Abstract
The aim of the present study was to assess survival rate, Zn accumulation, reactive oxygen species (ROS) levels, oxidative damage and antioxidant responses after Zn exposure (2 and 8 mg L−1 Zn) at different exposure times (6, 12, 24, 48 and 96 h) in the liver of large yellow croaker. Survival rate was reduced at 96 h, and hepatic Zn content increased during 24–96 by 8 mg L−1 Zn. In the 2 mg L−1 Zn group, no fish died and the increase in Zn content merely occurred at 96 h. Exposure to 8 mg L−1 Zn induced accumulation of ROS, lipid peroxidation and protein carbonylation during the late stage of exposure. In contrast, exposure to 2 mg L−1 Zn did not result in oxidative damage, which may result from the up-regulation of antioxidant defenses. Although exposure to 8 mg L−1 Zn increased activities and mRNA levels of antioxidant enzymes during the early stage of exposure, including Cu/Zn–SOD, Mn–SOD, CAT, GPx and GR, the activities of these enzymes except Cu/Zn–SOD were inhibited at 96 h. Furthermore, a sharp increase in Nrf2 expression was observed in fish exposed to 8 mg L−1 at 6 and 12 h, and 2 mg L−1 at 12 h and 24 h, suggesting that Nrf2 was required for the protracted induction of these genes. The late increase in Keap1 expression may support its role in switching off the Nrf2 response. In conclusion, the present study demonstrated different effects of low- and high-dose waterborne Zn on antioxidant responses, which could contribute to the understanding of antioxidant and toxic roles of zinc on a molecular level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last few decades, a dramatic increase in environmental pollution by metals occurs as a consequence of industrial, agricultural and anthropogenic activities, and thus aquatic organisms are exposed to a significant amount of these pollutants (Heath 1995). Among the heavy metals, essential heavy metals are an interesting subject of research because they are required for the function of various biological processes, but become toxic at increased levels. Zn is an essential micronutrient required for the various functions in biological development such as cell structure and enzyme activities of fish (Watanabe and Kiron 1994). But, excessive Zn in aquatic environment can be toxic because Zn can induce oxidative stress by accelerating the generation of highly reactive oxygen species (ROS) (Zheng et al. 2011).
Organisms have developed an antioxidant defense system that helps them cope with ROS. The antioxidant system is composed of a complex network of low molecular weight scavengers and enzymatic defenses, interacting via both direct and indirect mechanisms (Regoli and Giuliani 2014). It is well established that the most important antioxidant enzymes are superoxide dismutase (SOD), which detoxifies superoxide anions, catalase (CAT), which reduces H2O2, glutathione peroxidase (GPx), which reduces both H2O2 and organic peroxides by a glutathione-dependent reaction, and glutathione reductase (GR), which catalyzes the NADPH-dependent regeneration of glutathione (GSH) from the oxidized form (GSSG) generated by GPx. It has also been widely reported that the intracellular levels of some nonenzymatic antioxidants, such as glutathione, influence the activity of the enzymatic antioxidants (Halliwell and Gutteridge 1999). The complexity of antioxidant responses reflects a likewise complex mechanism of regulation, which involves different pathways modulating either the catalytic activity of pre-existing enzymes or the de novo synthesis of new molecules. There have been numerous studies on the effects of waterborne Zn exposure on oxidative stress and the activity of antioxidant enzymes in fish (Atli et al. 2006; Gallego et al. 2007; Zheng et al. 2011; Loro et al. 2012; Eroglu et al. 2014; Qu et al. 2014). Some studies have focused on the effects of waterborne Zn exposure on the expression of antioxidant genes in fish (Cho et al. 2006; Hansen et al. 2006; Cho et al. 2009). However, these studies were limited in that they only investigated a single aspect of antioxidant responses (mRNA or enzymatic levels). The underlying mechanisms of response to Zn exposure at both enzymatic and molecular levels in fish are poorly understood. On the other hand, numerous experimental studies, mainly driven by mammalian models, have demonstrated that antioxidant-related genes are under the control of numerous transcription factors activated by ROS, such as Nrf2 (NF-E2-related factor 2), which represents a critical mechanism of protection against chemically induced oxidative stress through the Nrf2–Keap1 (Kelch-like-ECH-associated protein 1) system (Baird and Dinkova-Kostova 2011). Although there have recently been a few studies that addressed Nrf2 antioxidant defense induced by waterborne copper or cadmium in fish (Wang and Gallagher 2013; Jiang et al. 2014, 2015), little information is available regarding the role of Nrf2–Keap1 signaling molecules in Zn-induced oxidative stress in fish.
