Abstract
In this study, CA I and II isoenzymes were purified from Van Lake fish gills by using Sepharose-4B-L-tyrosine-sulfanilamide affinity chromatography and to determine the effects of some metals on the enzyme activities. For purified CA I isoenzyme, yield, specific activity, and purification fold were obtained as 42.07%, 4948.12 EU/mg protein, and 116.61 and for CA II isoenzyme, 7%, 1798.56 EU/mg protein, and 42.38 respectively. Activity of CA was determined by measuring “CO2-hydratase activity”. Purity control was checked by SDS-PAGE. In vitro inhibitory effect of Cu2+, Ag+, Cd2+, Ni2+ metal ions, and arsenic (V) oxide were also examined for both isozymes activities. Whereas Cu2+, Ag+, Cd2+, and Ni2+ ions showed inhibitory effects on both isozymes, arsenic (V) oxide showed activation effect. IC50 values were calculated by drawing activity %-[I] graphs for metal ions exhibiting inhibitory effects. IC50 values were determined as 3.39, 6.38, 13.52, and 206 μM for CA I isozyme and 6.16, 20.29, 46, and 223 μM for CA II isozyme respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lake Van fish (Chalcalburnus tarichi, Pearl mullet) is a member of the Cypriniformes family, which is an endemic species living in the Lake Van. The Lake Van is one of the saltiest lakes known in the world. Due to these extreme conditions of the lake (salinity 22% and pH 9.8), only a vertebrate species and a few invertebrate species live in the lake. The only living vertebrate species in the lake is the endemic Lake Van fish (Kuzu et al. 2016).
Carbonic anhydrase (EC 4.2.1.1 CA) is ubiquitous metalloenzyme family that catalyzes the reversible hydration/dehydration of carbon dioxide to HCO3− and H+, being involved in many physiological processes. CA isozymes participate in important biological processes such as acid-base regulation, respiration, carbon dioxide and ion transport, gluconeogenesis, lipogenesis, ureogenesis, electrolyte secretion, bone resorption, and tumorigenesis in different tissues by simplifying interconversion of carbon dioxide to HCO3− (Şentürk et al. 2011). Up to now, 16 different CA isoforms have been determined with their biochemical properties, subcellular location, and sequence. Additionally, novel isoforms have recently been determined in non-mammalian vertebrates (Esbaugh and Tufts 2006). Carbonic anhydrase is found abundantly in fish gills, a complex organ that plays a role in acid-base balance homeostasis, respiration, gas exchange, and ion transport (Ceyhun et al. 2010).
Metals are components found in trace quantities in the aquatic environment. However, development in industry, agriculture, and mining has led to an increase in their levels (Kalay and Canlı 2000). This increase has become one of the most important problems of environmental toxicology. This has become very dangerous for living organisms, particularly for living organisms in aquatic environments containing specific enzymes. It is known that enzymes catalyze almost all of the reactions that occur in living systems. Contaminants such as metal ions take effect by increasing or decreasing enzyme activity at very low concentrations. This can be accomplished by binding to nonmetals containing an unbound electron pair such as N, O, and S present in the structure of the active enzymes (Ekinci et al. 2007; Çomaklı et al. 2013) Some heavy metals such as nickel, silver, and cadmium, which fish are exposed to, accumulate in their tissues. The human, at the top of the food chain, is at the greatest risk of exposure to health problems as a result of consuming these living things (Kaya et al. 2015).
Biochemical and physiological characteristics of aquatic organisms exposed to Cd2+ may vary. This ion causes changes in the biochemical activity of the enzyme by binding to functional structures such as sulfhydryl and carboxyl group in the structure of the enzymes, (Wang et al. 2009). Cu2+ has an important function in living organisms, such as the presence of many proteins. However, the excessive concentration of this metal ion may be toxic to organisms living in the aquatic environment (Manyin and Rowe 2009).
Ni2+ is one of the most abundant pollutants in nature (Wo-Niak and Basiak 2003). The excessive concentration of this metal ion is toxic to many living species. Researchers have reported little information about the toxic effect of a pollutant with such toxic effects on aquatic systems, particularly on fish (Pandey and Sharma 2002; Pane et al. 2005).
