Abstract
A continuous fibroblast-like cell line, TMF (turbot muscle fibroblasts), was established from juvenile turbot Scophthalmus maximus muscle with the method of trypsin digestion. It has been subcultured more than 60 passages for over 150 days. The TMF cells were cultured in L-15 medium supplemented with HEPES, fetal bovine serum (FBS), GlutaMAX, and basic fibroblast growth factor (bFGF). The optimal temperature for TMF culture was 24 °C. TMF cells were predominantly composed of fibroblastic-like cells, and the transcription factor 4 (TCF-4) was highly expressed in TMF cells. Chromosome analysis revealed that it had a diploid chromosome number of 2n = 44. The transfection efficiency achieved 54.95 ± 6.59%, and the cell mortality rate was about 8.70% when transfected with the nucleofection method. Meanwhile, the TMF cells showed a sensitive response to amino acid levels and activation target of rapamycin (TOR) signaling pathway. These results indicate that TMF was a potential tool to explore the signal transduction of teleost in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish cell line has been a powerful tool to explore the immune response (Falco et al. 2009), toxic effect (Bian et al. 2017), and cellular signaling (Lansard et al. 2010; Trevino et al. 2008). The availability of fish cell lines, since the 1960s, has begun to make impacts in scientific research, while at a much slower rate than mammalian cell lines (Lakra and Joy 2011). Till now, there are more than 283 cell lines isolated from fishes, and they were widely used in fish immunology (Bols et al. 2001), ecotoxicology (Kristin 2006), and aquaculture (Conde-Sieira and Soengas 2016). However, only about one-third cell lines were derived from marine fish species, and few studies about signal transduction were reported using fish cell lines (Lakra and Joy 2011).
Recently, replacement of fish meal in aqua feed with alternative protein sources has been a major goal for aquaculture nutritionist (Fuchs et al. 2015; Liu et al. 2014). Numerous studies have been carried out, from feeding trials (Lee et al. 2015; Wang et al. 2016a) to primary cell culture studies (Gregory et al. 2011; Minghetti et al. 2011), to explore the molecular mechanism involved in the limited usage of alternative protein sources. However, the results were highly influenced by feeding environments (Bœuf and Payan 2001), animal health (Oliva-Teles 2012), diet palatability (Yamamoto et al. 2015), and digestibility (Eusebio et al. 2004). Establishing a cell line and characterizing its availability in fish signal transduction studies would promote the progress of fish nutrition.
Muscle is the major site of protein deposition and dynamic organ for nutrient metabolism. It contains substantial cell types, such as myogenic precursor cells, fibroblasts, and adipocytes. Myogenic precursor cells play a critical role in muscle growth, maintenance, and regeneration (Schienda et al. 2006). However, establishing a pure myoblast culture is a challenging task. Fibroblast cells tend to adhere earlier as compared to the myoblasts (Li and Huard 2002). To date, cell lines isolated from fish muscle tissue have been developed from spotted weakfish (Cynoscion nebulosus) (Middlebrooks et al. 1979), fathead minnow (Pimephales promelas) (Gravell and Malsberger 2010), Indian major carp (Catla catla) (Ahmed et al. 2010; Ahmed et al. 2009), and zebrafish (Danio rerio) (Kumar et al. 2016). Most of these cell lines were fibroblastic-like. In mammal, the muscle fibroblast cell lines, like NIH/3T3 and mouse embryonic fibroblasts (MEFs), have been potent models used to study cell proliferation (Thomopoulos et al. 2005), collagen synthesis (Wood et al. 1993), and cellular signaling (Duran et al. 2011). The establishment and characterization of a fibroblast-like cell line from fish muscle might be a potential tool to study the gene function of fish.
Turbot (Scophthalmus maximus) is an economically valuable marine carnivorous fish species in Europe and China featured by its rapid growth, good market value, and high production while strongly relied on fish meal. Up to now, several cell lines were derived from turbot, such as fin (Fernández-Puentes et al. 1993), posterior end (Tocher et al. 1989), embryo, and kidney (Wang et al. 2010a). In the present study, we established a fibroblastic-like cell line from the dorsal muscle of turbot and the culture condition was optimized. Meanwhile, a transfection method with high transfection efficiency was developed. Its potential application in signal transduction study was also explored.
