Abstract
Water temperature directly affects the body temperature in fish, so increasing water temperatures in oceans and rivers will lead to increases in fish body temperatures. Whilst a range of responses of fish to increases in water temperature have been measured, amino acid metabolism in a fish under high water temperature (HT) conditions has not been investigated. The aim of this study was to determine the effects of an acute increase in water temperature on oxygen consumption, plasma cortisol concentrations, and free amino acid concentrations in plasma and several tissues in goldfish (Carassius auratus). Oxygen consumption and plasma cortisol concentrations were increased in goldfish exposed to HT (30 ± 1 °C) for 200 min compared with goldfish at a control water temperature (CT 17 ± 1 °C). Oxygen consumption and plasma cortisol concentrations in both groups of fish combined were positively correlated. When goldfish were exposed to HT for 300 min oxygen consumption and plasma concentrations of 15 free amino acids were increased compared with goldish at CT. Concentrations of several free amino acids were increased to varying extents in the brain, liver, and muscle tissues. In conclusion, an acute increase in water temperature affected amino acid metabolism differently in the brain, liver, and muscle tissues. Goldfish will be a useful species for further studies of the possible roles of various amino acids in the brain, muscle, and liver during acute increases in water temperature in fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amino acids play various important roles, not only as constituents of protein but also as regulators of many physiological functions. In particular, much attention has been paid to their regulation of stress-related physiology and behaviors (Kurata et al. 2011; Erwan et al. 2014), including heat stress responses in chicks (Chowdhury et al. 2014; Ito et al. 2014, 2015; Han et al. 2017, 2018). Several lines of evidence have demonstrated that free amino acids are also important regulators of many vital metabolic pathways that are necessary for stress responses in fish (Bystriansky et al. 2007). Therefore, further information of amino acid metabolism in fish upon environmental challenges is relevant to understanding their contribution in stress responses.
Global warming and the rising temperature of water in oceans and rivers are of increasing concern worldwide. High water temperature (HT) can affect energy metabolism and stress responses in fish (Pörtner and Knust 2007; Madeira et al. 2016). The rate of oxygen consumption can be used as a basic parameter to evaluate metabolic activities (Yahav 2015). Besides, cortisol is the principal corticosteroid and is the most commonly measured indicator of stress in humans (Peckins et al. 2016), other mammals (Kala and Nivsarkar 2016; Carlton and Demas 2017; Sivakumar et al. 2010; Sejian et al. 2014), and fish (Cockrem 2013). Cortisol affects the metabolism of carbohydrates, proteins, and lipids. Cortisol concentration in fish increases rapidly in response to a wide variety of stressors (Cockrem 2013). For instance, Eslamloo et al. (2014) have reported significantly increased plasma cortisol concentration in goldfish after recurrent acute chasing and air-exposure stress. Ryan (1995) showed that plasma cortisol was increased in Antarctic fish (Pagothenia borchgrevinki) at 5 or 8 °C above the control water temperature (CT − 1.9 °C) for 6 h.
Jin et al. (2009) reported that free amino acid concentrations in the brain, liver, and plasma were closely related to each other in rats. Aragão et al. (2008) and Powell et al. (1982) found that the concentration of β-alanine in Senegalese sole plasma and in oyster gill tissue was elevated in response to stressors that included handling, air exposure, salinity, turbidity, low O2 concentration, and polluted water. Heat stress reduced plasma citrulline in chicks (Chowdhury et al. 2014), and oral administration of citrulline afforded thermotolerance (Chowdhury et al. 2017). Ito et al. (2015) found that heat stress increased brain leucine in chicks; however, Han et al. (2017) found that the amount of leucine in the brain decreased in embryos as a result of thermal manipulation during egg incubation. Interestingly, in ovo administration of leucine afforded thermotolerance in broiler chicks (Han et al. 2018). Therefore, it was suggested that some amino acids serve as a biomarker of heat stress in chickens (Chowdhury 2018). Amino acid supplements were reported to reduce the negative effects of muscle damage caused by stress in fish (Kraemer et al. 2006). Previous research reported that dietary supplement of indispensable amino acids, together with tyrosine and cysteine, were resulted in decrease of stress responses in Senegalese sole (Solea senegalensis) in high stocking density (Costas et al. 2013). Hoseini et al. (2012) reported that dietary tryptophan is capable of increasing tolerance to copper toxicity in common carp. However, on the whole, the changes in the metabolism of amino acids in heat-exposed fish are unknown, so there was no clear basis for the choice of amino acids to use as supplements to potentially reduce the adverse effects of heat stress on fish.
The aim of this study was to measure free amino acid concentrations in the blood, brain, liver, and muscles in goldfish exposed to high temperature conditions, to determine the differences in the amino acid metabolic profile in the central and peripheral tissues. To this end, two experiments were conducted to investigate the effects of heat stress on free amino acid levels in plasma and different tissues in goldfish. Oxygen consumption and plasma cortisol were also measured as indicators of stress.