In the study, it was aimed to purify the CA-I and CA-II isoenzymes electrophoretically homogeneously from the gill tissues of the Van Lake fish for the first time. In addition, toxic effects of heavy metals induced by CA enzyme inhibition were determined by examining the in vitro effects of some heavy metals on enzyme activities.
Material and methods
Purification of carbonic anhydrase isozymes from Van Lake fish (Chalcalburnus tarichi) gill by affinity chromatography
Gill samples were homogenized by liquid nitrogen, transferred to 25 mM Tris-HCl/0.1 M Na2SO4, 1 mM EDTA, and 1 mM DTT pH = 8.7and centrifuged at 4 °C, 13000 rpm for 60 min (Soyut and Beydemir 2008). Supernatant was used in further studies. The pH of homogenate was adjusted to 8.7 with solid Tris. The homogenate was applied to the prepared Sepharose 4B-L-tyrosine-sulfanylamide affinity column equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH 8.7). Van Lake fish gills CA-I and CA-II isozymes were eluted with 1 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M NaCH3COO/0.5 M NaClO4 (pH 5.6), respectively. All procedures were performed at 4 °C (Ekinci et al. 2007).
Measurement of CA activity
Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson (1948). CO2–Hydratase activity as an enzyme unit (EU) was calculated by using the equation (to − tc/tc) where t0 and tc are the times for pH change of the non-enzymatic and the enzymatic reactions, respectively.
Protein determination
The absorbance at 280 nm was used to monitor the protein in the column effluents. Quantitative protein determination was achieved by absorbance measurements at 595 nm according to Bradford, with bovine serum albumin as a standard (Bradford 1976).
SDS polyacrylamide gel electrophoresis
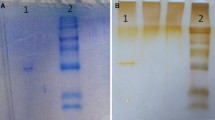
SDS polyacrylamide gel electrophoresis was performed after the purification of the enzyme. It was carried out in 10 and 3% acrylamide concentrations for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli (1970). Gels were stained for 1.5 h in 0.1% Coommassie Brillant Blue R- 250 in 50% methanol and 10% acetic acid, then destained with several changes of the same solvent without the dye. The electrophoretic pattern was photographed (see Fig. 1).
SDS polyacrylamide gel electrophoresis of Van Lake fish gills CA-I and CA-II purified by Sepharose 4B-tyrosine-sulfanilamide affinity gel. Line 1; Bio-Rad: The Precision Plus Protein™ Kaleidoscope™ standards (250, 150,100, 75, 50, 37, 25, and 20 kDa), Line 2; Van Lake fish gills CA-II, Line 3; Van Lake fish gills CA-I
In vitro inhibition assays
The effects of increasing concentrations of Cu2+, Ag+, Cd2+, Ni2+, and arsenic (V) oxide on Van Lake fish gills CA-I and CA-II isozymes activities were determined colorimetrically using CO2-hydratase assay. The metal ions were also tested in the hydratase activity assay in triplicate at each concentration used. Different concentrations of metal ions were examined in preliminary assays.
Result
The current study concerns the purification of CA I and II isoenzymes and investigation of the inhibitory influences of some metals, including Cu2+, Ag+, Cd2+, Ni2+, and arsenic (V) oxide on the enzymatic activity. In this study, CA I and II isoenzymes were purified from Van Lake fish gills by using Sepharose-4B-L-tyrosine-sulfanilamide affinity chromatography. Obtaining the single band by SDS-PAGE showed that the enzyme was obtained pure (Fig. 1). An Rf-MW chart was drawn to determine the molecular mass of the isoenzymes. According to the results obtained, the molecular mass of CA I was calculated as 28.4 kDa and the molar mass of CA II as 27.8 kDa. Carbonic anhydrase I isoenzyme (CA-I) was purified 42 times with 4948.12 EU/mg protein specific activity and carbonic anhydrase II isoenzyme (CA-II) was purified 7 times with 1798.56 EU/mg protein specific activity (Table 1).