Materials and methods
Primary culture and subculture
Juvenile turbot (~ 10 g) was immersed in 75% ethanol for 30 s and rinsed by sterilized Dulbecco’s phosphate-buffered saline (DPBS) with 4% antibiotics (penicillin 400 U/ml, streptomycin 400 μg/ml). White dorsal muscle tissue was excised and collected in cold L-15 medium (Sigma, #L5520) with 4% antibiotics. The tissue was cut into 1.0-mm3 pieces, and the fragments were collected through centrifugation (300×g, 10 min at 4 °C). The pellet was digested with 0.05% trypsin at room temperature on a shaker for 30 min. The enzymatic digestion was neutralized with L-15 medium containing 10% fetal bovine serum (FBS; Gibco). The isolated muscle cells were purified through a 70-μm nylon cell strainer (BD Falcon) and collected through centrifugation (300×g, 10 min at 4 °C). The pellet was resuspended with cold growth medium (L-15 medium with 20% FBS, 20 mM HEPES, 2 mM GlutaMAX™, 1% antibiotics, and 2.5 ng/ml fibroblast growth factor). The cells were seeded in the 100-mm dish at 2 × 106 cells/ml and cultured at 24 °C in a humidified incubator without CO2. Medium was changed every 2 or 3 days. When the cell confluence reached about 90%, cells were splited with trypsinization method. Trypsin-EDTA solution (0.25%) was used, and the digestion reaction was performed for 5 min at 24 °C. Trypsinization was stopped by adding growth medium, and the cells were subcultured at a ratio of 1:3–1:4. After ten passages, cell proliferation became more rapid and the FBS level of growth medium was reduced to 10%.
Cryopreservation and recovery
Cryopreservation of TMF cells was performed as follows. Cells were collected with trypsinization method when the cell confluence reached 90% and centrifuged (90×g, 2 min, 4 °C). The supernatant was discarded, and the cell pellet was resuspended with Recovery™ Cell Culture Freezing Medium (Gibco, #12648-010) to 5 × 106 cells/ml. The suspensions were divided into sterile cryovials and placed in the freezing container (Thermo Fisher Scientific). The container was stored at − 20 °C for 2 h and − 80 °C for overnight and then transferred the cells to − 152 °C cryogenic freezer for prolonged storage.
For cell recovery, the cryovial was warmed up in a 37 °C water bath for 1 min and the cell suspensions were transferred in a tube containing warm growth medium and centrifuged at 90×g for 2 min to discard the DMSO in the freezing medium. The pellet was gently resuspended and seeded in the 100-mm cell culture dish.
Effects of temperature on cell growth
TMF cells were seeded in 60-mm culture dish at approximately 2.5 × 104 cells and incubated at either 20, 24, or 28 °C. The cells were dispersed by trypsin at 1, 2, 3, 4, 5, 7, and 10 days after seeding, and a number of cells were counted using an automated cell counter (Merck Millipore). All cell culture experiments were repeated at least three times.
Chromosome analysis
The chromosome analysis of TMF cells was performed as described before (Chen et al. 2005). Briefly, TMF cells were seeded in 100-mm dish and incubated for 36 h. Colchicine was added to the cells at a final concentration of 0.5 μg/ml, and the cells were harvested after 6 h. The hypotonic solution (75 mM KCl) was added to the cells and treated for 25 min and adding Carnoy’s fixative for pre-fixing. After centrifugation, the cell pellet was fixed with cold Carnoy’s fixative and then air-dried. Five percent Giemsa was added to stain the chromosomes. The chromosome karyotype was analyzed as described previously (Nichols et al. 1964), and 100 cells at metaphase were analyzed.
Optimization of the transfection methods for TMF cells
Three kinds of transfection methods, calcium phosphate method, liposome method, and nucleofection method, were carried out to determine the transfection efficient of TMF cells. pmaxGFP vector (Lonza) was used as the reporter, and the transfection efficiency and cell mortality were measured by flow cytometry (Thermo).
The calcium phosphate transfection was carried out according to the instruction of the transfection kit (Beyotime, China). Cells were seeded in the plate for 24 h, and the medium was refreshed prior to transfection. The pmaxGFP vector was mixed with the calcium phosphate solution and the transfection solution (Beyotime). The mixture was added to the cell plate and then incubated for 24 h before detecting the expression of GFP. Two commercial kit of liposome transfection method, Lipofectamine 2000 and Lipofectamine 3000 (Thermo Scientific), were used in this study. According to the manufacturer’s instructions, the transfection operation was contacted when the cell confluence reached 80%. The pmaxGFP vector was mixed with the Lipofectamine™ transfection solution and added to the cell. The expression of GFP was detected after 24 h using the fluorescence microscope (Nikon).