Material and methods
Animals
Wakin-strain goldfish (Carassius auratus) 8–10 cm in length were purchased from a goldfish supplier (Rare Kobe, Kobe, Japan) and kept in a 200-L (diameter = 35 cm) housing aquarium for 1 week to acclimatize them prior to the experiment in Kyushu University Fishery Research Laboratory, Fukuoka, Japan. This aquarium was called the home tank. The water was aerated and dechlorinated prior to introducing the goldfish into the aquarium. Half of the water was renewed every day. The water temperature was maintained at 17 ± 2 °C under a natural photoperiod. The aquarium contained an air pump to aerate the water. The fish were fed commercial goldfish pellets equivalent to 1% of body weight per day and were maintained for 2 weeks for acclimatization to the new environment before the start of the experiments, according to the Guidelines for the Care and Use of Fish in Research by DeTolla et al. (1995). No mortality occurred during the acclimation period. This study was performed according to the guidelines for the care and use of laboratory animals (8th edition) of the National Research Council (https://grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf).
Experimental design
In Experiment 1, we examined the correlation between oxygen consumption and plasma cortisol in fish at a control temperature (17 °C) and in fish exposed to 200 min of high water temperature (30 °C). The measurement of oxygen consumption was performed when fish were housed individually in respiration chambers with a separate water flow into and out of each chamber. Twenty fish were randomly caught in the home tank and placed into one of four 30-L (diameter = 20 cm; height = 24 cm) tanks (n = 5 fish per 30-L tank) for acclimation. The water temperature was maintained at 17 ± 2 °C under a natural photoperiod. After one week, fish were individually moved into glass respiration chambers (diameter = 7 cm). These chambers were placed in another tank containing water at 17 ± 1 °C (control temperature, CT) or water at 30 ± 1 °C (high temperature, HT). There were five chambers in each of the tanks. Two tanks (10 fish in 10 chambers) had water at CT and two tanks (10 fish in 10 chambers) had water at HT. The experimental design showing the transfer of fish between tanks and into the individual chambers is illustrated in Fig. 1.
The experimental design showing the transfer of fish from the home tank (20 fish) to four acclimatization tanks (5 fish per tank). After 7 days, fish were removed from each acclimatization tank, placed into individual respiration chambers, and then the individual chambers were placed into experimental tanks where the water temperature was either 17 ± 1 °C or 30 ± 1 °C
Each individual chamber had its own water flow. Water pumped from the tank entered the respiration chamber through a pipe, flowed through the chamber, and then returned to the tank in another pipe. Water from the outlet pipe could be diverted into a container for the measurement of dissolved oxygen (DO) concentration. Each of the four tanks used in the experiment contained a heater, a cooler, a temperature regulator, an air pump, individual chambers, and a water circulation system. The water temperature in these tanks had been maintained at either CT or HT for one day before the experiment began.
After the fish had been kept in the individual chambers at CT or HT for 200 min, the dissolved oxygen (DO) concentration in the outlet water from the individual chambers was measured in order to calculate the oxygen consumption as described below. After the DO concentration had been measured, the fish were removed from the individual chambers and placed in a bucket containing an anesthetic solution (MS-222, TCI, Japan; 1 g/L). The fish were removed from the bucket once they had been euthanized, and blood was collected into heparinized capillary tubes by cutting off the caudal peduncle. Blood was expelled into small centrifuge tubes that were placed on ice. The body weights of the fish were measured after blood collection had been completed. Heparinized blood in small centrifuge tubes was centrifuged at 15,000×g for 5 min at 4 °C to collect plasma which was stored at − 80 °C for later measurement of plasma cortisol concentrations.
In Experiment 2, we measured oxygen consumption several times after fish were moved into individual respiration chambers, then collected blood and tissues for the subsequent measurement of free amino acid concentrations. The experimental conditions were the same as those in Experiment 1. Fish were moved from the home tank to the 30-L acclimatization tank and then to individual chambers as in Experiment 1. Ten fish were moved to chambers in tanks at 17 ± 1 °C (CT), and 10 fish were moved to chambers in tanks at 30 ± 1 °C (HT). The DO concentration in water flowing from the individual chambers was measured 120, 180, 240, and 300 min after fish were moved into the chambers. After the final measurement of DO concentration, the goldfish were removed from the individual chambers, euthanized, and blood collected as described in Experiment 1. The liver, muscle (red muscle and white muscle), and different regions of brain tissue (the telencephalon, the optic lobe, the diencephalon, the cerebellum (including a partial part of the mesencephalon), and the vagal lobe (including the medulla oblongata)) were removed and placed in small centrifuge tubes following procedures described elsewhere (Ikegami et al. 2015). Plasma and tissues were immediately frozen in liquid nitrogen and then stored at − 80 °C for later measurement of concentrations of plasma and tissue free amino acids.
