Abstract
Amino acids (AA) regulate key metabolic pathways, including some immune responses. Therefore, this study aimed to assess whether an increased availability of dietary AA can mitigate the expected increase in plasma cortisol and metabolites levels due to high stocking density and its subsequent immunosuppression. Senegalese sole (Solea senegalensis) were maintained at low stocking density (LSD; 3.5 kg m−2) or high stocking density (HSD; 12 kg m−2) for 18 days. Additionally, both treatments were fed a control or a high protein (HP) diet (LSD, LSD HP, HSD and HSD HP). The HP diet slightly increased the levels of digestible indispensable AA, together with tyrosine and cysteine. HSD was effective in inducing a chronic stress response after 18 days of treatment since fish held at HSD presented higher plasma cortisol, glucose and lactate levels. Moreover, this increase in stress indicators translated in a decrease in plasma lysozyme, alternative complement pathway (ACP) and peroxidase activities, suggesting some degree of immunosuppression. Interestingly, while plasma glucose and lactate levels in HSD HP specimens decreased to similar values than LSD fish, plasma lysozyme, ACP and peroxidase activities increased, with even higher values than LSD groups for ACP activity. It is suggested that the HP diet may be used as functional feed since it may represent a metabolic advantage during stressful events and may counteract immunosuppression in sole.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish welfare is recognized as an important issue in aquaculture, due both to ethical issues and production advantages. For instance, good welfare may lead to lower susceptibility to stress and diseases (e.g. Conceição et al. 2012; Prunet et al. 2012; Segner et al. 2012). The effect of stress on the immune system has been widely investigated, and chronic stress was found to inhibit an optimal immune response in mammals and fish, leading to increased susceptibility to pathogens (Dhabhar 2009; Tort 2011). In particular, cortisol alone can influence multiple aspects of the innate immune defence mechanisms in fish (Verburg-van Kemenade et al. 2009). Several in vitro studies demonstrated that cortisol affects the cytokine production of immune cells. For instance, cortisol inhibits LPS-induced expression of acute-phase protein serum amyloid S and pro-inflammatory cytokines interleukin (IL)-1β, tumour necrosis factor-α, IL-11 and inducible nitric oxide synthase (Saeij et al. 2003; Fast et al. 2008; Stolte et al. 2008). Cortisol also affects apoptosis and proliferation of immune cells (Verburg-van Kemenade et al. 2009). Macrophages isolated from Atlantic salmon (Salmo salar) submitted to repeated handling for 3 and 4 weeks showed decreased survival when exposed to Aeromonas salmonicida (Fast et al. 2008). Interestingly, cortisol can induce apoptosis in activated B lymphocytes but not in neutrophils (Weyts et al. 1999). Still, the effects of stress on the immune system are difficult to interpret in vivo, since a number of different hormones are involved via the hypothalamic-pituitary-interrenal axis (Wendelaar Bonga 1997).
Stress conditions that induced high plasma cortisol levels also modified fish amino acid (AA) metabolism in several teleost species (Milligan 1997; Pinto et al. 2007; Aragão et al. 2008, 2010; Costas et al. 2008, 2011a, 2012a). Changes in plasma-free AA levels may be indicative of AA requirements in fish (Wilson 2002). In fact, it has been suggested that fish under stressful conditions present additional AA requirements, due to either increased energetic demands or for the synthesis of stress-related proteins and other compounds related with the stress response (Aragão et al. 2008, 2010; Costas et al. 2008). The role of specific AA and their metabolites on key metabolic pathways that are necessary for growth, immunity or resistance to environmental stressors and pathogens has been recently reviewed in mammals and fish (Li et al. 2007, 2009). Thus, AA not only serves as constituents of proteins and energy sources, but also can be converted into important biochemically active substances in vivo. In particular, arginine is the precursor for the synthesis of nitric oxide (NO) and polyamines in higher vertebrates. In fish, NO production plays an important role in cellular defence mechanisms and has been demonstrated in stimulated macrophages in several fish species (Neumann et al. 1995; Tafalla and Novoa 2000), including Senegalese sole (Solea senegalensis) (Costas et al. 2011b). Moreover, dietary tryptophan supplementation can inhibit aggression or reduce cannibalism and stress-induced anorexia and cortisol augmentation in several teleosts (Hseu et al. 2003; Lepage et al. 2003; Höglund et al. 2007). Furthermore, an increase in all dietary indispensable AA appears to minimize some negative effects attributed to cortisol release in chronically handled Senegalese sole (Costas et al. 2012a).