In this study, we measured the in vitro inhibition effects of Cu2+, Ag+, Cd2+, Ni2+ metal ions, and arsenic (V) oxide on Van Lake fish gills of CA I and II. Enzyme activities were measured in the presence of different concentrations of Cu2+, Ag+, Cd2+, Ni2+, and arsenic (V) oxide. Control enzyme activity in the absence of a metal ion was taken as 100%. For each metal ion, an activity % vs. inhibitor concentration tube was drawn (Fig. 2). Metal ion concentrations that produced 50% inhibition (IC50) were calculated from graphs (Table 2). The half maximal inhibitory concentration (IC50) is a measurement of the effectiveness of an inhibitor in inhibiting a specific biochemical function. According to the results obtained, CA I and CA II isoenzymes were inhibited with IC50 values by Cu2+ 3.39–6.16 μM, Ag+ 6.38–20.3 μM, Cd2+ 13.5–46 μM, and Ni2+ 206–223 μM, respectively.
Discussion
Heavy metal pollution has become a major environmental problem in the world due to the continuity and permanency of heavy metals in the environment. This pollution creates various toxic effects in living organisms. In particular, scientists have reported that it causes the toxic effects such as the inhibition effect of metal ions on various enzymes in protein structure, which play an important role in the execution of metabolic activities in living organisms (Çomaklı et al. 2013) In studies investigating the acute toxic effect of copper, lead, and iron ions, O. mossambicus fry and fingerlings have been reported to exhibit toxic effects depending on the species in all the analytical methods applied (Mashifane and Moyo 2014). It has also been stated that metals have negative effects on metabolism and embryonic development and genotoxic effect in fish (Jezierska et al. 2009; Witeska et al. 2014; Teta et al. 2017). In recent years, industrial development, rapid population growth, agricultural activities, and mining have also caused the aquatic environment to be exposed to this pollution (Kaya et al. 2015). As a result, in fish that are at the head of the aquatic food chain, heavy metals accumulate in body tissues and reach high levels and become toxic (Sorsa et al. 2016).
Carbon dioxide, produced in fish tissues, is hydrated rapidly by carbonic anhydrase enzyme, converted into bicarbonate, and transported in the blood. CA has previously been purified and characterized from many living organisms including animals (Kaya et al. 2015). To the best of our knowledge, although the toxic effects of the metal ions have been described, in particular with hydratase activity, the effects of metals on Van Lake fish gills CA-I and CA-II enzymes have not been studied, yet.
In this study, the CA I and CA II isoenzymes were purified for the first time in electrophoretic purity from the gill tissues of Van Lake fish. The purity of the isoenzymes and monomeric molecular masses were determined by SDS-PAGE. Accordingly, the molecular mass of CA I was calculated to be 28.4 kDa and CA II as 27.8 kDa. CA I isoenzyme has been described in the gills, heart, and intestine tissues of rainbow trout and the molecular mass is reported to be 29 kDa by Kho and colleagues. It is also reported that CA I concentration is higher than other studied tissues in the gills and heart (Kho et al. 2015). CA II isoenzyme is described in the gills of the spotted green pufferfish by immunoblotting. It was found that this isoenzyme was present in both membrane and cytosol, and the molecular mass was reported as 30 kDa (Tang and Lee 2007). In a study by Ceyhun et al., CA enzyme was purified from the gill tissues of European seabass and reported molecular mass as 30 kDa (Ceyhun et al. 2011). In other studies, it was determined as 29.9 kDa (Demirdag et al. 2015) for Ağrı Balık Lake Trout Gill CA (Demirdag et al. 2015) and as 27.5 kDa for Rainbow Trout Lens CA (Beydemir et al. 2006). The values found for the gill CA isoenzyme of Van Lake are consistent with this data. Also, CA-I was purified with 4948.12 EU/mg protein specific activity 42 times and CA-II was purified with 1798.56 EU/mg protein specific activity 7 times. Our results show that the enzyme has been purified with a high specific activity when compared to the reported results for the enzyme obtained from different fish species and tissues in the literature (Hisar et al. 2006; Kaya et al. 2013; Kucuk and Gulcin 2016).
