Abstract
The objective of this study was to evaluate the effects of the supplementation of vitamin D3 on the growth, vitamin D metabolites, and osteocalcin secretion in juvenile Siberian sturgeon (Acipenser baerii). A 90-day growth trial was conducted with juvenile Siberian sturgeon (initial body weight 3.47 ± 0.14 g) fed seven isonitrogenous and isoenergetic practical diets (45% CP and 13% lipid) containing 60 (basal diet), 240, 450, 880, 1670, 3300, or 1.0 × 105 IU/kg feed (D60~D 1.0 × 105) vitamin D3. The results showed that weight gain and specific growth rate increased as the dietary vitamin D3 levels increased from 450 to 3300 IU/kg (P < 0.05). The fish fed with D1670 and D3300 diets had higher crude lipid and ash levels than the fish fed the D60 diet (P < 0.05). The fish fed D880, D1670, or D3300 diets had higher 25-OH-D3 and 1,25-(OH)2-D3 levels than the fish fed the D60 diet (P < 0.05). The fish fed D880, D1670, D3300, or D1.0 × 105 diets had higher osteocalcin levels than the fish fed the D60 diet (P < 0.05). Based on the broken line method analysis of weight gain and osteocalcin, the dietary vitamin D3 requirement of juvenile Siberian sturgeon was estimated to be 1683.30 and 1403.27 IU/kg per diet, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin D, a fat-soluble vitamin, is crucial for the preservation of calcium and phosphorus homeostasis and to protect skeletal integrity (Darias et al. 2011). The two major natural sources of vitamin D are ergocalciferol (vitamin D2, which occurs predominantly in plants) and cholecalciferol (vitamin D3, which occurs in animals). Cholecalciferol is at least three times more effective than ergocalciferol in meeting the vitamin D requirement of rainbow trout (Barnett et al. 1982). Formulated diets are used in aquaculture, and vitamin D intake can be manipulated. Vitamin D studies of different types have been performed in juveniles of several fish species, and the dietary requirements of this vitamin have been reviewed by Lock et al. (2010).
Most fish species contain a large amount of vitamin D3 in their livers (Takeuchi et al. 1984). After hydroxylation in the liver into 25-OH-D and kidney into 1,25-(OH)2-D3, the active metabolite can enter the cell and bind to the vitamin D-receptor and subsequently to a responsive gene such as that of calcium binding protein or osteocalcin. Osteocalcin regulates the incorporation of calcium phosphates within the bone thus playing a mineralization and remodeling role (Fraser and Price 1988). Teleosts inhabiting freshwater and seawater are able to convert vitamin D3 to 25-OH-D3, 1,25-(OH)2-D3, and 24,25-(OH)2-D3, which are found in the plasma of several species (Rao and Raghuramulu 1995; Horvli et al. 1998; Darias et al. 2011).
Sturgeon is the common name used for some 25 species of fish in the family Acipenseridae, including the genera Acipenser, Huso, Scaphirhynchus, and Pseudoscaphirhynchus. The Siberian sturgeon (Acipenser baerii) is a species of sturgeon in the Acipenseridae family. While wild catches have been generally declining, the Siberian sturgeon is increasingly farmed for both the meat and to produce caviar from its roe; the largest meat producers are Russia and China. Some studies have been performed on the nutrient requirement of Siberian sturgeon such as protein (Hung 1991; Médale et al. 1995), amino acids (Kaushik et al. 1991), ascorbic acid (Moreau et al. 1996), phosphorus (Xu et al. 2011), and vitamin K3 (Wang et al. 2015). To our knowledge, no information has been published concerning the vitamin D requirement of Siberian sturgeon. Some studies showed that vitamin D deficiency decreases growth, body Ca and P levels and bioavailability for lipid, and delay on skeletogenesis in fish (Lovell and Li 1978; Barnett et al. 1982; Darias et al. 2010). Therefore, the purpose of the present study was to determine the effects of the supplementation of vitamin D3 on the growth, vitamin D metabolites, and osteocalcin secretion in juvenile Siberian sturgeon and to determine the dietary vitamin D requirement.