Senegalese sole is a very attractive candidate for marine aquaculture and has a very big potential for future farming at commercial scale. However, growth and survival from juvenile to market-size fish is not fully controlled with regard to rearing technology and husbandry conditions, feeding behaviour and nutritional requirements (Imsland et al. 2003). Among the different factors that may induce high mortality during the juvenile stage, stress might be one of the key issues. In particular, this species appears to be highly susceptible to opportunistic pathogens when stocked at high density (Costas et al. 2008). Therefore, the main objective of this study is to assess whether an increased availability of dietary AA can mitigate the expected increase in plasma cortisol and metabolites levels due to high stocking density. Moreover, it is also intended to verify whether the expected decrease in plasma non-specific immune parameters due to cortisol action can also be mitigated through dietary treatment.
Materials and methods
Experimental diets
Two diets were formulated, a moderate protein reference diet (Control) containing 44.1 % fish meal as the main protein source and a high protein diet (HP) where fish meal was increased up to 46.5 %. Therefore, HP diet had a slightly increase in the levels of digestible indispensable AA, together with tyrosine and cysteine, when compared to the control diet. In addition, l-tryptophan [0.5 % on a dry matter (DM) basis] was added to the HP diet since this indispensable AA is known to reduce cortisol augmentation in fish (see introduction). Wheat gluten and corn gluten were chosen as complementary protein sources due to their high protein content and potential high digestibility in fish, while soybean meal is known to have both high crude protein content (44 % DM) and a reasonably balanced AA profile (Gatlin et al. 2007). In the absence of specific data on vitamin, mineral and trace element requirements for Senegalese sole, requirement data for other species were applied (NRC 1993; Kaushik 1998). All dietary ingredients were supplied by Sorgal S.A. (Ovar, Portugal) and were finely ground, mixed and dry pelleted through a 3.2 mm die at 50 °C (CPM, C-300 model, San Francisco, CA, USA). The diets were dried at 37 °C for 24-h and stored in a refrigerator (4 ± 1 °C) until use. Formulation and proximate composition of the experimental diets are presented in Table 1 and the corresponding AA profile in Table 2.
Fish
Senegalese sole juveniles (109.6 ± 16.9 g wet weight) originated from the natural spawning of wild broodstock and were reared according to standard larval and juvenile rearing protocols (Dinis et al. 1999). Before the experiment, fish were acclimated for 15 days using a flow-through seawater system (temperature: 20 ± 1 °C; salinity: 36 g l−1; dissolved oxygen: above 90 % saturation level), comprised by a flat-bottomed fibreglass tank.
This study was directed by trained scientists (following FELASA category C recommendations) and conducted according to the guidelines on the protection of animals used for scientific purposes from the European directive 2010/63/UE.
Experimental design
At the beginning of the experiment, fish were fasted for 24 h, anaesthetized with 2-phenoxyethanol (500 ml l−1, Sigma-Aldrich, Germany) and individually measured and weighed. Fish were distributed in eight flat-bottomed tanks (70 cm length × 30 cm width × 20 cm depth, volume 20 l), using a partial-recirculated seawater system (temperature: 20 ± 1 °C; salinity: 36 g l−1; dissolved oxygen: 90 % above saturation level), to achieve two different initial densities in quadruplicate tanks: 3.5 and 12 kg m−2 with 8 and 20 specimens per tank, respectively. Fish maintained at low stocking density (3.5 kg m−2) were regarded as control while fish held at high stocking density (12 kg m−2) were considered chronically stressed. Moreover, fish held at both densities were fed either the Control or the HP diets. Therefore, this experimental set-up comprised four treatments randomly assigned to duplicate tanks: fish held at low stocking density fed the control diet (LSD) or the HP diet (LSD HP), and fish held at high stocking density fed the control diet (HSD) or the HP diet (HSD HP). The experimental period lasted for 18 days and fish were fed daily to apparent satiety by automatic feeders, over a 24-h period.
Sampling
Fish were fasted 24-h prior to sampling in order to avoid any influence of feeding on cortisol and glucose levels (Arends et al. 1999). Five fish were quickly taken out from each tank at a time and anaesthetized with lethal doses of 2-phenoxyethanol (1,000 ml l−1, Sigma-Aldrich). Blood was withdrawn from the caudal vein of each fish using heparinized syringes. After each sampling, blood was centrifuged at 2,000×g during 5 min at room temperature. The collected plasma was stored at −25 °C for further analysis.