For the nucleofection, cells were subculcured 48 h prior to transfection. Cells were harvested by trypsinization, counted using the automated cell counter, and centrifuged at 200×g for 10 min. Cells (2 × 106) were resuspended with SG solution (Lonza) and mixed with 2 μg pmaxGFP plasmid. The mixture was transferred into the cuvette and electroporated using the 4D-Nucleofector™ X (Lonza). After the electroporation, 500 μl of pre-warmed growth medium was added and gently mixed. The mixture was transferred to the 6-well cell culture plate. The expression of GFP was detected after 24-h incubation.
Cell treatment
To identify the cell type, TMF cells (at passage 16) were collected and analyzed with immunoblotting. The expression of desmin, paired box protein 7 (Pax-7), and transcription factor 4 (TCF-4) was determined. The mouse muscle myoblast cell line C2C12 and mouse fibroblast cell line NIH/3T3 were referred as the control. Both C2C12 and NIH/3T3 were obtained from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. C2C12 and NIH/3T3 cells were maintained in DMEM (Invitrogen) supplemented with 10% FBS (Invitrogen), 1% penicillin-streptomycin, 2 mM GlutaMAX™, and 1 mM sodium pyruvate in a humidified incubator at 37 °C and 5% CO2. The cells were collected and analyzed when the confluence reached 90%.
The response of target of rapamycin (TOR) signaling pathway was measured to assess the available of TMF cells in fish nutrient sensing study. Cells were seeded at a density of 2 × 106 cells/well and incubated for 48 h. Then, cells were starved without serum or amino acids for 2 h. The amino acid solution, according to the composition of L-15 medium, was added to the medium while rapamycin (10 nM, Selleck) was added to block the activity of TOR. Cells were incubated for 1 h before being harvested. All cell culture experiments were repeated at least three times.
Western blot analyses
Cells were rinsed with ice-cold DPBS and lysed in ice-cold lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 0.5% NP-40, 0.1% SDS, 1 mM EDTA) with protease and phosphatase inhibitors (Roche). Turbot muscle (about 20 mg) was homogenized with Glass Tenbroeck Tissue Grinders (Kimble Chase) on ice and lysed in ice-cold lysis buffer with protease inhibitors. Lysates were centrifuged at 13,000×g for 10 min at 4 °C. The supernatants were collected and used for immunoblotting analysis. Protein concentrations were determined by a BCA protein assay kit (Beyotime Biotechnology) with bovine serum albumin as standard. Aliquots of 10 μg of total protein were loaded and separated by 12% SDS-PAGE gel and then transferred to 0.45-μm PVDF membrane (Millipore). After blocking with 5% nonfat milk, the membrane was incubated with primary antibodies overnight at 4 °C. Horseradish peroxidase (HRP)–conjugated secondary antibodies were detected using ECL reagents (Beyotime Biotechnology). The antibodies used in this study were as follows: antibodies against desmin (# sc-23879), paired box protein (Pax-7) (# sc-365843), and transcription factor 4 (TCF-4) (# sc-166699) were purchased from Santa Cruz Biotechnology Inc.; mTOR (# 2972), phospho-mTOR (Ser2448) (# 2971S), S6 kinase (# 9202), phospho-S6k (Thr389) (# 9205), S6 (# 2217), phospho-S6 (Ser235/236) (# 4856S), and β-tubulin (# 2146) were from Cell Signaling Technology Inc. All the antibodies used in this study have been confirmed cross-reactive with turbot. All experiments were repeated at least three times.
Result
Primary culture and subculture of TMF cells
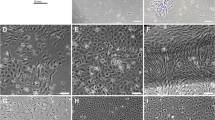
In the primary culture, the single cells were isolated from turbot dorsal muscle using trypsin digestion method and adhered the dish after 48 h (Fig. 1a, b) and achieved confluence in a week. Cells were subcultured in L-15 medium with 20% FBS at a ratio of 1:3 every 3 days. After four subcultures, most of cells were fibroblastic-like (Fig. 1c). The serum level was reduced to 10%, and the cells were splitted at a ratio of 1:4 every 3 days after ten subcultures. TMF cells recovered from storage at the 20th subcultures adhered to the substratum after 12 h and grew to confluency in 3 days (Fig. 2). To date, TMF cells have been subcultured for more than 60 passages over 150 days.