Materials and methods
Experimental design and diets
Fishmeal-soybean-based meals supplemented with seven levels of vitamin D3 (233,000 IU/g, Yuxin Chemical Products Ltd., Dalian, China) were used at levels of 60 (basal diet), 240, 450, 880, 1670, 3300 or 1.0 × 105 IU/kg feed (D60~D 1.0 × 105) vitamin D3. All of the ingredients were mixed homogeneously, pelleted into a 2.0-mm-diameter size, and stored at −20 °C. The formula and analyzed proximate composition of the basal diet are shown in Table 1. The dietary vitamin D3 content was measured using high-performance liquid chromatography (HPLC) (Agilent 1100, California, USA) (GB/T 17818–2010). The separation was performed on an Agilent C18 column (150 mm × 4.6 mm, 5 μm) using methanol–water (95:5 v:v −1) for the mobile phase. The flow rate was 1.0 mL/min, and the column temperature was 25 °C.
Fish and feeding
Juvenile Siberian sturgeon from the Fangshan Station of the Chinese Academy of Fishery Sciences was transferred to 21 tanks (220 L of water, 30 fish/tank, and three replicates), which were connected to an automatically controlled recirculation system. The experimental fish from the broodstock originated from cultured Siberian sturgeons from Russia and reared in flowing-water ponds with 25 m × 4 m, water depth of 1 m. Every three aquaria were connected to a separate recycling system and supplied with aerated water, which was filtered through zeolum, corallite, and activated carbon. The water temperature and dissolved oxygen during this period averaged 21.4 ± 1.0 °C and 8.0 ± 0.5 mg/L, respectively. The pH values were 7.6 ± 0.1, the ammonia-N concentrations were 0.3 ± 0.05 mg/L, and the N-NO2 concentrations were 0.1 ± 0.02 mg/L. Approximately one third of the water in each aquarium was replaced daily with aerated freshwater. The fish were fed with a basal diet three times daily for 2 weeks. With an average initial weight of 3.47 ± 0.14 g, all of the groups of fish were fed their respective diets at the same fixed rate to apparent satiety for 90 days. The fish were weighed every fortnight and feed quantities were adjusted according to fish body weight. The fish were fed three times daily (09:00, 13:00, and 17:00). Any uneaten food was collected 1 h after feeding, and the dry matter content was determined for both supplied and uneaten food (AOAC 1995).
Sample collection
At the end of the feeding trial, all of the fish from each aquarium were collectively weighed to obtain a final biomass. The growth and feed conversion were determined according to Li et al. (2015). A total of 18 fish from each treatment (six fish per tank) were anesthetized with tricaine methane sulfonate (MS-222,200 mg/L, Western Chemical Inc., Ferndale, WA).
Blood was collected from three fish from each tank at the caudal vasculature location using a 1-mL heparinized syringe. The plasma was separated by centrifugation (3500×g for 30 min at 4 °C). The other three fish per tank were killed to collect the liver and the bone for biochemical measurement. All of the samples were stored at −40 °C until analysis.
-
Weight gain (WG, %) = 100 × (final total weight − initial total weight)/initial total weight
-
Feed conversion ratio (FCR, %) = 100 × dry feed intake/wet weight gain
-
Specific growth rate (SGR, %) = 100 × (ln(final body weight) − ln(initial body weight)/days).
Carcass and bone composition
The carcass samples (three per tank) were collected and assayed for dry matter (at 105 °C for 24 h), crude protein (Kjeldahl apparatus, nitrogen × 6.25), crude fat (extraction with petroleum ether by a Soxhlet apparatus), and ash (incineration at 550 °C for 6 h) (AOAC 1995). The bones were defatted by ether influxing overnight and were then measured and ashed at 550 °C for 8 h; the bone ash weight was determined and then dissolved in 6 N hydrochloric acid and diluted 100-fold. The vanadium molybdenum yellow colorimetric method was used to determine the bone phosphorus content. The bone calcium content was determined by the ethylene diamine tetraacetic acid (EDTA) method.