Analytical procedures
Diets were analysed for total AA contents. Diet samples were hydrolysed in 6 M HCl at 106 °C over 24-h in nitrogen-flushed glass vials. After deproteinization, samples were pre-column derivatized with phenylisothiocyanate (PITC; Pierce), using the PicoTag method (Waters, USA) according to Cohen et al. (1989). External standards were prepared along with the samples, using physiological AA standard solutions (acid/neutral and basics from Sigma) and a glutamine solution. Norleucine was used as an internal standard. Samples and standards were analysed by high performance liquid chromatography (HPLC) in a Waters Reversed-Phase Amino Acid Analysis System equipped with a PicoTag column (3.9 × 300 mm), using the conditions described by Cohen et al. (1989). Resulting peaks were analysed with the Breeze software (Waters). During acid hydrolysis, asparagine is converted to aspartate and glutamine to glutamate, so the reported values for these AA (Asx and Glx) represent the sum of the respective amine and acid. Moreover, tryptophan was not determined since it is destroyed by acid hydrolysis. Due to technical constraints, cysteine and methionine in the samples were not quantified.
Plasma cortisol levels were determined by radioimmunoassay (RIA) as described by Rotllant et al. (2006). Briefly, 50 μl of plasma samples was diluted in 950 μl phosphate buffer containing 1 g l−1 gelatin, pH 7.6, and denatured at 80 °C for 1 h. Duplicate aliquots (100 μl) of diluted denatured plasma were then used in the assay. Plasma glucose and lactate were assessed using commercially available Spinreact kits (Glucose HK Ref. 1001200; Lactate Ref. 1001330), adapted for 96-well microplates. Plasma total proteins were determined in 1:50 (v/v) diluted plasma samples using the bicinchoninic acid (BCA) Protein Assay Kit (Pierce #23225, Rockford, USA) for microplates. Bovine serum albumin served as a standard. All analyses were conducted in triplicates.
Total nitrite plus nitrate in plasma was analysed using a nitrite/nitrate colorimetric method (Roche Diagnostics GmbH, Mannheim, Germany). Briefly, nitrate was reduced to nitrite with nitrate reductase and nitrite was determined colorimetrically after addition of sulfanilamide and N-naphthyl-ethylenediamine. Nitrite concentration was calculated by comparison with a sodium nitrite standard curve. Since nitrite and nitrate are endogenously produced as oxidative metabolites of the messenger molecule NO, these compounds are considered as indicative of NO production (Saeij et al. 2003). All analyses were conducted in duplicates.
Alternative complement pathway (ACP) activity was estimated as described by Sunyer and Tort (1995). The following buffers were used: GVB (Isotonic veronal buffered saline), pH 7.3, containing 0.1 % gelatin; EDTA-GVB, as previous one but containing 20 mM EDTA; and Mg-EGTA-GVB, which is GVB with 10 mM Mg+2 and 10 mM EGTA. Rabbit red blood cells (RaRBC; Probiologica Lda, Portugal) were used for ACP determination. RaRBC were washed four times in GVB and resuspended in GVB to a concentration of 2.5 × 108 cells ml−1. Ten microlitre of RaRBC suspension was then added to 40 μl of serially diluted plasma in Mg-EGTA-GVB buffer. Samples were incubated at room temperature for 100 min with regular shaking. The reaction was stopped by adding 150 μl of cold EDTA-GVB. Samples were then centrifuged, and the extent of haemolysis was estimated by measuring the optical density of the supernatant at 414 nm. The ACH50 units were defined as the concentration of serum giving 50 % haemolysis of RaRBC. All analysis was conducted by triplicates.
Lysozyme activity was measured using a turbidimetric assay as described by Costas et al. (2011a). Briefly, a solution of Micrococcus lysodeikticus (0.5 mg ml−1 0.05 M sodium phosphate buffer; pH 6.2) was prepared. To a microplate, 15 μl of plasma and 250 μl of the above suspension were added to give a final volume of 265 μl. The reaction was carried out at 25 °C, and the absorbance (450 nm) is measured after 0.5 and 4.5 min. Lyophilized hen egg white lysozyme (Sigma) was serially diluted in sodium phosphate buffer (0.05 M; pH 6.2) and used to develop a standard curve. The amount of lysozyme in the sample was calculated using the formula of the standard curve. All analyses were conducted in triplicate.
Total peroxidase activity in plasma was measured following the procedure described by Quade and Roth (1997). Briefly, 15 μl of plasma (triplicates per fish) was diluted with 135 μl of HBSS without Ca+2 and Mg+2 in flat-bottomed 96-well plates. Then, 50 μl of 20 mM 3,3′,5,5′-tetramethylbenzidine hydrochloride (Sigma) and 50 μl of 5 mM H2O2 were added. The colour-change reaction was stopped after 2 min by adding 50 μl of 2 M sulphuric acid, and the optical density was read at 450 nm in a Powerwave™ microplate spectrophotometer (BioTek, Winooski, USA). The wells without plasma were used as blanks. The peroxidase activity (units ml−1 plasma) was determined defining one unit of peroxidase as that which produces an absorbance change of 1 optic density.
