two HSP70 genes in the prawn, Macrobrachium rosenbergii. Cell Stress Chaperones 9:313–323
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Qin JG, Zhang D (2014) Ontogenetic development of digestive functionality in golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem 40:1157–1167
Ma Z, Hu J, Liu Y, Yang R, Qin JG, Sun D (2016) Molecular cloning and response to water temperature and nutrient manipulation of insulin-like growth factor (IGF) genes in golden pompano Trachinotus ovatus (Linnaeus 1758) larvae. Isr J Aquacult- Bamid, IJA_68.2016.1336:14 pages
Ma Z, Hu J, Yu G, Qin JG (2018) Gene expression of bone morphogenetic proteins and jaw malformation in golden pompano Trachinotus ovatus larvae in different feeding regimes. J Appl Anim Res 46:164–177
Manos-Turvey A, Brodsky JL, Wipf P (2015) The effect of structure and mechanism of the Hsp70 chaperone on the ability to identify chemical modulators and therapeutics. 1–49
Matranga V, Toia G, Bonaventura R, Müller WE (2000) Cellular and biochemical responses to environmental and experimentally induced stress in sea urchin coelomocytes. Cell Stress Chaperones 5:113–120
Multhoff G, Pockley AG, Schmid TE, Schilling D (2015) The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett 368:179–184
Nakai A (2016) Heat shock factor. Springer
Nie H, Liu L, Huo Z, Chen P, Ding J, Yang F, Yan X (2017) The HSP70 gene expression responses to thermal and salinity stress in wild and cultivated Manila clam Ruditapes philippinarum. Aquaculture 470:149–156
Niu J, Du Q, Lin HZ, Cheng YQ, Huang Z, Wang Y, Wang J, Chen YF (2013) Quantitative dietary methionine requirement of juvenile golden pompano Trachinotus ovatus at a constant dietary cystine level. Aquac Nutr 19:677–686
Ooi FK, Prahlad V (2017) Olfactory experience primes the heat shock transcription factor HSF-1 to enhance the expression of molecular chaperones in C. elegans. Sci Signal 10:eaan4893
Papetti C, Harms L, Windisch HS, Frickenhaus S, Sandersfeld T, Jürgens J, Koschnick N, Knust R, Pörtner HO, Lucassen M (2015) A first insight into the spleen transcriptome of the notothenioid fish Lepidonotothen nudifrons: resource description and functional overview. Mar Genomics 24:237–239
Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496
Pirkkala L, Nykänen P, Sistonen L (2001) Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J 15:1118–1131
Rabindran SK, Haroun RI, Clos J, Wisniewski J, Wu C (1993) Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science 259:230–234
Ransangan J, Manin BO, Abdullah A, Roli Z, Sharudin EF (2011) Betanodavirus infection in golden pompano, Trachinotus blochii, fingerlings cultured in deep-sea cage culture facility in Langkawi, Malaysia. Aquaculture 315:327–334
Rebl A, Brietzke A, Goldammer T, Seyfert HM (2014) GRP94 is encoded by two differentially expressed genes during development of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 40:1917–1926
Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY (2010) Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: a review. J Fish Dis 33:789–801
Rønnestad I, Yúfera M, Ueberschär B, Ribeiro L, Sæle Ø, Boglione C (2013) Feeding behaviour and digestive physiology in larval fish: current knowledge, and gaps and bottlenecks in research. Rev Aquac 5:S59–S98
Sagstad A, Sanden M, Haugland Ø, Hansen AC, Olsvik PA, Hemre GI (2007) Evaluation of stress- and immune-response biomarkers in Atlantic salmon, Salmo salar L., fed different levels of genetically modified maize (Bt maize), compared with its near-isogenic parental line and a commercial suprex maize. J Fish Dis 30:201–212
Schreck CB, Tort L (2016) The concept of stress in fish. In: Fish Physiology, vol 35. Elsevier, p 1–34
Sfakianakis DG, Renieri E, Kentouri M, Tsatsakis AM (2015) Effect of heavy metals on fish larvae deformities: a review. Environ Res 137:246–255
Shabtay A, Arad Z (2006) Reciprocal activation of HSF1 and HSF3 in brain and blood tissues: is redundancy developmentally related? Am J Physiol Regul Integr Comp Physiol 291:R566–R572