Biochemical measurements
The 25-OH-D3 and 1,25-(OH)2-D3 concentrations in the serum and liver samples were measured simultaneously using a Nichols Advantage chemiluminescent assay developed by Nichols Institute Diagnostics (San Clemente, CA, USA) (Roth et al. 2001). The total osteocalcin was analyzed by commercially available ELISA (enzyme-linked immunosorbent assay) kits (Biorbyt Ltd., UK) for fish osteocalcin using the following steps: 50 μl of diluted (1:1) fish serum sample was added to a 96-well microtitration polystyrene plate, and then 50 μl of biotinylated anti-OC was added to all of the wells. The plate was covered and incubated for 1 h at 37 °C. The plate was washed with 0.3 mL of washing solution in each well three times. Next, 60 μl of streptavidin-HRP solution was distributed to all of the wells, and the plate was covered and incubated for 30 min at 37 °C. The well strips were washed according to the step mentioned above, and 50 μl of substrate was immediately added and incubated for 10 min at 37 °C. The enzyme-substrate reaction was stopped by quickly pipetting 50 μl of H2SO4, and the absorbance was immediately read on a spectrophotometer using 450 nm as the primary wavelength.
Statistical analysis
The data are presented as treatment means ± SE. The data were analyzed by one-way ANOVA, and the least significance difference (LSD) was used for comparison of the means using SPSS 19.0 (SPSS Inc., Chicago IL) software. Multiple regression analyses (step-wise method) were used to determine the vitamin D3 requirement of juvenile Siberian sturgeon based on weight gain, and P < 0.05 was considered significant.
Results
Growth and feed utilization
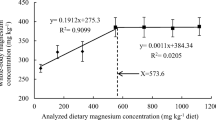
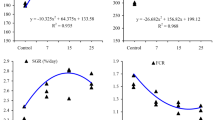
The results of the growth and feed utilization of the juvenile Siberian sturgeon fed with graded concentrations of dietary vitamin D3 are shown in Table 2. Dietary vitamin D3 levels significantly affected growth (P < 0.05). The fish fed with D60 to D450 diets had lower weight gain and specific growth rate than the fish fed the D1670 diet (P < 0.05), and the fish fed the D1670 diet presented the highest WG. The lowest weight gain rate was observed in fish fed the D60 and D240 diets (P < 0.05). There was no significant difference between the D1.0 × 105 and the D1670 diet (P > 0.05). The feed conversion ratio was not significantly affected among the different dietary groups (P > 0.05). As shown in Fig. 1, based on the broken line method analysis of weight gain, the dietary vitamin D3 requirement of juvenile Siberian sturgeon was estimated to be 1683.30 IU/kg diet.
Body composition
The whole body composition of juvenile Siberian sturgeon fed graded concentrations of dietary vitamin D3 for 90 days is shown in Table 3. There was a significant effect of diet on body crude lipid and ash content (P < 0.05). There was no effect of dietary vitamin D3 supplementation on body crude protein and moisture contents (P > 0.05).
Vitamin D metabolite concentrations in the liver and serum
The concentration of vitamin D3 metabolites in the serum and liver of Siberian sturgeon fed with graded concentrations of dietary vitamin D3 is shown in Table 4. There was a significant effect of diet on 25-OH-D3 and 1,25-(OH)2-D3 in the serum (P < 0.05). The fish fed the D880, D1670, or D3300 diets had higher 25-OH-D3 and 1,25-(OH)2-D3 than the fish fed the D60 diet (P < 0.05). The 1,25-(OH)2-D3 in the liver increased as the dietary vitamin D3 levels increased from 450 to 3300 IU/kg (P < 0.05). There was no effect of dietary vitamin D3 supplementation on the 25-OH-D3 concentration in the liver (P > 0.05). The fish fed with the D880, D1670, D3300, or D1.0 × 105 diets had higher osteocalcin levels than the fish fed the D60 diet (P < 0.05). As shown in Fig. 2, based on the broken line method analysis of osteocalcin, the dietary vitamin D3 requirement of juvenile Siberian sturgeon was estimated to be 1403.27 IU/kg per diet.