Over the past years, undesired impacts of metal ions on various enzymes have been reported increasingly. It has been reported that Ag+, Cu2+, and Ni2+ ions inhibited the cytosolic thioredoxin reductase enzyme purified from the Van Lake fish gills at micromolar levels. (Akyol and Kuzu 2017). In another study, researchers previously reported the inhibitory effects of heavy metal ions as lead, cobalt, and mercury on the activity of cytosolic human carbonic anhydrase isoenzymes I and II (Ekinci et al. 2007) and fish CAs (Hisar et al. 2006). Kucuk and Gulcin (2016) investigated the in vitro effects of some heavy metals (Fe2+, Pb2+, Co2+, Ag+, and Cu2+) on the purified Black sea trout kidney CA and reported that metals inhibited the enzyme at millimolar levels. In another study, the effects of Al3+, Cu2+, Pb2+, Co3+, Ag+, Zn2+, and Hg2+ metal ions on enzyme activity were investigated after the enzyme was purified and characterized from the liver of the teleost fish. In particular, Al3+ and Cu2+ were expressed as potent inhibitors of the enzyme with IC50 concentrations of 0.0692 and 0.0715 mM, respectively (Ceyhun et al. 2011). However, no study has been found in the literature on the effect of arsenic (V) oxide on CA I and CA II isoenzymes. Besides, it has been reported that arsenic (V) oxide inhibits glucose 6-phosphate dehydrogenase enzyme purified from rainbow trout liver (Comakli et al. 2015) and activates NADPH-cytochrome P450 reductase purified from Van Lake fish liver microsomes (Kuzu and Ciftci 2015).
Providing acid-base balance in fish depends on metabolic events in which acid-base equivalents are transferred between the animal and the external environment. This happens largely in gills. It has been emphasized that gill CA isoforms may differ according to fish species and therefore the role of CA in the branchial acid-base balance should be considered according to the groups (Gilmour and Perry 2009). For this reason, it is very important to study different fish species to determine the effects of heavy metals on the CA enzyme. As shown in Fig. 2, except for arsenic (V) oxide, other metal ions seem to inhibit both isoenzymes. In particular that Cu2+ and Ag+ inhibit the enzymes at very low micromolar levels suggests that the contamination of these metal ions may be toxic to living organisms, which is presented with the Van Lake fish sample.
Consequently, we purified carbonic anhydrase I and II from Van Lake fish (Chalcalburnus tarichi, Pearl mullet) gills for the first time and analyzed some characteristic features as specific activity, yield %, and purification coefficient. The specific activity values that we have obtained are higher than those reported in the literature. In addition, inhibitory effects of some heavy metals on CA-I and CA-II enzyme activity were investigated. Our findings and the relevant literature show that organisms have different sensitivities to metal ions. These metal ions, which cause inhibition at the micromolecular level, can cause toxic effects in fish via inhibition of CA-I and CA-II isoenzymes.
References
Akyol H, Kuzu M (2017) In vitro effects of some heavy metal ions on cytosolic thioredoxin reductase purified from rainbow trout gill tissues. Fresenius Environ Bull 26(7):4677–4683
Beydemir Ş, Bülbül M, Hisar O, Söyüt H, Yanık T (2006) Carbonic anhydrase: affinity purification and kinetic properties from rainbow trout lens. IJAC 2(1):45–55
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram. Anal Biochem 72(1–2):248–251. https://doi.org/10.1016/0003-2697(76)90527-3
Ceyhun SB, Şentürk M, Erdoğan O, Küfrevioğlu Öİ (2010) In vitro and in vivo effects of some pesticides on carbonic anhydrase enzyme from rainbow trout (Oncorhynchus mykiss) gills. Pestic Biochem Physiol 97(3):177–181. https://doi.org/10.1016/j.pestbp.2010.01.003