Statistics
All results are expressed as mean ± standard error of the mean (SEM). Data were analysed for normality (Kolmogorov–Smirnov test) and homoscedasticity of variance (Levene’s test) and, when necessary, they were log-transformed before being treated statistically. Data from experimental diets were analysed by t test, while fish data were analysed by two-way analysis of variance (ANOVA) with stress and dietary treatment as dependent variables. When significant differences were obtained from the ANOVA, Student’s t tests were carried out for paired-comparisons to analyse the effect of stress and dietary treatment. All statistical analyses were performed using the computer package SPSS 15.0 for WINDOWS. The level of significance used was P ≤ 0.05 for all statistical tests.
Results
Diets
Feed ingredients and feed composition are given in Table 1. HP diet presented higher protein level, while lipids, DM, ash and energy values were similar in both diets. In addition, digestible protein from Control and HP diets was calculated according to Dias et al. (2010), being 41.1 and 43.2 %, respectively. The AA patterns (g 100 g−1 AA) presented significant differences between experimental diets, with arginine, threonine and lysine being significantly higher in the HP diet (Table 2).
Experimental diets were well accepted, and no significant differences were found in growth and feed intake among the experimental groups (results not shown). Moreover, survival was 99.1 % for fish held at HSD and 100 % for fish in treatments LSD, LSD HP and HSD HP at the end of the experimental period.
Stress indicators
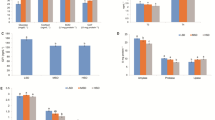
Plasma cortisol levels were significantly higher in specimens from HSD when compared to both LSD groups. Although cortisol levels also increased in fish held at HSD HP treatment, those values were not significantly different than the observed in specimens held at LSD treatment (Fig. 1). Plasma glucose and lactate levels showed a similar pattern, being significantly higher in fish held at HSD when compared to specimens held at LSD, LSD HP and HSD HP treatments. In addition, plasma protein levels were not significantly different among treatments (Table 3).
Innate humoral parameters
ACP activity decreased significantly in fish held at HSD when compared to all other treatments, while specimens from HSD HP treatment showed higher values than those in fish from LSD and HSD treatments (Fig. 2). Moreover, plasma NO levels showed a trend to decrease in fish held at HSD treatment, though no significant changes were observed (Fig. 3). Similar to that observed for ACP values, plasma lysozyme activity decreased significantly in fish held at HSD when compared to all other treatments (Fig. 4). Furthermore, plasma peroxidase activity also showed a similar tendency with lower values in fish held at HSD when compared to both LSD and LSD HP treatments. In addition, specimens from LSD HP presented higher peroxidase activity than fish from all other treatments (Fig. 5).
Alternative complement pathway activity in S. senegalensis after 18 days held at different treatments. Data are presented as ACH50 values in plasma, expressed as mean ± SEM (n = 10). *Significant differences attributed to stress, and †significant differences attributed to HP diet (Student’s t test; P < 0.05)
Discussion
High stocking density clearly increased plasma cortisol, glucose and lactate levels in fish held at HSD treatment from the current study. Moreover, those changes are in agreement with those previously described for Senegalese sole and other fish species under similar stressful conditions (Montero et al. 1999; Costas et al. 2008; Herrera et al. 2009; Conde-Sieira et al. 2010; Salas-Leiton et al. 2010). Measurement of plasma cortisol, glucose and lactate levels provides an effective method to monitor primary and secondary stress responses in fish (Mommsen et al. 1999; Pottinger 2008). Therefore, results from the current study validate the experimental design since HSD elicited a chronic stress response, including primary (plasma cortisol) and secondary (plasma glucose and lactate) responses, after 18 days of treatment.
Interestingly, plasma glucose and lactate levels decreased significantly in fish held at HSD HP up to similar values than LSD and LSD HP specimens, while plasma cortisol levels were not significantly different than those from controls. Therefore, dietary treatment seems to influence the Senegalese sole primary and secondary stress responses by minimizing cortisol release and the subsequent mobilization of energy substrates, at least after 18 days of feeding and under this particular stressful condition. Similarly, Senegalese sole submitted to repeated handling stress and fed a diet with a slightly increase in arginine, methionine, lysine and phenylalanine for 14 days also decreased plasma glucose and lactate levels (Costas et al. 2012a). Moreover, rainbow trout (Oncorhynchus mykiss) fed diets supplemented with tryptophan (4 and 8 times the tryptophan content of the control diets) for 7 days, and thereafter subjected to an acute stress, showed significantly lower cortisol levels than stressed fish fed the control diets (Lepage et al. 2002, 2003). Although HP diet from the current study was supplemented with l-tryptophan, this indispensable AA was not analysed. However, the HP diet presented higher levels of arginine, threonine and lysine than the control diet. Dietary arginine supplementation decreased the level of cortisol in serum of finishing pigs and weaned piglets (Ma et al. 2010; Yao et al. 2011). Additionally, Costas et al. (unpublished results) observed that dietary arginine supplementation beyond minimum requirement for optimal growth can decrease cortisol levels in chronically stressed turbot (Scophthalmus maximus). In the present study, it is possible that arginine metabolites or arginine itself may have minimized the release of the adrenocorticotropic hormone from pituitary and/or interfered in the brain monoaminergic system. For instance, an intracerebroventricular injection of arginine induced sedative or hypnotic effects in chicks exposed to a social isolation stress (Suenaga et al. 2008). However, the underlying mechanisms are unknown and the function of endogenous arginine under stressful husbandry conditions in fish deserves further attention.