Large yellow croaker (Pseudosciaena crocea) is a commercially important marine species, which has been widely cultured in China since the success of artificial hatchery. However, the high mortality of farmed fish, partly caused by poor water quality, has created significant barriers for commercial aquaculture (Liu et al. 1991). Therefore, understanding the defense characteristics of the large yellow croaker against environmental stress from pollutants may contribute to the development of strategies for its long-term sustainability in aquaculture. Earlier work in our laboratory (Zheng et al. 2016) pointed out that a 96-h pre-exposure to 2 mg L−1 Zn mitigated the oxidative stress induced by exposure to 8 mg L−1 Zn in large yellow croaker, indirectly suggesting low- and high-dose Zn might affect antioxidant responses differently. As a continuation, we evaluated the effects of 2 and 8 mg L−1 Zn exposure on survival rate, Zn accumulation, oxidative damage and antioxidant responses at different exposure times (6, 12, 24, 48 and 96 h) in the liver of large yellow croaker. Oxidative stress was evaluated by determining the levels of ROS, LPO and PC, and by investigating the activity and expression of antioxidant enzyme genes including Cu/Zn–SOD, Mn–SOD, CAT, GPx and GR. To understand the regulatory mechanism of antioxidant enzyme genes, the mRNA levels of Nrf2–Keap1 signaling molecules were also analyzed.
Materials and methods
Zinc exposure
Large yellow croaker were obtained from a local pond of the Dahaiyang Fisheries Co. Ltd, Zhejiang Province, China. Large yellow croaker were exposed to 2 and 8 mg Zn L−1 for 96 h (corresponding to 10 and 40 % of the 96-h LC50, respectively), with three replicates for each treatment. Zn concentrations for the control group, 2 and 8 mg Zn L−1 groups measured using flame atomic absorption spectroscopy (FAAS) were 0.02 ± 0.01, 1.96 ± 0.07 and 8.12 ± 0.13 mg Zn L−1, respectively. Prior to the experiment, large yellow croaker were maintained in 500-L circular fiberglass tanks for the 2-wk acclimatization. At the beginning of the trial, uniform-sized fish (initial body weight: 103.3 ± 5.7 g, mean ± SEM) were transferred into nine fiberglass tanks with a total of 30 fish in each. The acclimatization and the actual exposure were conducted in the static aquarium system with continuous aeration. The fish were fed commercial diets (lipid and protein contents of 11 and 48 % on a dry matter basis, respectively) twice daily at the rate of 1 % of average body weight. During the 96-h experiment, water was aerated continuously and was renewed 50 % every day. Water temperature was maintained at 25.7 ± 4.3 °C and normal photoperiod at 14L:10D. The hardness of the water was 101 mg L−1 as CaCO3. Dissolved oxygen, pH and salinity (‰) were 6.76 ± 0.38 mg L−1, 7.47 ± 0.45 and 30.9 ± 1.6, respectively.
Sampling and analysis
Control and Zn-treated fish were sampled at 0, 6, 12, 24, 48 and 96 h. At each sampling point, 4 fish per tank were randomly selected and dissected on ice. The liver was then removed immediately using sterile forceps, frozen in liquid nitrogen and stored at −80 °C until biochemical determinations and RNA extraction. All experiments, animal care and protocols followed Zhejiang Ocean University’s ethical guidelines for the care and use of laboratory animals.
Hepatic Zn content
The liver was dried at 80 °C to constant weight and then digested in 3 ml concentrated nitric acid at 110 °C for 72 h. The digested samples were diluted prior to FAAS measurements. The detection limit of Zn was 0.028 µg/ml. Quality control blanks and standards were run every 20th sample. The accuracy of the method was evaluated by calibration against an international standard (National Research Council of Canada). Recovery of Zn ranged from 94 to 106 %. The hepatic Zn contents were expressed as mg/kg wet weight. The analyses were conducted in duplicate.
Oxidative stress analysis
Tissues were homogenized in an ice-cold 20 mM Tris buffer, pH 7.6, containing 1 mM of ethylene diamine tetraacetic acid (EDTA), 0.5 M of saccharose, 0.15 M of KCl and 1 mM of dithiothreitol (DTT). The homogenates were centrifuged at 500×g for 15 min at 4 °C to precipitate large particles and centrifuged again at 12,000×g for 30 min at 4 °C. The supernatant was maintained at 4 °C until being measured for biochemical analysis.
ROS were measured using the carboxy-2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA) according to LeBel et al. (1992). Lipid peroxidation was determined by the thiobarbituric reactive species (TBARS) assay, which measured the production of malondialdehyde that reacts with thiobarbituric acid, according to the method described by Livingstone et al. (1990). Carbonyl groups of proteins (PC) were determined as previously described (Lushchak et al. 2005). Hydrogen peroxide (H2O2) content was determined according to the methods of Pick and Keisari (1981).
Glutathione reductase activity (GR, EC 1.6.4.2) was assayed according to methods described by Tanaka et al. (1994). The activity of glutathione peroxidase (GPx, EC 1.11.1.9) was determined according to methods described by Drotar et al. (1985), using H2O2 as the substrate. Catalase activity (CAT, EC 1.11.1.6) was determined according to methods described by Beutler (1982). Superoxide dismutase (SOD, EC 1.15.1.1) activity was measured according to the method of Beauchamp and Fridovich (1971) based on aerobic reduction of NBT at 535 nm by superoxide radicals. In order to assess Mn–SOD activity, the supernatant was preincubated for 60 min at 0 °C in the presence of 5 mM KCN, which produced total inhibition of Cu/Zn–SOD. The latter activity was calculated as the difference between the activities in the absence and the presence of KCN. Soluble protein content in the cytosolic fractions was determined according to the method of Bradford (1976) using bovine serum albumin (BSA) as a standard. All enzyme activities were expressed as U (units) per mg of soluble protein.