Effect of temperature on TMF cell growth
TMF cells were able to grow at temperature between 20 °C and 28 °C. The highest growth rate was obtained at 24 °C. The cell number increased from 2.5 × 104 to 2.33 × 105 in 5 days (Fig. 3).
Chromosome analysis of turbot muscle fibroblasts
The chromosome number of TMF cells ranged from 26 to 74, and about 70% of the cell chromosome number was 44 (Fig. 4a). The metaphase with a normal diploid number displayed the normal karyotype morphology consisting of 2 pairs of mediocentrics, 6 pairs of subtelocentrics, and 14 pairs of telocentrics (2n = 4m + 12st + 28t) (Fig. 4b, c).
Characterization of the cell type of TMF cells
As shown in Fig. 5a, TCF-4, a marker of fibroblast cells, was highly expressed in TMF cells and NIH-3T3 cells. However, no expression of Pax-7 or desmin, markers of myoblast cells, was detected. Pax-7 was highly expressed in turbot muscle as a positive control to indicate that the antibody does work in turbot (Fig. 5b).
Cell transfection
Among the transfection methods used in this study, nucleofection showed the highest transfection efficiency (54.95 ± 6.59%, Figs. 6 and 7a) and lowest cell mortality rate (8.70 ± 2.42%, Figs. 6 and 7b). Transfection with calcium phosphate in TMF cells showed the lowest transfection efficiency (0.26 ± 0.05%, Figs. 6 and 7a). Both Lipofectamine 2000 and Lipofectamine 3000 showed a low transfection efficiency (about 10%) in TMF cells.
The transfection efficiency and cell mortality rate with different transfection methods. Transfection efficiency (a) and mortality rate (b) of turbot muscle fibroblasts after calcium phosphate transfection, Lipofectamine 2000, Lipofectamine 3000, and nucleofection. a The transfection efficiency of cells were analyzed using flow cytometry at 24 h after transfection (abscissa, fluorescence intensity; ordinate, cell numbers; R1, untransfected cells; R2, transfected cells). b The transfected cells were stained with propidium iodide (PI) for mortality rate analyses using flow cytometry (abscissa, fluorescence intensity; ordinate, cell numbers; G, mortality rate). The results were expressed as means ± SE (n = 3)
Cell signaling response in TMF cells
As shown in Fig. 8, rapamycin treatment totally blocked the phosphorylation of S6K (Thr389) and S6 (Ser235/236) and reduced the phosphorylation of TOR (Ser2448). Amino acid supplement significantly activated the TOR-S6K-S6 pathway.
Discussion
To date, several cell lines have been developed from turbot (Scophthalmus maximus), such as embryonic (Chen et al. 2005), kidney (Wang et al. 2010a), and fin (Fan et al. 2010). These turbot cells have been used in fish immunology studies (Tafalla and Novoa 2001; Yang et al. 2013). In this study, we established a fibroblastic-like cell line from turbot muscle, and it has been subcultured for more than 60 times in 150 days. The optimal temperature for TMF cell growth was 24 °C, which was higher than the fish growth temperature (Wang et al. 2010a). Similar results were obtained in the turbot kidney cell line (Wang et al. 2010a) and turbot fin cell line (Fan et al. 2010). The differential optimal growth temperature between in vivo and in vitro was also observed in other fish species such as rainbow trout liver cell line (Schnell et al. 2009), flounder gill cell line (Tong et al. 1997), flounder embryonic cell line (Song et al. 2004), and half smooth tongue sole heart cell line (Wang et al. 2010b). However, the exact mechanism was unclear.
As reported in this study, TMF cells were primarily consisted of fibroblastic-like cells, which was similar with zebrafish muscle cell line (Kumar et al. 2016), TP-1 cell line from golden mahseer (Lakra et al. 2006), and SICH cell line from Indian major carp (Ahmed et al. 2009). Primary cultures of muscle cells were usually composed of myoblasts, non-myogenic cells, and fibroblasts. However, the growth rate was different, and it was highly influenced by the growth medium. In mammals, fibroblast cells doubled more rapidly and became the predominant cell type when the growth medium consisted of DMEM supplemented with 20% serum after several days (Rando and Blau 1994). The additional of basic fibroblast growth factor might also be a factor improving the growth of fibroblasts. The frequent overgrowth of fibroblasts limits the yield of myoblasts (Rando and Blau 1994). For pure myoblast culture, special procedures like flow cytometry and Percoll density gradient centrifugation were needed (Singh et al. 2013).