The innate humoral immune responses evaluated in this study generally depend on the species and type and duration of the stress imposed. In some cases, plasma lysozyme and ACP activities decrease or no changes are observed (Cuesta et al. 2005; Costas et al. 2011a; Mauri et al. 2011), while in other studies, plasma lysozyme and peroxidase activities significantly increased in stressed specimens (Demers and Bayne 1997; Rotllant et al. 1997; Cuesta et al. 2005; Caipang et al. 2009; Costas et al. 2011b, 2012a). These differential effects may be achieved by differences in overall glucocorticoid sensitivity or receptivity of the immune response being affected (Dhabhar 2009). In the current study, the decrease in plasma lysozyme, ACP and peroxidase activities observed in fish held at HSD suggests an impairment of the immune system. Moreover, this drop in humoral responses is consistent with that generally described for chronically stressed fish (Mauri et al. 2011; Tort 2011), including Senegalese sole held at high stocking density (Salas-Leiton et al. 2010). This lower plasma lysozyme, ACP and peroxidase values in fish held at HSD may be further associated with decreases in circulating phagocytes. Neutrophils are thought to be the source of plasma lysozyme and peroxidase (Murray and Fletcher 1976; Ellis 1999), and increases in lysozyme and peroxidase levels have been associated with increases in neutrophil numbers (Cerezuela et al. 2009; Costas et al. 2012b). In addition, complement proteins can stimulate phagocytosis by opsonizing pathogens, a process that is mediated by complement receptors on the surface of phagocytic cells (Holland and Lambris 2002). Considering the role of both macrophages and neutrophils on phagocytosing bacteria, it is likely that HSD specimens had a lower level of protection than specimens from the other treatments to resist a bacterial infection. In fact, this species appear to be highly susceptible to opportunistic pathogens under similar stressful conditions (Costas et al. 2008).
In the current study, the HP diet increased plasma lysozyme, ACP and peroxidase activities in fish held at HSD HP when compared to HSD specimens. ACP activity with HP diet was even higher than in LSD groups. Moreover, fish held at LSD HP also presented higher peroxidase levels than specimens from all other treatments. Therefore, arginine, threonine and/or lysine (and possibly tryptophan) present important roles in non-specific immune mechanisms. Particularly in this study, one or more of these indispensable AA appear to mitigate the immunosuppressive effects attributed to chronic stress action. Similarly, Costas et al. (unpublished results) have recently observed that Senegalese sole fed a diet with higher arginine, isoleucine, leucine, threonine, valine and methionine levels than the control diet increased plasma lysozyme, ACP and peroxidase activities after 12 weeks of feeding. As already specified above (see introduction), many AA regulates key metabolic pathways that are crucial for immune responses. In particular, leucine is an activator of the mTOR signalling pathway that regulates protein synthesis and degradation in cells and appears to exert a greater effect on immune function than isoleucine and valine, which may be explained in part by their differential actions on the mTOR signalling (Li et al. 2007). Furthermore, serotonin, melatonin and N-acetylserotonin, products of tryptophan catabolism, can enhance host immunity by inhibiting the production of superoxide, scavenging free radicals and attenuating the production of tumour necrosis factor-alpha (Perianayagam et al. 2005). Since there is a progressive decline in tryptophan concentrations in plasma of animals with inflammation, its catabolism plays a critical role in the functions of both macrophages and lymphocytes (Melchior et al. 2004). At present, a potential use of tryptophan supplementation for animal health management is not fully developed. However, it has been reported that dietary supplementation with tryptophan can inhibit aggression, reduce cannibalism and prevent cortisol-mediated immune suppression in fish (Li et al. 2009). Moreover, a large body of evidence from animal studies indicates that adequate provision of arginine is required for lymphocyte development and that dietary arginine supplementation enhances immune function in various models of immunological challenges (Li et al. 2007). In particular, a positive effect of arginine-enriched diets on disease resistance has even been observed in Senegalese sole (Costas et al. 2011b). Therefore, high protein diets may be used as functional feeds for chronically stressed sole, as an alternative to chemotherapeutic and antibiotic treatments. The term functional feeds is used to describe fish feeds that have added benefits beyond the fish essential nutritional requirements, being both health status and growth expected to improve (Li et al. 2009).