Analysis of oxygen consumption
Oxygen consumption was determined using a continuous flow method as described elsewhere (Yagi et al. 2010). In brief, the continuous flow method was based on the loss of oxygen and the rate of water flow through an individual respiration chamber (diameter = 8 cm; length = 30 cm). The water flow rate was controlled by a screw cock at the end of a tube connected to each individual respiration chamber.
A respiration chamber without fish was used as a blank chamber. To confirm that the water was air-saturated, the DO concentration in water flowing out of the blank chamber was measured by an oxygen electrode (DO meter UD-1, Central Kagaku Corp., Tokyo, Japan; the unit of DO was mg/L). Water flowed simultaneously through the blank chamber and through each individual respiration chamber containing fish at the same rate (3.6 ± 0.3 L per h). After the fish were exposed to either CT or HT for as long as designated in each experiment, the water flowing out of each chamber was collected to measure the DO concentration. The water flow rate of each chamber was also measured by collecting water into a measuring cylinder for a measured length of time.
The formula for calculating the oxygen consumption (units of mg/g/h) was:
In Experiment 1, one fish in the HT group died during the heat exposure. Thus, for the analysis of oxygen consumption, the total number of goldfish was 10 in the CT group and 9 in the HT group.
Measurement of plasma cortisol
Cortisol concentrations were measured using an enzyme immunoassay kit (Cortisol ELISA Kit, Enzo Life Science Inc., Farmingdale, NY) previously used for the measurement of cortisol in goldfish (Cockrem et al. 2018). Concentrations were expressed as nanogram per milliliter (ng/mL) plasma. Each plasma sample was thawed and diluted with an assay buffer by a factor of 5. Standards, samples, and quality controls were assayed in duplicate wells. The limit of detection of the cortisol assay, determined as the hormone concentration at the mean minus two standard deviations from the zero hormone point on the standard curves, was 2.34 ng/mL. Two doses of cortisol solution in the assay buffer, which gave around 80% and 50% binding on the standard curve, were used as low- and medium-quality controls in every assay, respectively. The intra-assay coefficients of variation were 3.93% and 2.26%, and the interassay coefficients of variation were 6.25% and 2.41% for low and medium cortisol concentration quality controls, respectively. Two samples from the CT group were outside the standard curve so the number of valid samples used as data for statistical analysis was 8 in the CT group and 9 in the HT group.
Analysis of free amino acids and phosphoserine in plasma and tissues
The concentrations of free amino acids in the plasma, brain, liver, and muscle as well as phosphoserine levels were measured by HPLC according to the method of Boogers et al. (2008), with some modifications as described by Ito et al. (2014). Plasma was filtered through a 0.2-μm filter (Millipore, Bedford, MA, USA). The brain, muscle, and liver tissue samples were homogenized in an ice-cold 0.2 M perchloric acid solution containing 0.01 mM ethylenediaminetetraacetic acid disodium salt (EDTA·2Na) and left for deproteinization on ice. After 30 min, the mixtures were centrifuged at 20,000×g for 15 min at 4 °C, and the supernatants were then adjusted to pH 7 with 1 M sodium hydroxide. Each plasma (10 μL) and tissue sample (20 μL) was then completely dried under reduced pressure. The dried residues were dissolved in 10 μL of 1 M sodium acetate-methanol-triethylamine (2:2:1) and re-dried under reduced pressure, and then converted to their phenylthiocarbamoyl derivatives by dissolving them in 20 μL of methanol-distilled water-triethylamine-phenyl isothiocyanate (7:1:1:1). After 20 min at room temperature, phenyl isothiocyanate was allowed to react with the amino groups and the samples were dried again and then dissolved in 200 μL of Pico-Tag Mobile Phase A solution. The samples were filtrated through a 0.2-μm filter (Millipore, Bedford, MA, USA). The same methods were performed on standard solutions, which were prepared by diluting a commercially available l-amino acid solution (type ANII, type B, l-asparagine, l-glutamine, and l-tryptophan; Wako, Osaka, Japan) with distilled water. These derivatized samples were applied to a Waters HPLC system (Pico-tag free amino acid analysis column (3.9 mm × 300 mm), Alliance 2690 separation module, 2487 dual-wavelength UV detector, and Millennium 32 chromatography manager; Waters, Milford, CT, USA). They were equilibrated with mobile phase A (70 mM sodium acetate adjusted to pH 6.45 with 10% acetic aceto-acetonitrile, ratio 975:25) and eluted with a linear gradient of mobile phase B (water-acetonitrile-methanol (40:45:15) (0%, 3%, 6%, 9%, 40%, and 100%)) at a flow rate of 1 mL/min at 46 °C. Absorbances at a wavelength of 254 nm were measured to determine concentrations of free amino acids (leucine, isoleucine, valine, threonine, methionine, phenylalanine, tryptophan, GABA, aspartate, taurine, proline, serine, tyrosine, lysine, 3-methyl-histidine, arginine, alanine, glycine, asparagine, glutamine, citrulline, histidine, β-alanine, α-aminoadipic acid, cystathionine, ornithine, hydroxyproline, and glutamate) and phosphoserine.