The calcium and phosphorus levels in the serum and bone
No significant differences were found among the different dietary groups regarding calcium and phosphorus levels in the serum or bones of the fish (P > 0.05). The calcium and phosphorus levels in the bones with average values 1.02 ± 0.02 or 0.95 ± 0.03% and 2.72 ± 0.21 or 5.71 ± 0.35 mmol/l in the serum, respectively.
Discussion
Vitamin D is crucial to preserve calcium and phosphorus homeostasis and to protect the skeletal integrity. Besides, vitamin D also acts directly on osteoblasts, the resident bone-forming cells of the skeleton, to inhibit proliferation, modulate differentiation, and regulate mineralization of the extracellular matrix (Darias et al. 2011). Vitamin D is essential for the normal growth of fish, although the metabolic roles of vitamin D and its analogs in fish are not well characterized (Vandenberg et al. 2012). In the present study, all of the dietary treatments were adequate to develop the juvenile sturgeon in suitable conditions, as confirmed by the high percentage of weight gain (>739%). A vitamin D3 level higher than 450 IU/kg is needed to maximize growth. The weight gain data obtained in this experiment showed that vitamin D3 is essential for the maximal growth of juvenile Siberian sturgeon. Similarly, Andrews et al. (1980) found that the average weight gain of channel catfish (6.0 g) in the control group was significantly lower than that of the groups fed supplemented with 2000 IU/kg vitamin D3. Lovell and Li (1978) found that a basal diet (0 IU/kg vitamin D3) resulted in significantly lower weight gain and body Ca and P of channel catfish (0.5 g) compared to those fed diets supplemented with 1000 IU/kg vitamin D3. Another study showed that inadequate levels of dietary vitamin D3 negatively influenced the intestinal tract maturation of European sea bass larvae (Darias et al. 2010). The excessive intake of vitamin D3 depressed the growth rate of channel catfish (6.0 g) (Andrews et al. 1980) and rainbow trout (51.6 g) (Vielma et al. 1998) fed diets supplemented with 50,000 or 250,000 IU/kg vitamin D3, respectively. High levels of vitamin D (3750 IU vitamin D3/g diet) caused impaired growth, lethargy, and dark coloration in brook trout, Salvelinus fontinalis (Poston 1969). Our results, together with previous reports, also demonstrated that no effect of excess vitamin D3 was detected in feed conversion, specific growth rate, and survival rate in our trial, and the results were the same as those of a vitamin D excess study on rainbow trout with a dose of up to 1.004 × 106 (Hilton and Ferguson 1982). Yellow catfish that were fed a 2000 IU/kg diet of vitamin D3 may have already met their growth requirements, and higher levels of vitamin D3 are not necessary to improve growth performance in juvenile yellow catfish (Zhu et al. 2015).
Different vitamin D studies have been performed in adults and juveniles of several fish species and dietary requirements of this vitamin D have been recently reviewed by Lock et al. (2010). The NRC (2011) recommends 1600 IU vitamin D3/kg diet for an optimal performance of juvenile fish based on analyses made on young rainbow trout, which is the actual value of the reference in the diet formulation for fish. The minimum dietary requirement for vitamin D has been established in Monopterus albus (Tan and He 2007), Salmo gairdneri (Barnett et al. 1982), Salmo salar (Woodward 1994), Ictalurus punctatus (Andrews et al. 1980), Oreochromis niloticus × Oreochromis aureus (Shiau and Hwang 1993), and Megalobrama amblycephala (Miao et al. 2015) as 5000, 1600, 2400, 2000, 375, and 5430 IU/kg per diet, respectively. The dietary vitamin D3 requirement of juvenile Siberian sturgeon was estimated to be 1683.30 or 1403.27 IU/kg per diet by weight gain or osteocalcin, respectively. It might be worth to note that vitamin D3 requirements at juvenile and larval stage are different; for instance, the optimal amount has been shown to be higher during the larval stage for European sea bass than that recommended by the NRC (1993) for juveniles.