Shi HN, Liu Z, Zhang JP, Kang YJ, Wang JF, Huang JQ, Wang WM (2015) Short communication: effect of heat stress on heat-shock protein (Hsp60) mRNA expression in rainbow trout Oncorhynchus mykiss. Genet Mol Res 14:5280–5286
Skugor S, Grisdale-Helland B, Refstie S, Afanasyev S, Vielma J, Krasnov A (2011) Gene expression responses to restricted feeding and extracted soybean meal in Atlantic salmon (Salmo salar L.). Aquac Nutr 17:505–517
Sun X et al (2016) Feed type regulates the fatty acid profiles of golden pompano Trachinotus ovatus (Linnaeus 1758). J Appl Anim Res 1–4
Tateishi Y, Ariyoshi M, Igarashi R, Hara H, Mizuguchi K, Seto A, Nakai A, Kokubo T, Tochio H, Shirakawa M (2009) Molecular basis for SUMOylation-dependent regulation of DNA binding activity of heat shock factor 2. J Biol Chem 284:2435–2447
Tutman P, Glavic N, Kozul V, Skaramuca B, Glamuzina B (2004) Preliminary information on feeding and growth of pompano, Trachinotus ovatus (Linnaeus, 1758) (Pisces; Carangidae) in captivity. Aquac Int 12:387–393
Urán PA, Schrama JW, Rombout JHWM, Taverne-Thiele JJ, Obach A, Koppe W, Verreth JAJ (2009) Time-related changes of the intestinal morphology of Atlantic salmon, Salmo salar L., at two different soybean meal inclusion levels. J Fish Dis 32:733–744
Wang Y, Xu J, Sheng L, Zheng Y (2007) Field and laboratory investigations of the thermal influence on tissue-specific Hsp70 levels in common carp (Cyprinus carpio). Comp Biochem Physiol A Mol Integr Physiol 148:821–827
Wang T-Y, Chen Y-M, Chen T-Y (2016) Molecular cloning of orange-spotted grouper (Epinephelus coioides) heat shock transcription factor 1 isoforms and characterization of their expressions in response to nodavirus. Fish Shellfish Immunol 59:123–136
Werner I, Viant MR, Rosenblum ES, Gantner AS, Tjeerdema RS, Johnson ML (2006) Cellular responses to temperature stress in steelhead trout (onchorynchus mykiss) parr with different rearing histories. Fish Physiology & Biochemistry 32(3):261–273
Werner I, Nagel R (2010) Stress proteins HSP60 and HSP70 in three species of amphipods exposed to cadmium, diazinon, dieldrin and fluoranthene. Environ Toxicol Chem 16:2393–2403
Werner I, Linares-Casenave J, Eenennaam JPV, Doroshov SI (2007) The effect of temperature stress on development and heat-shock protein expression in larval green sturgeon ( Acipenser mirostris ). Environ Biol Fish 79:191–200
Xu XY, Shen YB, Fu JJ, Liu F, Guo SZ, Yang XM, Li JL (2011) Molecular cloning, characterization and expression patterns of HSP60 in the grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol 31:864–870
Yang Y, Li Z (2005) Roles of heat shock protein gp96 in the ER quality control: redundant or unique function? Mol Cells 20:173
Yang Q, Zheng P, Ma Z, Li T, Jiang S, Qin JG (2015) Molecular cloning and expression analysis of the retinoid X receptor (RXR) gene in golden pompano Trachinotus ovatus fed Artemia nauplii with different enrichments. Fish Physiol Biochem 41:1449–1461. https://doi.org/10.1007/s10695-015-0098-x
Yeh FL, Hsu LY, Lin BA, Chen CF, Li IC, Tsai SH, Hsu T (2006) Cloning of zebrafish (Danio rerio) heat shock factor 2 (HSF2) and similar patterns of HSF2 and HSF1 mRNA expression in brain tissues. Biochimie 88:1983–1988
Zhu G, Lee AS (2015) Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol 230:1413–1420
Funding
This project was supported by the National Natural Science Foundation of China (31502186), Pearl River S&T Nova Program of Guangzhou (201610010166), and Ministry of Human Resources and Social Security of China (2016 High-level Overseas Researcher Come Back to Work Funds).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Trachinotus ovatus is not endangered or protected species, and there is no requirement for permission to perform experiments involving this species in China.
Rights and permissions
About this article
Cite this article
Allais, L., Zhao, C., Fu, M. et al. Nutrition and water temperature regulate the expression of heat-shock proteins in golden pompano larvae (Trachinotus ovata, Limmaeus 1758). Fish Physiol Biochem 45, 485–497 (2019). https://doi.org/10.1007/s10695-018-0578-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0578-x