Amino acid and phosphoserine concentrations in the brain, muscle, and liver were expressed as pmol/mg wet tissue, and plasma amino acid concentrations were expressed as pmol/μL. The system applied here could not separate l- and d-forms of amino acids. Therefore, the results for the amino acids that were determined only use the names of the amino acids.
Statistical analysis
Comparisons between groups were made with Student’s t tests for oxygen consumption and plasma cortisol concentrations in Experiment 1 and free amino acid concentrations in Experiment 2. Linear regression analysis of O2 consumption and plasma cortisol concentration in Experiment 1 was carried out using GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA) software. A two-way repeated measures analysis of variance (ANOVA) was used for the analysis of O2 consumption in Experiment 2. For the repeated measures ANOVA, time was a within-subjects factor and temperature a between-subjects factor. Values are expressed as means ± SEM. Significant differences were denoted as p < 0.05. The ANOVA and t tests were performed using IBM SPSS Statistics (SPSS, Inc., Chicago, IL, USA) software.
Results
Oxygen consumption and plasma cortisol concentrations
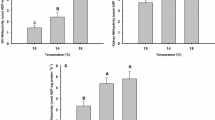
Oxygen consumption and plasma cortisol concentrations were significantly higher in goldfish exposed to HT for 200 min than goldfish at CT (p < 0.01; Fig. 2a, b). There was a significant (p < 0.001) positive correlation between plasma cortisol concentrations and oxygen consumption for goldfish of both groups combined (Fig. 2c). In Experiment 2, a two-way repeated measures ANOVA showed that there were significant effects of temperature but not of time on oxygen consumption, with no significant interaction between temperature and time (F1,18 = 94.15, p < 0.001; F3,54 = 2.291, p > 0.05; F3,54 = 1.804, p > 0.05, respectively; see Fig. 3). These results indicate that oxygen consumption did not change significantly over the experimental period (120–300 min).
Oxygen consumption (a) and plasma cortisol concentration (b) of goldfish after they were moved from a home tank to chambers individually at either control water temperature (CT, 17 ± 1 °C) or high water temperature (HT, 30 ± 1 °C) for 200 min. A linear regression analysis of the relationship between oxygen consumption and plasma cortisol concentration is shown in (c). Results are mean ± S.E.M. of 8–10 fish in each group. Comparisons between means were made using Student’s t test. **p < 0.01; ***p < 0.001
Oxygen consumption of goldfish after they were moved from a home tank to chambers at either control water temperature (CT, 17 ± 1 °C) or high water temperature (HT, 30 ± 1 °C) for 300 min. Results are mean ± S.E.M. of 10 fish in each group, and they were analyzed using a two-way repeated measures analysis of variance (ANOVA)
Free amino acid concentrations in plasma
Free amino acid concentrations in the plasma of goldfish exposed to either HT or CT for 300 min are shown in Table 1. Concentrations of 15 free amino acids (p < 0.001: leucine, isoleucine, valine, methionine, tyrosine; p < 0.01: tryptophan, glutamate, GABA; p < 0.05: threonine, phenylalanine, aspartate, taurine, proline, serine, and phosphoserine) in the plasma were significantly higher in fish exposed to HT than in fish exposed to CT.
Free amino acid concentrations in the brain
There were significant differences between CT- and HT-exposed goldfish in the concentrations of 8 free amino acids in some or all of the five brain regions (see Fig. 4). Glutamine, asparagine, and threonine in the telencephalon, the optic lobe, the diencephalon, and the cerebellum were significantly higher in HT than in CT goldfish (p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.01, p < 0.05, p < 0.01, p < 0.05, p < 0.05, p < 0.05, p < 0.05, p < 0.05, respectively; see Fig. 4a–c). Glutamate was significantly higher in HT goldfish in the telencephalon (p < 0.01) and the optic lobe (p < 0.05; Fig. 4d). Leucine and isoleucine concentrations in the telencephalon and optic lobe were approximately twice as high in HT than those in CT goldfish (p < 0.001, p < 0.001, p < 0.001, p < 0.001, respectively; see Fig. 4e, f). Leucine (p < 0.001) was also significantly higher in the diencephalon in HT goldfish (Fig. 4e). Notably, only phosphoserine (p < 0.05) in the cerebellum and methionine (p < 0.01) in the vagal lobe were significantly lower in HT than those in CT goldfish (Fig. 4g, h). The other amino acids and phosphoserine did not show any statistical significant differences between CT and HT groups for any of the brain regions.