A decrease in carcass ash was reported in channel catfish and Wuchang bream fed low levels of vitamin D3 (Lovell and Li 1978; Miao et al. 2015). Consistent with the abovementioned studies, the fish fed with 1670 and 3300 IU/kg vitamin D3 diets had higher crude lipid and ash levels than the fish fed the 60 IU/kg diet in the present study. This demonstrated that appropriate dietary vitamin D3 promoted the deposition of minerals in fish, and deficient or excessive dietary vitamin D3 inhibited mineral accumulation. However, there was little change in carcass ash between the vitamin D deficient and supplemented groups after 6 months of feeding for Labeo rohita (Rora) (Ashok et al. 1998).
Then, 25-OH-D3 is processed into the most active molecule, 1,25-dihydroxy vitamin D, after two hydroxylation steps. The 1,25-(OH)2-D3 compound is the most active metabolite of vitamin D3 and regulates the calcium and phosphorus balance in mammals (Fraser 1980; De Luca and Schnose 1983). The salmon responded to the two high dietary levels of vitamin D3 tested (88.4 and 1147.2 IU/g per diet) by accumulating the 1,25-(OH)2-D3 in a dose-dependent manner, and similar results were obtained in the investigated serum and liver. This dietary dose-dependent accumulation of 1,25-(OH)2-D3 was also found by Takeuchi et al. (1991) in the livers of the bastard halibut and carp, Cyprinus carpio. Similar to this study, the fish fed 880, 1670, or 3300 IU/kg vitamin D3 diets had higher 1,25-(OH)2-D3 levels than the fish fed the 60, 240, and 1.0 × 105 IU/kg vitamin D3 diets. One possible reason for the lower 1,25-(OH)2-D3 level is that 1.0 × 105 IU/kg vitamin D3 dietary inhibited the kidney microsomers that metabolize either vitamin D3 or 25-(OH)D3 to 1,25-(OH)2-D3 for juvenile Siberian sturgeon.
Vitamin D is also needed for bone growth and bone remodeling by osteoblasts and osteoclasts. Osteocalcin is one of the marker genes for the progression of osteoblastic differentiation and is associated with the mineralization of the extracellular matrix (Lian and Stein 1995). In human osteoblast cell culture, vitamin D, particularly 1,25-(OH)2-D3, stimulates the production of osteocalcin (Price and Baukol 1980). Compared with the control group, the serum osteocalcin significantly increased in the D880 group, and the variation trend was similar to the 1,25-(OH)2-D3 content in serum. Similarly, the supplementation of vitamin D3 promoted the expression of the osteocalcin gene of sea bass larvae (Darias et al. 2010).
Some reports have shown that vitamin D had no marked effect on the utilization of dietary calcium for tissue mineralization (O’Connell and Gatlin 1994; Graff et al. 2002). Similarly, Zhu et al. (2015) reported that supplementation with vitamin D3 did not significantly affect vertebrae and serum Ca or P contents. The present study also demonstrates the same results, i.e., that no significant differences were recorded in calcium and phosphorus both in the plasma and bone. This fact may indicate that calcium and phosphorus in the surrounding water in our trial were collected by the gills and satisfied the need of calcium and phosphorus to maintain homeostasis and support normal mineralization of bone for juvenile sturgeon. Furthermore, similar calcium and phosphorous levels in all dietary treatments are more alike due to the fact that juvenile sturgeon did not feed the vitamin D3-deficient diet.
In conclusion, the differences observed may indicate that supplementation of vitamin D3 could enhance weight gain, crude lipid, and ash in the carcass, the concentration of 25-OH-D3, 1,25-(OH)2-D3 and osteocalcin in serum, and the concentration of 1,25-(OH)2-D3 in the liver. Based on the broken line method analysis of weight gain and osteocalcin, the dietary vitamin D3 requirement of juvenile Siberian sturgeon was estimated to be between 1683.30 and 1403.27 IU/kg per diet.