Ceyhun SB, Sentürk M, Yerlikaya E, Erdogan O, Küfrevioglu OI, Ekinci D (2011) Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 32(1):69–74. https://doi.org/10.1016/j.etap.2011.03.013
Comakli V, Ciftci M, Kufrevioglu OI (2013) Effects of some metal ions on rainbow trout erythrocytes glutathione S-transferase enzyme: an in vitro study. J Enzyme Inhib Med Chem. 28(6):1261–1266. https://doi.org/10.3109/14756366.2012.729829
Comakli V, Akkemik E, Ciftci M, Küfrevioğlu Öİ (2015) Purification and characterization of glucose 6-phosphate dehydrogenase enzyme from rainbow trout (Oncorhynchus mykiss) liver and investigation of the effects of some metals on enzyme activity. Toxicol Ind Health 1(5):403–411. https://doi.org/10.1177/0748233713475514
Çomaklı V, Kuzu M, Demirdağ R (2013) Characterization and purification of glutathione S-transferase from the liver and gill tissues of Ağrı Balık Lake trout Salmo trutta labrax and the effects of heavy metal ions on its activity. J Aquat Anim Health 27(3):145–151. https://doi.org/10.1080/08997659.2015.1032441
Demirdag R, Comakli V, Kuzu M, Yerlikaya E, Şentürk M (2015) Purification and characterization of carbonic anhydrase from Ağrı Balık Lake Trout Gill (Salmo trutta labrax) and effects of sulfonamides on enzyme activity. J Biochem Mol Toxicol 29(3):123–128. https://doi.org/10.1002/jbt.21675
Ekinci D, Beydemir S, Kufrevioglu OI (2007) In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 22(6):745–750. https://doi.org/10.1080/14756360601176048
Esbaugh AJ, Tufts BL (2006) The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir Physiol Neurobiol 154(1–2):185–198. https://doi.org/10.1016/j.resp.2006.03.007
Gilmour KM, Perry SF (2009) Carbonic anhydrase and acid–base regulation in fish. J Exp Biol 212:1647–1661. https://doi.org/10.1242/jeb.029181
Hisar O, Beydemir S, Bulbul M, Yanik T (2006) Kinetic properties of carbonic anhydrase purified from gills of rainbow trout (Oncorhynchus mykiss). J Appl Anim Res 30(2):185–188. https://doi.org/10.1080/09712119.2006.9706615
Jezierska B, Ługowska K, Witeska M (2009) The effects of heavy metals on embryonic development of fish (a review). Fish Physiol Biochem 35(4):625–640. https://doi.org/10.1007/s10695-008-9284-4
Kalay M, Canlı M (2000) Elimination of essential (Cu, Zn) and nonessential (Cd, Pb) metals from tissues of a freshwater fish Tilapia zillii following an uptake protocol. Turk J Zool 24:429–436
Kaya ED, Söyüt H, Beydemir Ş (2013) Carbonic anhydrase activity from the gilthead sea bream (Sparus aurata) liver: the toxicological effects of heavy metals. Environ Toxicol Pharmacol 36(2):514–521. https://doi.org/10.1016/j.etap.2013.05.019
Kaya ED, Söyüt H, Beydemir Ş (2015) The toxicological impacts of some heavy metals oncarbonic anhydrase from gilthead sea bream (Sparus aurata) gills. Environ Toxicol Pharmacol 39(2):825–832. https://doi.org/10.1016/j.etap.2015.01.021
Kho KH, Kim JW, Kim SC, Choi MR, Han KH, Lee WK, Choi KS (2015) Identification and intracellular localization of carbonic anhydrase I in gills, heart, muscle, and intestine of rainbow trout, Oncorhynchus mykiss. J Korean Soc Appl Biol Chem 58(5):729–733. https://doi.org/10.1007/s13765-015-0098-7
Kucuk M, Gulcin I (2016) Purification and characterization of the carbonic anhydrase enzyme from Black Sea trout (Salmo trutta Labrax Coruhensis) kidney and inhibition effects of some metal ions on enzyme activity. Environ Toxicol Pharmacol 44:134–139. https://doi.org/10.1016/j.etap.2016.04.011