Glutamine (a), asparagine (b), threonine (c), glutamate (d), leucine (e), isoleucine (f), phosphoserine (g), and methionine (h) concentrations in brain regions of goldfish after they were individually moved from a home tank to chambers at either control water temperature (CT, 17 ± 1 °C) or high water temperature (HT, 30 ± 1 °C) for 300 min. Results are mean ± S.E.M. of 8–10 fish in each group. Mean concentrations in each brain region were compared between CT and HT using Student’s t test. Tel, telencephalon; OL, optic lobe; Dien, diencephalon; Cb, cerebellum; VL, vagal lobe; u.d.: undetectable. *p < 0.05; **p < 0.01; ***p < 0.001
Free amino acid concentrations in the muscle and liver
Concentrations of three free amino acids in the liver and skeletal muscle are shown in Fig. 5. Threonine concentrations were significantly (p < 0.05) higher in red muscle (Fig. 5a) in goldfish exposed to HT for 300 min compared with goldfish at CT. Glutamate in the liver (p < 0.001), white muscle (p < 0.05; Fig. 5b), and hydroxyproline in the liver (p < 0.05; Fig. 5c) were significantly higher in HT than in CT goldfish (Fig. 5c). There were no significant differences between CT and HT fish in concentrations in the liver, red muscle, and white muscle of any of the other amino acids or phosphoserine (data not shown).
Threonine (a), glutamate (b), and hydroxyproline (c) concentrations in muscle and liver tissues of goldfish after they were moved from a home tank to an individual chamber at either control water temperature (CT, 17 ± 1 °C) or high water temperature (HT, 30 ± 1 °C) for 300 min. Results are mean ± S.E.M. of 10 fish in each group. Mean concentrations in each tissue were compared between CT and HT using Student’s I test. *p < 0.05; ***p < 0.001
Discussion
This study has shown that oxygen consumption and plasma cortisol concentrations were increased in goldfish exposed to warm water (30 ± 1 °C) for 200 min compared with goldfish at a control water temperature (CT 17 ± 1 °C). When goldfish were exposed to HT for 300 min oxygen consumption and plasma concentrations of 15 free amino acids were increased compared with goldfish at CT. Concentrations of several free amino acids were increased to varying extents in the brain, liver, and muscle tissues.
The increased oxygen consumption in goldfish exposed to warm water is consistent with the report by Heath and Hughes (1973) that oxygen consumption was increased during warm water (26 °C) treatment in rainbow trout (Salmo gairdneri). An increase in oxygen consumption suggests that the metabolic rate of the fish was increased when they experienced increased water temperatures. The hypothalamic-pituitary-adrenal axis in mammals or birds, which is known as the hypothalamic-pituitary-interrenal (HPI) axis in fish (Bernier and Peter 2001), becomes activated to integrate the body’s response to stress (Turnbull and Rivier 1997; Cockrem 2013). Activation of the HPI results in increased levels of corticotropin-releasing factor, adrenocorticotropic hormone, glucocorticoids, and glucose (Sapolsky et al. 2000). The glucocorticoids are generally used as biomarkers of various types of stress in fish (Ellis et al. 2004; Ciji et al. 2012; Fanouraki et al. 2016; Wu et al. 2017). In the current study, plasma cortisol concentration was higher in goldfish after 200 min of HT exposure compared with those exposed to CT for that length of time (Fig.2b). The mean plasma cortisol concentration (~100 ng/mL) in goldfish 200 min after they experienced an abrupt change in water temperature from 17 to 30 °C was a little higher than mean cortisol concentrations in goldfish 240 min after the start of a gradual increase in water temperature over one hour from 20 to 30 °C (~80 ng/mL; Cockrem et al. 2018) and similar to mean cortisol (~96 ng/mL) in goldfish held at 32 °C for 4 days after experiencing an increase in water temperature from 22 to 32 °C over the preceding 10 days (Gandar et al. 2017).
Fifteen free amino acids were significantly increased in the plasma due to the exposure to high temperature. A human study demonstrated that cortisol can promote amino acid transport from muscle to the liver and other viscera where amino acids are needed during stress (Brillon et al. 1995). Thus, protein in the cells keeps degrading and amino acids are released, thereby increasing plasma amino acid concentrations. In the current study, whilst 15 free amino acids increased in plasma, only 4 amino acids (threonine, glutamate, leucine, and isoleucine) increased and phosphoserine and methionine decreased in the brain, indicating that changes in plasma free amino acid concentrations generally did not correspond with the changes in brain content of the free amino acids. These findings suggest that there is tissue-specific amino acid metabolism in the brain during thermal stress. Among all the upregulated amino acids in the central or peripheral tissues, there were some essential amino acids (leucine, isoleucine, and threonine) that cannot be synthesized by goldfish, which further indicates that increased concentrations of these free amino acids were due to the degradation of protein.