Expressing exogenous DNA or eliminating endogenous expression has been a potent tool to determine the function of specific gene and been widely used in cell signaling studies (Gregory 2012). However, as reported previously, the transfection efficiency in fish cells were extremely low (usually < 10%) with the transfection methods which performed well in mammalian cells (Lakra and Joy 2011). Developing a high transfection efficiency was critical for fish cell studies (Falco et al. 2009). In this study, neither the calcium phosphate method nor the liposome method showed a satisfactory transfection efficiency. The frustrating results might be related to the working temperature, as culture temperature of fish cells was much lower than that of mammalian cells. Nucleofection has been a popular transfection method for its high efficiency. Schiøtz reported the transfection efficiency using the nucleofection method achieved to 90% in Atlantic salmon (Schiøtz et al. 2011). In this study, the transfection efficiency of nucleofection method was significantly higher than other two methods. These results will dramatically extend the application of fish cells.
Target of rapamycin (TOR) signaling was the key checkpoint to integrate nutritional signals and evolutionary conserved, from fungi, nematodes, teleost, and human (Chantranupong et al. 2015). The activation of TOR signaling pathway was critical for the anabolic metabolism and diverse cellular progress (Polak and Hall 2009). Our previous studies have demonstrated that TOR signaling pathway was involved in the regulation of growth performance by diet in turbot (Wang et al. 2016b; Xu et al. 2016). An in vitro study in rainbow trout hepatocytes also showed that TOR signaling pathway was regulated by amino acid (Trevino et al. 2008). In the present study, exogenous amino acids induced a valid activation of TOR signaling and it can be attenuated by rapamycin, a TOR inhibitor widely used in cell signaling research (Saxton and Sabatini 2017). It indicated that TMF cell was a sensitive model for amino acid sensing and other cell signaling research in fish.
We established a continuous cell line from turbot muscle, and it was predominantly composed of fibroblastic-like cells. A transfection method with nucleofection could achieve more than 50% transfection efficiency. Moreover, the TMF cells were sensitive in responding to amino acid levels. Our results showed that TMF was a powerful tool to study fish signal transduction in vitro.
References
Ahmed VPI, Babu VS, Chandra V et al (2009) A new fibroblastic-like cell line from heart muscle of the Indian major carp (Catla catla): development and characterization. Aquaculture 293:180–186
Ahmed VPI, Chandra V, Parameswaran V et al (2010) A new epithelial-like cell line from eye muscle of catla Catla catla (Hamilton): development and characterization. J Fish Biol 72:2026–2038
Bian F, Jiang H, Man M, Mai K, Zhou H, Xu W, He G (2017) Dietary gossypol suppressed postprandial TOR signaling and elevated ER stress pathways in turbot (Scophthalmus maximus L.). Am J Physiol Endocrinol Metab 312:37–47
Bœuf G, Payan P (2001) How should salinity influence fish growth? Comp Biochem Physiol C-Toxicol Pharmacol 130:411–423
Bols NC, Brubacher JL, Ganassin RC, Lee LEJ (2001) Ecotoxicology and innate immunity in fish. Dev Comp Immunol 25:853–873
Chantranupong L, Wolfson R, Sabatini D (2015) Nutrient-sensing mechanisms across evolution. Cell 161:67–83
Chen SL, Ren GC, Sha ZX, Hong Y (2005) Development and characterization of a continuous embryonic cell line from turbot (Scophthalmus maximus). Aquaculture 249:63–68
Conde-Sieira M, Soengas JL (2016) Nutrient sensing systems in fish: impact on food intake regulation and energy homeostasis. Front Neurosci 10:603
Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT (2011) P62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell 44:134–146