In conclusion, feeding sole a diet with a slightly increase in all indispensable AA, together with tyrosine and cysteine, with respect to a reference diet results in a decrease in post-stress plasma cortisol, glucose and lactate levels after 18 days of treatment, thus minimizing the negative effects attributed to cortisol release after hypothalamic-pituitary-interrenal axis activation. In addition, the decrease in plasma lysozyme, ACP and peroxidase activities due to high stocking density was minimized through HP diet after 18 days. Therefore, it is suggested that a high-quality protein diet may represent a functional feed for chronically stressed sole. A large number of additives or feed ingredients (e.g. prebiotics, probiotics, glucans or nucleotides) are available for inclusion in functional feeds. However, little attention has been paid to the role of individual AA as potential immunostimulants. According to the results from this study and to that already reported by the same authors, supplementing key AA in the diet may represent a metabolic advantage during predictable stressful events (e.g. crowding associated with grading procedures), which may have a significant effect on growth, immunity and welfare in the longer term.
References
Aragão C, Corte-Real J, Costas B, Dinis MT, Conceição LEC (2008) Stress response and changes in amino acid requirements in Senegalese sole Solea senegalensis Kaup 1758. Amino Acids 34:143–148
Aragão C, Costas B, Vargas-Chacoff L, Ruiz-Jarabo I, Dinis MT, Mancera JM, Conceição LEC (2010) Changes in plasma amino acid levels in a euryhaline fish exposed to different environmental salinities. Amino Acids 38:311–317
Arends RJ, Mancera JM, Muñoz JL, Wendelaar Bonga SE, Flik G (1999) The stress response of the gilthead sea bream (Sparus aurata L.) to air exposure and confinement. J Endocr 163:149–157
Caipang CMA, Berg I, Brinchmann MF, Kiron V (2009) Short-term crowding stress in Atlantic cod, Gadus morhua L. modulates the humoral immune response. Aquaculture 295:110–115
Cerezuela R, Cuesta A, Meseguer J, Esteban MA (2009) Effects of dietary vitamin D3 administration on innate immune parameters of seabream (Sparus aurata L.). Fish Shellfish Immunol 26:243–248
Cohen SA, Meys M, Tarvin TL (1989) The pico-tag method—a manual of advanced techniques for amino acids analysis. Waters, Division of Milipore, Bedford
Conceição LEC, Aragão C, Dias J, Costas B, Terova G, Martins C, Tort L (2012) Dietary nitrogen and fish welfare. Fish Physiol Biochem 38:119–141
Conde-Sieira M, Aguilar AJ, López-Patiño MA, Míguez JM, Soengas JL (2010) Stress alters food intake and glucosensing response in hypothalamus, hindbrain, liver, and Brockmann bodies of rainbow trout. Physiol Behav 101:483–493
Costas B, Aragão C, Mancera JM, Dinis MT, Conceição LEC (2008) High stocking density induces crowding stress and affects amino acid metabolism in Senegalese sole Solea senegalensis (Kaup 1858) juveniles. Aquac Res 39:1–9
Costas B, Conceição LEC, Aragão C, Martos JA, Ruiz-Jarabo I, Mancera JM, Afonso A (2011a) Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 316:68–76
Costas B, Conceição LEC, Dias J, Novoa B, Figueras A, Afonso A (2011b) Dietary arginine and repeated handling increase disease resistance and modulate innate immune mechanisms of Senegalese sole (Solea senegalensis Kaup, 1858). Fish Shellfish Immunol 31:838–847
Costas B, Aragão C, Soengas JL, Míguez JM, Rema P, Dias J, Afonso A, Conceição LEC (2012a) Effects of dietary amino acids and repeated handling on stress response and brain monoaminergic neurotransmitters in Senegalese sole (Solea senegalensis) juveniles. Comp Biochem Physiol A 161:18–26
Costas B, Rêgo PCNP, Simões I, Marques JF, Castro-Cunha M, Afonso A (2012b) Cellular and humoral immune responses of Senegalese sole (Solea senegalensis Kaup, 1858) following challenge with two Photobacterium damselae subsp. piscicida strains from different geographical origins. J Fish Dis (in press). doi:10.1111/jfd12033
Cuesta A, Laiz-Carrión R, Martín del Río MP, Meseguer J, Mancera JM, Esteban MA (2005) Salinity influences the humoral immune parameters of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 18:255–261