Leucine, isoleucine, and valine, which are branched-chain amino acids (BCAAs), increased in the plasma, and leucine and isoleucine were sharply increased in the telencephalon and optic lobe. Notably, leucine concentration was also increased in the diencephalon in HT-exposed goldfish. The finding of increased leucine concentration in the diencephalon was similar to results obtained from heat-exposed chicks (Ito et al. 2015). The diencephalon is an important brain region which contains many neuroendocrine regulators. For example, neuropeptide Y (NPY) neurons are located in the diencephalon, and leucine can regulate brain NPY in mammals (Cota et al. 2006). Moreover, BCAAs can act as important substrates for energy metabolism when there is carbohydrate depletion (Kim et al. 2013). Therefore, as well as contributing to energy metabolism, the increased brain leucine may have other unknown functions in stress responses of heat-exposed goldfish.
Glutamine and asparagine levels were significantly higher in the brain (telencephalon, optic lobe, diencephalon, and cerebellum) of HT fish compared with CT fish; in particular, a robust increment in glutamine was found. A previous study showed that ammonia and glutamate can form glutamine in the brain of fish, with glutamine formation considered to be a main method of detoxification of ammonia (Randall and Tsui 2002). In the present study, glutamate concentrations were increased in the telencephalon and the optic lobe. Meanwhile, a higher concentration of glutamine was observed in all brain regions except the vagal lobe in the HT group. This might have been a consequence of higher brain ammonia concentrations in fish under HT than in those under CT, leading to the formation of glutamine in HT fish.
Phosphoserine and methionine concentrations were lower in HT than in CT goldfish in the cerebellum and the vagal lobe, respectively. Since phosphoserine is the precursor of serine, phosphoserine concentration was also measured in the current study. Serine concentration was higher in the plasma but not in the brain tissues of HT goldfish. Ito et al. (2015) reported that brain serine concentration was increased in heat-exposed chicks. Central injection of serine induced sedative and hypnotic effects under acute stress in chicks (Asechi et al. 2006). Skiba-Cassy et al. (2016) reported that methionine regulates lipid metabolism in rainbow trout by affecting gene transcription. Therefore, the reduced phosphoserine and methionine concentrations in the brain of goldfish indicates that thermal stress might have activated the utilization of these amino acids to contribute to the stress response and to energy metabolic processes. Threonine has been said to prevent cellular apoptosis and enhance cellular expression of heat shock protein (HSP)-25 and -70 in rat intestinal epithelial-18 cells in an in vitro study of heat stress (Baird et al. 2013). The increased threonine content in the muscle may be postulated to have some functions in inducing the expression of HSP.
In conclusion, oxygen consumption and plasma cortisol concentrations were increased in goldfish 200 min after exposure to an abrupt change in water temperature from 17 to 30 °C. Increased concentrations of some free amino acids in the plasma and some free amino acids in the brain, liver, and muscle may have resulted from catabolic metabolism and may have important physiological significance in heat-exposed goldfish.
References
Aragão C, Corte-Real J, Costas B, Dinis MT, Conceição LEC (2008) Stress response and changes in amino acid requirements in Senegalese sole (Solea senegalensis Kaup 1858). Amino Acids 34:143–148
Asechi M, Tomonaga S, Tachibana T, Han L, Hayamizu K, Denbow DM, Furuse M (2006) Intracerebroventricular injection of L-serine analogs and derivatives induces sedative and hypnotic effects under an acute stressful condition in neonatal chicks. Behav Brain Res 170:71–77
Baird CH, Niederlechner S, Beck R, Kallweit AR, Wischmeyer PE (2013) L-threonine induces heat shock protein expression and decreases apoptosis in heat-stressed intestinal epithelial cells. Nutrition 29:1404–1411
Bernier NJ, Peter RE (2001) The hypothalamic-pituitary-interrenal axis and the control of food intake in teleost fish. Comp Biochem Physiol B Biochem Mol Biol 129:639–644
Boogers I, Plugge W, Stokkermans YQ, Duchateau AL (2008) Ultra-performance liquid chromatographic analysis of amino acids in protein hydrolysates using an automated pre-column derivatisation method. J Chromatogr A 1189:406–409