References
Andrews JW, Murai T, Page JW (1980) Effects of dietary cholecalciferol and ergocalciferol on catfish. Aquaculture 19:49–54
Ashok A, Rao DS, Raghuramulu N (1998) Vitamin D is not an essential nutrient for rora (Labeo rohita) as a representative of freshwater fish. J Nutr Sci 44:195–205
Association of Official Analytical Chemists (AOAC) (ed) (1995) Official analytical chemists, official methods of analysis, 16th edn. AOAC, Arlington
Barnett BJ, Cho CY, Slinger SJ (1982) Relative biopotency of dietary ergocalciferol and cholecalciferol and the role of and requirement for vitamin D in rainbow trout (Salmo gairdneri). J Nutr 112:2011–2019
Darias MJ, Mazurais D, Koumoundouros G, Glynatsi N, Christodoulopoulou S, Huelvan C, Desbruyeres E, Le Gall MM, Quazuguel P, Cahu CL, Zambonino-Infante JL (2010) Dietary vitamin D3 affects digestive system ontogenesis and ossification in European sea bass (Dicentrachus labrax, Linnaeus, 1758). Aquaculture 298:300–307
Darias MJ, Mazurais D, Koumoundouros G, Cahu CL, Zambonino-Infante JL (2011) Overview of vitamin D and C requirements in fish and their influence on the skeletal system. Aquaculture 315:49–60
De Luca HF, Schnose HK (1983) Vitamin D: recent advances. Annu Rev Biochem 52:411–439
Fraser DR (1980) Regulation of the metabolism of vitamin D. Physiol Rev 60:551–613
Fraser JD, Price PA (1988) Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla-protein. Implications for the possible functions of matrix Gla-protein and for the possible distribution of the gamma carboxylase. J Biol Chem 263:11033–11036
Graff IE, Høie S, Totland GK, Lie Ø (2002) Three different levels of dietary vitamin D3 fed to first-feeding fry of Atlantic salmon (Salmo salar L.): effect on growth, mortality, calcium content and bone formation. Aquac Nutr 8:103–111
Hilton JW, Ferguson HW (1982) Effect of excess vitamin D3 on calcium metabolism in rainbow trout Salmo gairdneri Richardson. J Fish Biol 21:373–379
Horvli O, Lie A, Aksnes L (1998) Tissue distribution of vitamin D3 in Atlantic salmon Salmo salar: effect of dietary level. Aquac Nutr 4:127–131
Hung SSO (1991) Sturgeon, Acipenser spp. In: Wilson RP (ed) Handbook of nutrient requirements of finfish. CRC Press, Boca Raton, pp 153–160
Kaushik SJ, Breque J, Blanc D (1991) Requirement for protein and essential amino acids and their utilization by Siberian sturgeon (Acipenser baeri). In: Williot P (ed) Proceedings of the first international symposium on the sturgeon, CEMAGREF, France, pp 25–39
Li JN, Xu QY, Wang CA, Wang LS, Zhao ZG, Luo L (2015) Effects of dietary glucose and starch levels on the growth, haematological indices and hepatic hexokinase and glucokinase mRNA expression of juvenile mirror carp (Cyprinus carpio). Aquac Nutr. doi:10.1111/anu.12278
Lian JB, Stein GS (1995) Development of the osteoblast phenotype, molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J 15:118–140
Lock EJ, Waagbø R, Wendelaar Bonga S, Flik G (2010) The significance of vitamin D for fish: a review. Aquac Nutr 16:100–116
Lovell RT, Li Y (1978) Essentiality of vitamin D in diets of channel catfish (Ictalurus punctatus). Trans Am Fish Soc 107:809–811
Médale F, Corraze G, Kaushik SJ (1995) Nutrition of farmed Siberian sturgeon. In: Gershanovic AD, Smith TIJ (eds) Proceedings of the 3rd international symposium on sturgeons. VNIRO Publishing, Moscow, pp 289–298
Miao LH, Ge XP, Xie J, Liu B, Wang KB, Zhu J, Ren MC, Zhou QL, Pan LK, Chen RL (2015) Dietary vitamin D3 requirement of Wuchang bream (Megalobrama amblycephala). Aquaculture 436:104–109