Eusebio PS, Coloso RM, Mamauag REP, Rimmer MA, Mcbride S, Williams KC (2004) Apparent digestibility of selected feed ingredients in diets for grouper (Epinephelus coioides) juveniles. Aquac Res 35:1261–1269
Falco A, Encinas P, Carbajosa S, Cuesta A, Chaves-Pozo E, Tafalla C, Estepa A, Coll JM (2009) Transfection improvements of fish cell lines by using deacylated polyethylenimine of selected molecular weights. Fish Shellfish Immunol 26:559–566
Fan TJ, Ren BX, Geng XF, Yu QT, Wang LY (2010) Establishment of a turbot fin cell line and its susceptibility to turbot reddish body iridovirus. Cytotechnology 62:217–223
Fernández-Puentes C, Novoa B, Figueras A (1993) Initiation of a cell line from turbot (Scophthalmus maximus L.). In Vitro Cell Dev Biol-Anim 29:899–900
Fuchs VI, Schmidt J, Slater MJ, Zentek J, Buck BH, Steinhagen D (2015) The effect of supplementation with polysaccharides, nucleotides, acidifiers and bacillus strains in fish meal and soy bean based diets on growth performance in juvenile turbot (Scophthalmus maximus). Aquaculture 437:243–251
Gravell M, Malsberger RG (2010) A permanent cell line from the fathead minnow (Pimephales promelas). Annnyacadsci 126:555–565
Gregory MK, King HW, Bain PA, Gibson RA, Tocher DR, Schuller KA (2011) Development of a fish cell culture model to investigate the impact of fish oil replacement on lipid peroxidation. Lipids 46:753–764
Gregory P (2012) Gene overexpression: uses, mechanisms, and interpretation. Genetics 190:841–854
Kristin S (2006) Proposal to improve vertebrate cell cultures to establish them as substitutes for the regulatory testing of chemicals and effluents using fish. Toxicology 224:163–183
Kumar A, Singh N, Goswami M, Srivastava JK, Mishra AK, Lakra WS (2016) Establishment and characterization of a new muscle cell line of zebrafish (Danio rerio) as a model for gene expression studies. Anim Biotechnol 27:166–173
Lakra WS, Bhonde RR, Sivakumar N, Ayyappan S (2006) A new fibroblast like cell line from the fry of golden mahseer Tor putitora (Ham). Aquaculture 253:238–243
Lakra WS, Joy KP (2011) Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem 37:1–20
Lansard M, Panserat S, Plagnesjuan E, Seiliez I, Skibacassy S (2010) Integration of insulin and amino acid signals that regulate hepatic metabolism-related gene expression in rainbow trout: role of TOR. Amino Acids 39:801–810
Lee JK, Cho SH, Park SU, Kim KD, Lee SM (2015) Dietary protein requirement for young turbot (Scophthalmus maximus L.). Aquac Nutr 9:283–286
Li Y, Huard J (2002) Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol 161:895–907
Liu Y, He G, Wang Q, Mai K, Wei X, Zhou H (2014) Hydroxyproline supplementation on the performances of high plant protein source based diets in turbot (Scophthalmus maximus L.). Aquaculture 433:476–480
Middlebrooks BL, Ellender RD, Wharton JH (1979) Fish cell culture: a new cell line from Cynoscion nebulosus. Vitro 15:109–111
Minghetti M, Leaver MJ, Tocher DR (2011) Transcriptional control mechanisms of genes of lipid and fatty acid metabolism in the Atlantic salmon (Salmo salar L.) established cell line, SHK-1. Biochim Biophys Acta 1811:194–202
Nichols WW, Levan A, Coriell LL, Goldner H, Ahlström CG (1964) Chromosome abnormalities in vitro in human leukocytes associated with Schmidt-Ruppin Rous sarcoma virus. Science 146:248–250
Oliva-Teles A (2012) Nutrition and health of aquaculture fish. J Fish Dis 35:83–108
Polak P, Hall MN (2009) mTOR and the control of whole body metabolism. Curr Opin Cell Biol 21:209–218
Rando TA, Blau HM (1994) Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125:1275–1287
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168:960–976
Schienda J, Engleka KA, Jun S, Hansen MS, Epstein JA, Tabin CJ, Kunkel LM, Kardon G (2006) Somitic origin of limb muscle satellite and side population cells. Proc Natl Acad Sci U S A 103:945–950
Schiøtz BL, Rosado EG, Baekkevold ES, Lukacs M, Mjaaland S, Sindre H, Grimholt U, Gjøen T (2011) Enhanced transfection of cell lines from Atlantic salmon through nucoleofection and antibiotic selection. BMC Res Notes 4:136–136