Brillon DJ, Zheng B, Campbell RG, Matthews DE (1995) Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol Endocrinol Metab 268:E501–E513
Bystriansky JS, Frick NT, Ballantyne JS (2007) Intermediary metabolism of Arctic char Salvelinus alpinus during short-term salinity exposure. J Exp Biol 210:1971–1985
Carlton ED, Demas GE (2017) Glucose and insulin modulate sickness responses in male Siberian hamsters. Gen Comp Endocrinol 242:83–91
Chowdhury VS (2018) Heat stress biomarker amino acids and neuropeptide afford thermotolerance in chicks. J Poult Sci 56:1–11. https://doi.org/10.2141/jpsa.0180024
Chowdhury VS, Tomonaga S, Ikegami T, Erwan E, Ito K, Cockrem JF, Furuse M (2014) Oxidative damage and brain concentrations of free amino acid in chicks exposed to high ambient temperature. Comp Biochem Physiol A Mol Integr Physiol 169:70–76
Chowdhury VS, Han G, Bahry MA, Tran PV, Do PH, Yang H, Furuse M (2017) L-citrulline acts as potential hypothermic agent to afford thermotolerance in chicks. J Therm Biol 69:163–170
Ciji A, Sahu NP, Pal AK, Dasgupta S, Akhtar MS (2012) Alterations in serum electrolytes, antioxidative enzymes and haematological parameters of Labeo rohita on short-term exposure to sublethal dose of nitrite. Fish Physiol Biochem 38:1355–1365
Cockrem JF (2013) Individual variation in glucocorticoid stress responses in animals. Gen Comp Endocrinol 181:45–58
Cockrem JF, Bahry MA, Chowdhury VS (2018) Cortisol responses of goldfish (Carassius auratus) to air exposure, chasing, and increased water temperature. Gen Comp Endocrinol 270:18–25
Costas B, Aragão C, Dias J, Afonso A, Conceição LE (2013) Interactive effects of a high-quality protein diet and high stocking density on the stress response and some innate immune parameters of Senegalese sole Solea senegalensis. Fish Physiol Biochem 39:1141–1151
Cota D, Proulx K, Smith KAB, Kozma SC, Thomas G, Woods SC, Seeley RJ (2006) Hypothalamic mTOR signaling regulates food intake. Science 312:927–930
DeTolla LJ, Srinivas S, Whitaker BR, Andrews C, Hecker B, Kane AS, Reimschuessel R (1995) Guidelines for the care and use of fish in research. ILAR J 37:159–173
Ellis T, James JD, Stewart C, Scott AP (2004) A non-invasive stress assay based upon measurement of free cortisol released into the water by rainbow trout. J Fish Biol 65:1233–1252
Erwan E, Chowdhury VS, Nagasawa M, Goda R, Otsuka T, Yasuo S, Furuse M (2014) Oral administration of D-aspartate, but not L-aspartate, depresses rectal temperature and alters plasma metabolites in chicks. Life Sci 109:65–71
Eslamloo K, Akhavan SR, Fallah FJ, Henry MA (2014) Variations of physiological and innate immunological responses in goldfish (Carassius auratus) subjected to recurrent acute stress. Fish Shellfish Immunol 37:147–153
Fanouraki E, Papandroulakis N, Pavlidis M (2016) Non-invasive stress indicators in Mediterranean mariculture: effect of fish size on water cortisol release rate. Symposium on Oceanography Fisheries 2009 Proc 9, pp 711–715
Gandar A, Laffaille P, Canlet C, Tremblay-Franco M, Gautier R, Perrault A, Gress L, Mormède P, Tapie N, Budzinski H, Jean S (2017) Adaptive response under multiple stress exposure in fish: from the molecular to individual level. Chemosphere 188:60–72
Han G, Yang H, Bahry MA, Tran PV, Do PH, Ikeda H, Furuse M, Chowdhury VS (2017) L-leucine acts as a potential agent in reducing body temperature at hatching and affords thermotolerance in broiler chicks. Comp Biochem Physiol A Mol Integr Physiol 204:48–56
Han G, Yang H, Bungo T, Ikeda H, Wang Y, Nguyen LT, Eltahan HM, Furuse M, Chowdhury VS (2018) In ovo L-leucine administration stimulates lipid metabolisms in heat-exposed male, but not female, chicks to afford thermotolerance. J Therm Biol 71:74–82
Heath AG, Hughes GM (1973) Cardiovascular and respiratory changes during heat stress in rainbow trout (Salmo gairdneri). J Exp Biol 59:323–338
Hoseini SM, Hosseini SA, Soudagar M (2012) Dietary tryptophan changes serum stress markers, enzyme activity, and ions concentration of wild common carp Cyprinus carpio exposed to ambient copper. Fish Physiol Biochem 38:1419–1426
Ikegami T, Takemura A, Choi E, Suda A, Tomonaga S, Badruzzaman M, Furuse M (2015) Increase in telencephalic dopamine and cerebellar norepinephrine contents by hydrostatic pressure in goldfish: the possible involvement in hydrostatic pressure-related locomotion. Fish Physiol Biochem 41:1105–1115