Moreau R, Kaushik SJ, Dabrowski K (1996) Ascorbic acid status as affected by dietary treatment in the Siberian sturgeon (Acipenser baeri Brandt): tissue concentration, mobilization and L-gulonolactone oxidase activity. Fish Physiol Biochem 15:431–438
NRC National Research Council (1993) Nutrient requirement of fish and shrimp. National Academy Press, Washington
NRC National Research Council (2011) Nutrient requirement of fish and shrimp. National Academy Press, Washington
O’Connell JP, Gatlin DM (1994) Effects of dietary calcium and vitamin D3 on weight gain and mineral composition of the blue tilapia Oreochromisaureus in low-calcium water. Aquaculture 125:107–117
Poston HA (1969) Effects of massive doses of vitamin D3 on fingerling brook trout. Fish Res Bull 32:48–50
Price PA, Baukol SA (1980) 1, 25-dihydroxyvitamin D3 increases synthesis of the vitamin K-dependent bone protein by steosarcoma cells. J Biol Chem 255:316–360
Rao DS, Raghuramulu N (1995) Vitamin D and its related parameters in fresh-water wild fishes. Comp Biochem Physiol A 111:191–198
Roth HJ, Zahn I, Alkier R, Schmidt H (2001) Validation of the first automated chemiluminescence protein-binding assay for the detection of 25-hydroxycalciferol. Clin Lab 47:357–365
Shiau SY, Hwang JY (1993) Vitamin D requirements of juvenile hybrid tilapia Oreochromis niloticus × O. aureus. Nippon Suisan Gakk 59:553–558
Takeuchi A, Okano T, Ayame M, Yoshikawa H, Teraoka S, Murakami Y, Kobayashi T (1984) High-performance liquid chromatographic determination of vitamin D3 in fish liver oils and eel body oils. J Nutr Sci Vitaminolo 30:421–430
Takeuchi A, Okano T, Kobayashi T (1991) The existence of 25-hydroxyvitamin D3-1-hydroxylase in the liver of carp and bastard halibut. Life Sci 48:275–282
Tan Q, He R (2007) Effect of dietary supplementation of vitamins A, D3, E, and C on yearling rice field eel, Monopterus albus: serum indices, gonad development, and metabolism of calcium and phosphorus. J World Aquac Soc 38:146–153
Vandenberg GW, Scott SL, De La Noüe J (2012) Factors affecting nutrient digestibility in rainbow trout (Oncorhynchus mykiss) fed a plant protein-based diet supplemented with microbial phytase. Aquac Nutr 18:369–379
Vielma J, Lall SP, Koskela J, Schöner FJ, Mattila P (1998) Effects of dietary phytase and cholecalciferol on phosphorus bioavailability in rainbow trout (Oncorhynchus mykiss). Aquaculture 163:309–323
Wang LS, Xu H, Wang Y, Wang CA, Li JN, Zhao ZG, Luo L, Du X, Xu QY (2015) Effects of dietary vitamin K3 on growth and antioxidation in Siberian sturgeon Acipenser baerii. J Ocean U Dalian 30:641–646
Woodward B (1994) Dietary vitamin requirements of cultured young fish, with emphasis on quantitative estimates for salmonids. Aquaculture 124:133–168
Xu QY, Xu H, Wang C, Zheng Q, Sun D (2011) Studies on dietary phosphorus requirement of juvenile Siberian sturgeon. J Appl Ichthyol 27:709–714
Zhu Y, Ding Q, Chan J, Chen P, Wang CF (2015) The effects of concurrent supplementation of dietary phytase, citric acid and vitamin D3 on growth and mineral utilization in juvenile yellow catfish Pelteobagrus fulvidrac. Aquaculture 436:143–150
Acknowledgements
This research was supported by the Central-level Non-profit Science Research Institutes Special Funds (HSY201408), the Special Fund for Agro-scientific Research in the Public Interest (201003055), and the Natural Science Funds of Heilongjiang (QC2015041).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Xu, H., Wang, Y. et al. Effects of the supplementation of vitamin D3 on the growth and vitamin D metabolites in juvenile Siberian sturgeon (Acipenser baerii). Fish Physiol Biochem 43, 901–909 (2017). https://doi.org/10.1007/s10695-017-0344-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0344-5