Schnell S, Bols NC, Barata C, Porte C (2009) Single and combined toxicity of pharmaceuticals and personal care products (PPCPs) on the rainbow trout liver cell line RTL-W1. Aquat Toxicol 93:244–252
Singh SP, Kumar R, Kumari P, Mitra A (2013) An alternate protocol for establishment of primary caprine fetal myoblast cell culture: an in vitro model for muscle growth study. In Vitro Cell Dev Biol-Anim 49:598–598
Song LC, Guo CR, Zhen XS, Cheng YS (2004) Establishment of a continuous embryonic cell line from Japanese flounder Paralichthys olivaceus for virus isolation. Dis Aquat Org 60:241–246
Tafalla C, Novoa B (2001) Respiratory burst of turbot (Scophthalmus maximus) macrophages in response to experimental infection with viral haemorrhagic septicaemia virus (VHSV). Fish Shellfish Immunol 11:727–734
Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH (2005) Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg-Am Vol 30:441–447
Tocher DR, Carr J, Sargent JR (1989) Polyunsaturated fatty acid metabolism in fish cells: differential metabolism of (n-3) and (n-6) series acids by cultured cells originating from a freshwater teleost fish and from a marine teleost fish. Comp Biochem Physiol B Comp Biochem 94:367–374
Tong SL, Li H, Miao HZ (1997) The establishment and partial characterization of a continuous fish cell line FG-9307 from the gill of flounder Paralichthys olivaceus. Aquaculture 156:327–333
Trevino JG, George SA, Hughes SJ, Chellappan SP (2008) An in vivo and in vitro assessment of TOR signaling cascade in rainbow trout (Oncorhynchus mykiss). Am J Phys 295:329–335
Wang L, Zhou H, He R, Wei X, Mai K, He G (2016a) Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). Aquaculture 464:87–94
Wang N, Wang XL, Sha ZX, Tian YS, Chen SL (2010a) Development and characterization of a new marine fish cell line from turbot (Scophthalmus maximus). Fish Physiol Biochem 36:1227–1234
Wang Q, He G, Mai K, Xu W, Zhou H, Wang X, Mei L (2016b) Chronic rapamycin treatment on the nutrient utilization and metabolism of juvenile turbot (Psetta maxima). Sci Rep 6:28068
Wang XL, Wang N, Sha ZX, Chen SL (2010b) Establishment, characterization of a new cell line from heart of half smooth tongue sole (Cynoglossus semilaevis). Fish Physiol Biochem 36:1181–1189
Wood CA, Padmore L, Radda GK (1993) The effect of phosphatidic acid on the proliferation of Swiss 3T3 cells. J Cell Biochem 21:369S
Xu D, He G, Mai K, Zhou H, Song F (2016) Postprandial nutrient-sensing and metabolic responses after partial dietary fishmeal replacement by soyabean meal in turbot (Scophthalmus maximus L). Br J Nutr 115:379–388
Yamamoto T, Shima T, Furuita H, Suzuki N, Sanchezvazquez FJ, Tabata M (2015) Self-selection and feed consumption of diets with a complete amino acid composition and a composition deficient in either methionine or lysine by rainbow trout. Oncorhynchus mykiss (Walbaum) Aquac Res 32:83–91
Yang CG, Liu SS, Sun B, Wang XL, Wang N, Chen SL (2013) Iron-metabolic function and potential antibacterial role of Hepcidin and its correlated genes (ferroportin 1 and transferrin receptor) in turbot (Scophthalmus maximus). Fish Shellfish Immunol 34:744–755
Acknowledgments
This study was supported by the National Key R&D Program of China (2018YFD0900400), National Natural Scientific Foundation of China grant (31702355 and 31772860), Aoshan Talents Cultivation Program supported by Qingdao National Laboratory for Marine Science and Technology (2017ASTCP-OS12), Fundamental Research Funds for the Central Universities (201822017) to GH, and China Agriculture Research System (CARS-47-G10) to KM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, Y., Zhou, H., Gao, Z. et al. Establishment and characterization of a fibroblast-like cell line from the muscle of turbot (Scophthalmus maximus L.). Fish Physiol Biochem 45, 1129–1139 (2019). https://doi.org/10.1007/s10695-019-00628-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00628-3