Ito K, Erwan E, Nagasawa M, Furuse M, Chowdhury VS (2014) Changes in free amino acid concentrations in the blood, brain and muscle of heat-exposed chicks. Br Poult Sci 55:644–652
Ito K, Bahry MA, Hui Y, Furuse M, Chowdhury VS (2015) Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comp Biochem Physiol A Mol Integr Physiol 187:13–19
Jin G, Kataoka Y, Tanaka M, Mizuma H, Nozaki S, Tahara T, Mizuno K, Yamato M, Watanabe Y (2009) Changes in plasma and tissue amino acid levels in an animal model of complex fatigue. Nutrition 25:597–607
Kala M, Nivsarkar M (2016) Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen Comp Endocrinol 225:117–124
Kim DH, Kim SH, Jeong WS, Lee HY (2013) Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J Exerc Nutr Biochem 17:169–180
Kraemer WJ, Ratamess NA, Volek JS, Häkkinen K, Rubin MR, French DN, Gómez AL, McGuigan MR, Scheett TP, Newton RU, Spiering BA, Izquierdo M, Dioguardi FS (2006) The effects of amino acid supplementation on hormonal responses to resistance training overreaching. Metab Clin Exp 55:282–291
Kurata K, Shigemi K, Tomonaga S, Aoki M, Morishita K, Denbow DM, Furuse M (2011) L-ornithine attenuates corticotropin-releasing factor-induced stress responses acting at GABAA receptors in neonatal chicks. Neuroscience 172:226–231
Madeira D, Vinagre C, Diniz MS (2016) Are fish in hot water? Effects of warming on oxidative stress metabolism in the commercial species Sparus aurata. Ecol Indic 63:324–331
Peckins MK, Susman EJ, Negriff S, Noll JG, Trickett PK (2016) Cortisol profiles: a test for adaptive calibration of the stress response system in maltreated and nonmaltreated youth. Dev Psychopathol 28:1563–1564
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97
Powell EN, Kasschau M, Chen E, Koenig M, Pecon J (1982) Changes in the free amino acid pool during environmental stress in the gill tissue of the oyster, Crassostrea virginica. Comp Biochem Physiol A Mol Integr Physiol 71:591–598
Randall DJ, Tsui TKN (2002) Ammonia toxicity in fish. Mar Pollut Bull 45:17–23
Ryan SN (1995) The effect of chronic heat stress on cortisol levels in the antarctic fish Pagothenia borchgrevinki. Experientia 51:768–774
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Sejian V, Singh AK, Sahoo A, Naqvi SMK (2014) Effect of mineral mixture and antioxidant supplementation on growth, reproductive performance and adaptive capability of Malpura ewes subjected to heat stress. J Anim Physiol Anim Nutr (Berl) 98:72–83
Sivakumar AVN, Singh G, Varshney VP (2010) Antioxidants supplementation on acid base balance during heat stress in goats. Asian-Australas J Anim Sci 23:1462–1468
Skiba-Cassy S, Geurden I, Panserat S, Seiliez I (2016) Dietary methionine imbalance alters the transcriptional regulation of genes involved in glucose, lipid and amino acid metabolism in the liver of rainbow trout (Oncorhynchus mykiss). Aquaculture 454:56–65
Turnbull AV, Rivier C (1997) Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med 215:1–10
Wu H, Ohnuki H, Ota S, Murata M, Yoshiura Y, Endo H (2017) New approach for monitoring fish stress: a novel enzyme-functionalized label-free immunosensor system for detecting cortisol levels in fish. Biosens Bioelectron 93:57–64
Yagi M, Kanda T, Takeda T, Ishimatsu A, Oikawa S (2010) Ontogenetic phase shifts in metabolism: links to development and anti-predator adaptation. Proc Biol Sci 277:2793–2801
Yahav S (2015) Regulation of body temperature: strategies and mechanisms. In: Scanes CG (ed) Sturkie’s avian physiology, 6th edn. Academic, London, pp 869–890
Acknowledgments
The diligent technical support of Shin Oikawa (Retired Associate Professor, Fishery Research Laboratory, Kyushu University) in the measurement of oxygen consumption in goldfish is gratefully acknowledged.
Funding
This study was supported by JSPS KAKENHI grant numbers JP15K07694 and JP18K19271 to VSC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was performed in accordance with the ethical guidelines for animal experiments as described in the materials and methods section.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Han, G., Pham, C.V. et al. An acute increase in water temperature can increase free amino acid concentrations in the blood, brain, liver, and muscle in goldfish (Carassius auratus). Fish Physiol Biochem 45, 1343–1354 (2019). https://doi.org/10.1007/s10695-019-00642-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00642-5