Kuzu M, Ciftci M (2015) Purification and characterization of NADPH-cytochrome P450 reductase from Lake Van fish liver microsomes and investigation of some chemical and metals’ effects on the enzyme activity. Turk J Chem 39(1):149–158. https://doi.org/10.3906/kim-1404-76
Kuzu M, Aslan A, Ahmed I, Comakli V, Demirdag R, Uzun N (2016) Purification of glucose-6-phosphate dehydrogenase and glutathione reductase enzymes from the gill tissue of Lake Van fish and analyzing the effects of some chalcone derivatives on enzyme activities. Fish Physiol Biochem 42(2):483–491. https://doi.org/10.1007/s1069
Laemmli DK (1970) Cleavage of structural proteins during the assembly of the head. Nature 227:680–685
Manyin T, Rowe CL (2009) Bioenergetic effects of aqueous copper and cadmium the grass shrimp, Palaemonetes pugio. Comp Biochem Physiol, B 150(1):65–71. https://doi.org/10.1016/j.cbpc.2009.02.007
Mashifane TB, Moyo NAG (2014) Acute toxicity of selected heavy metals to Oreochromis mossambicus fry and fingerlings. Afr J Aquat Sci 39(3):279–285. https://doi.org/10.2989/16085914.2014.960358
Pandey N, Sharma CP (2002) Effect of heavy metals Co2+ Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci 163(4):753–758. https://doi.org/10.1016/S0168-9452(02)00210-8
Pane EF, Bucking C, Patel M, Wood CM (2005) Renal function in the freshwater rainbow trout (Oncorhynchus mykiss) following acute and prolonged exposure to waterborne nickel. Aquat Toxicol 72(1–2):119–133. https://doi.org/10.1016/j.aquatox.2004.11.020
Şentürk M, Gülçin İ, Beydemir Ş, Küfrevioğlu Öİ, Supuran CT (2011) In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 77(6):494–499. https://doi.org/10.1111/j.1747-0285.2011.01104.x
Sorsa S, Gezahagn A, Dadebo E (2016) Bioaccumulation of heavy metals in two morphotypes of African large barb Labeobarbus intermedius (Osteichthyes: Cyprinidae) in Lake Hawassa, Ethiopia. Afr J Aquat Sci 41(4):427–434. https://doi.org/10.2989/16085914.2016.1218821
Soyut H, Beydemir S (2008) Purification and some kinetic properties of carbonic anhydrase from rainbow trout (Oncorhynchus mykiss) liver and metal inhibition. Protein Pept Lett 15(5):528–535. https://doi.org/10.2174/092986608784567627
Tang CH, Lee TH (2007) The novel correlation of carbonic anhydrase II and anion exchanger 1 in gills of the spotted green pufferfish, Tetraodon nigrovirids. J Exp Zool 307A:411–418. https://doi.org/10.1002/jez.391
Teta C, Ncube M, Naik SY (2017) Heavy metal contamination of water and fish in peri-urban dams around Bulawayo, Zimbabwe. Afr J Aquat Sci 42(4):351–358. https://doi.org/10.2989/16085914.2017.1392925
Wang Q, Liu BZ, Yang HS, Wang XY, Lin ZH (2009) Toxicity of lead, cadmium and mercury on embryogenesis, survival, growth and metamorphosis of Meretrix meretrix larvae. Ecotoxicology 18(7):829–837. https://doi.org/10.1007/s10646-009-0326-1
Wilbur KM, Anderson NG (1948) Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 176:147–154
Witeska M, Sarnowski P, Ługowska K, Kowal E (2014) The effects of cadmium and copper on embryonic and larval development of ide Leuciscus idus L. Fish Physiol Biochem 40(1):151–163. https://doi.org/10.1007/s10695-013-9832-4
Wo-Niak K, Basiak J (2003) Free radicals-mediated induction of oxidized DNA bases and DNA protein cross-links by nickel chloride. Mutat Res Genet Toxicol Environ Mutagen 514(1–2):233–243. https://doi.org/10.1016/S1383-5718(01)00344-8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuzu, M., Çomaklı, V., Akkemik, E. et al. Inhibitory properties of some heavy metals on carbonic anhydrase I and II isozymes activities purified from Van Lake fish (Chalcalburnus Tarichi) gill. Fish Physiol Biochem 44, 1119–1125 (2018). https://doi.org/10.1007/s10695-018-0499-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0499-8