Demers NE, Bayne CJ (1997) The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev Comp Immunol 21:363–373
Dhabhar FS (2009) A hassle a day may keep the pathogens away: the fight-or-flight stress response and the augmentation of immune function. Integr Comp Biol 49:215–236
Dias J, Yúfera M, Valente LMP, Rema P (2010) Feed transit and apparent protein, phosphorus and energy digestibility of practical feed ingredients by Senegalese sole (Solea senegalensis). Aquaculture 302:94–99
Dinis MT, Ribeiro L, Soares F, Sarasquete C (1999) A review on the cultivation potential of Solea senegalensis in Portugal and Spain. Aquaculture 176:27–38
Ellis AE (1999) Immunity to bacteria in fish. Fish Shellfish Immunol 9:291–308
Fast MD, Hosoya S, Johnson SC, Afonso LOB (2008) Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish Immunol 24:194–204
Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl Å, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza EJ, Stone D, Wilson R, Wurtele E (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579
Herrera M, Vargas-Chacoff L, Hachero I, Ruíz-Jarabo I, Rodiles A, Navas JI, Mancera JM (2009) Physiological responses of juvenile wedge sole Dicologoglossa cuneata (Moreau) to high stocking density. Aquac Res 40:790–797
Höglund E, Sorensen C, Bakke MJ, Nilsson GE, Øverli Ø (2007) Attenuation of stress-induced anorexia in brown trout (Salmo trutta) by pre-treatment with dietary l-tryptophan. Br J Nutr 97:786–789
Holland MCH, Lambris JD (2002) The complement system in teleosts. Fish Shellfish Immunol 12:399–420
Hseu JR, Lu FI, Su HM, Wang LS, Tsai CL, Hwang PP (2003) Effect of exogenous tryptophan on cannibalism, survival and growth in juvenile grouper, Epinephelus coioides. Aquaculture 218:251–263
Imsland AK, Foss A, Conceição LEC, Dinis MT, Delbare D, Schram E, Kamstra A, Rema P, White P (2003) A review of the culture potential of Solea solea and Solea senegalensis. Rev Fish Biol Fish 13:379–407
Kaushik SJ (1998) Whole body amino acid composition of European seabass (Dicentrarchus labrax), gilthead seabream (Sparus aurata) and turbot (Psetta maxima) with an estimation of their IAA requirement profiles. Aquat Living Resour 11:355–358
Lepage O, Tottmar O, Winberg S (2002) Time-course of the effect of dietary l-tryptophan on plasma cortisol levels in rainbow trout Oncorhynchus mykiss. J Exp Biol 206:3679–3687
Lepage O, Vílchez IM, Pottinger TG, Winberg S (2003) Elevated dietary intake of l-tryptophan counteracts the stress-induced elevation of plasma cortisol in rainbow trout (Oncorhynchus mykiss). J Exp Biol 205:3589–3599
Li P, Yin Y-L, Li D, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li P, Mai K, Trushenski J, Wu G (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43–53
Ma X, Lin Y, Jiang Z, Zheng C, Zhou G, Yu D, Cao T, Wang J, Chen F (2010) Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids 38:95–102
Mauri I, Romero A, Acerete L, MacKenzie S, Roher N, Callol A, Cano I, Alvarez MC, Tort L (2011) Changes in complement responses in Gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) under crowding stress, plus viral and bacterial challenges. Fish Shellfish Immunol 30:182–188
Melchior D, Seve B, Le Floc’h N (2004) Chronic lung inflammation affects plasma amino acid concentrations in pigs. J Anim Sci 82:1091–1099
Milligan CL (1997) The role of cortisol in amino acid mobilization and metabolism following exhaustive exercise in rainbow trout (Oncorhynchus mykiss Walbaum). Fish Physiol Biochem 16:119–128
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Montero D, Izquierdo MS, Tort L, Robaina L, Vergara JM (1999) High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata L, juveniles. Fish Physiol Biochem 20:53–60
Murray CK, Fletcher TC (1976) The immunohistochemical localization of lysozyme in plaice (Pleuronectes platessa L.) tissues. J Fish Biol 9:329–334
Neumann NF, Fagan D, Belosevic M (1995) Macrophage activating factor(s) secreted by mitogen stimulated goldfish kidney leucocytes synergize with bacterial lipopolysaccharide to induce nitric oxide production in teleost macrophages. Dev Comp Immunol 19:473–482
NRC (1993) Nutrient requirements of fish. National Academy Press, Washington
Perianayagam MC, Oxenkrug GF, Jaber BL (2005) Immune-modulating effects of melatonin, N-acetylserotonin, and N-acetyldopomine. Ann N Y Acad Sci 1053:386–393
Pinto W, Aragão C, Soares F, Dinis MT, Conceição LEC (2007) Growth, stress response and free amino acid levels in Senegalese sole (Solea senegalensis Kaup 1858) chronically exposed to exogenous ammonia. Aquac Res 38:1198–1204
Pottinger TG (2008) The stress response in fish-mechanisms, effects and measurement. In: Branson EJ (ed) Fish welfare. Blackwell Publishing, Oxford, pp 32–48
Prunet P, Øverli Ø, Douxfils J, Bernardini G, Kestemont P, Baron D (2012) Fish welfare and genomics. Fish Physiol Biochem 38:43–60
Quade MJ, Roth JA (1997) A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol 58:239–248
Rotllant J, Pavlidis M, Kentouri M, Abad ME, Tort L (1997) Non-specific immune responses in the red porgy, Pagrus pagrus after crowding stress. Aquaculture 156:279–290
Rotllant J, Ruane NM, Dinis MT, Canário AVM, Power DM (2006) Intra-adrenal interactions in fish: catecholamine stimulated cortisol release in sea bass (Dicentrarchus labrax L.). Comp Biochem Physiol A 143:375–381
Saeij JPJ, Verburg-van Kemenade LBM, van Muiswinkel WB, Wiegertjes GF (2003) Daily hangling stress reduces resistance of carp to Trypanoplasma borreli: in vitro modulatory effects of cortisol on leukocyte function and apoptosis. Dev Comp Immunol 27:233–245
Salas-Leiton E, Anguis V, Martín-Antonio B, Crespo D, Planas JV, Infante C, Cañavate JP, Manchado M (2010) Effects of stocking density and feed ration on growth and gene expression in the Senegalese sole (Solea senegalensis): potential effects on the immune response. Fish Shellfish Immunol 28:296–302
Segner H, Sundh H, Buchmann K, Douxfils J, Sundell KS, Mathieu C, Ruane N, Jutfelt F, Toften H, Vaughan L (2012) Health of farmed fish: its relation to fish welfare and its utility as welfare indicator. Fish Physiol Biochem 38:85–105
Stolte EH, Nabuurs SB, Bury NR, Sturm A, Flik G, Savelkoul HFJ, Verburg-van Kemenade BML (2008) Stress and innate immunity in carp: corticosteroid receptors and pro-inflammatory cytokines. Mol Immunol 46:70–79
Suenaga R, Tomonaga S, Yamane H, Kurauchi I, Tsuneyoshi Y, Sato H, Denbow DM, Furuse M (2008) Intracerebroventricular injection of l-arginine induces sedative and hypnotic effects under an acute stress in neonatal chicks. Amino Acids 35:139–146
Sunyer JO, Tort L (1995) Natural hemolitic and bactericidal activities of seabream Sparus aurata serum are affected by the alternative complement pathway. Vet Immunol Immunopathol 45:333–345
Tafalla C, Novoa B (2000) Requirements for nitric oxide production by turbot (Scophthalmus maximus) head kidney macrophages. Dev Comp Immunol 24:623–631
Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35:1366–1375
Verburg-van Kemenade LBM, Stolte EH, Metz JR, Chadzinska M (2009) Neuroendocrine-immune interactions in teleost fish. In: Bernier NJ, Van Der Kraak G, Farrell AP, Brauner CJ (eds) Fish physiology, vol 28. Academic Press Inc., San Diego, pp 313–364
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 7:591–625
Weyts FAA, Cohen N, Flik G, Verburg-van Kemenade BML (1999) Interactions between the immune system and the hypothalamo-pituitary-interrenal axis in fish. Fish Shellfish Immunol 9:1–20
Wilson RP (2002) Amino acids and proteins. In: Halver JE, Hardy RW (eds) Fish nutrition. Elsevier Science, San Diego, pp 144–179
Yao K, Guan S, Li T, Huang R, Wu G, Ruan Z, Yin Y (2011) Dietary l-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br J Nutr 105:703–709
Acknowledgments
This study was supported by project STRESSAA-POCTI/CVT/49324/2002 (FCT, Portugal and FEDER). Benjamín Costas and Cláudia Aragão were supported by Fundação para a Ciência e a Tecnologia, Portugal (SFRH/BD/38697/2007 and SFRH/BPD/37197/2007, respectively).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costas, B., Aragão, C., Dias, J. et al. Interactive effects of a high-quality protein diet and high stocking density on the stress response and some innate immune parameters of Senegalese sole Solea senegalensis . Fish Physiol Biochem 39, 1141–1151 (2013). https://doi.org/10.1007/s10695-013-9770-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-013-9770-1