Gene expression
Extractions of total RNA from liver and first strand cDNA synthesis were performed according to the methods in our recent study with slight modification (Zheng et al. 2016). The cDNA synthesis reactions were diluted to 200 μL in water. Q-PCR reactions (20 μL) were performed in 96-well plates in an Applied Biosystems Prism 7500 Sequence Detection System (Applied Biosystems, USA) with SYBR® Premix Ex Taq™ Kit(Takara), containing 10μL SYBR®Premix Ex Taq Master Mix, 2 μL of cDNA and 0.2 μM of each primer. The primer sequences of each gene used in this analysis are given in Table 1. The designed primers were based on the genomic sequences in the large yellow croaker genome data (Wu et al. 2014a).The thermal program included 1 min at 95 °C, 45 cycles at 95 °C for 5 s, 57 °C for 10 s and 72 °C for 30 s. All reactions were performed in duplicate, and each reaction was verified to contain a single product of the correct size using agarose gel electrophoresis. Standard curves were constructed for each gene using serial dilutions of stock cDNA to account for any differences in amplification efficiencies. A set of four housekeeping genes (EF 1α, β-Actin, GAPDH and Tubulin α) were selected from the literature to test their transcription stability. According to the geNorm software (Vandesompele et al. 2002), the two genes with the most stable levels of expression across experimental conditions were β-Actin and GAPDH. The relative expression levels were calculated with the “delta–delta Ct” method (Pfaffl 2001), when normalizing to the geometric mean of the best combination of two genes (β-Actin and GAPDH) as suggested by geNorm.
Statistical analysis
Results are presented as mean ± SEM. Prior to statistical analysis, all data were tested for normality of distribution using the Kolmogorov–Smirnov test. The homogeneity of variances among the different treatments was tested using Barlett’s test. The variables were subjected to one-way analysis of variance (ANOVA) and Tukey’s multiple range test. Analysis was performed using SPSS 18.0 for Windows (SPSS, Michigan Avenue, Chicago, IL, USA), and the minimum significant level was set at 0.05.
Results
Effects of Zn exposure on survival rate and Zn content
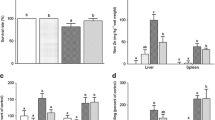
Exposure to 8 mg L−1 Zn significantly reduced survival rate at 96 h (Fig. 1a). In contrast, exposure to 2 mg L−1 Zn did not significantly survival rate. Zn content increased during 12–96 h in the 8 mg L−1 Zn group and at 96 h in the 2 mg L−1 Zn group (Fig. 1b).
Effects of Zn exposure on ROS and oxidative damage
Exposure to 8 mg L−1 Zn exposure induced an increase in the levels of ROS (Fig. 2a), LPO (Fig. 2b) and PC (Fig. 2c) during 24–96, 48–96 and 24–96 h, respectively. In contrast, exposure to 2 mg L−1 Zn did not significantly affect ROS levels and even reduced LPO levels at 96 h and PC levels during 48–96 h. H2O2 content increased at 6, 12, 48 and 96 h in the 8 mg L−1 Zn group and at 12, 24 and 48 h in the 2 mg L−1 Zn group.
Effects of Zn exposure on activities of antioxidant enzymes
Exposure to 2 mg L−1 Zn enhanced activities of Cu/Zn–SOD at 12 h (Fig. 3a), Mn–SOD at 24, 48 and 96 h (Fig. 3b), CAT at 96 h (Fig. 3c), GR at 6, 12, 24 and 48 h (Fig. 3d), GPx at 6, 12, 24 and 48 h (Fig. 3e). Similarly, exposure to 8 mg L−1 Zn induced an increase in activities of Cu/Zn–SOD at 6 h and 12 h (Fig. 3a), Mn–SOD at 6 h and 12 h (Fig. 3b), CAT at 48 h (Fig. 3c), GR at 6 h (Fig. 3d), GPx at 6, 12 and 24 h (Fig. 3e). However, activities of Mn–SOD, CAT, GR and GPx significantly declined at 96 h in the 8 mg L−1 Zn group.
Effects of Zn exposure on expression of antioxidant genes
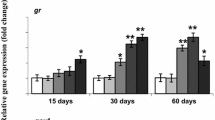
Exposure to 2 mg L−1 Zn up-regulated mRNA levels of Cu/Zn–SOD at 24 h (Fig. 4a), Mn–SOD at 24 h (Fig. 4b), CAT at 24 h and 48 h (Fig. 4c), GR at 24 h (Fig. 4d), GPx1a at 12, 24 and 48 h (Fig. 4e), GPx1b at 48 h and 96 h (Fig. 4f), Nrf2 at 12 h and 24 h (Fig. 4g) and Keap1 at 48 h (Fig. 4h). Similarly, exposure to 8 mg L−1 Zn increased expression levels of Cu/Zn–SOD at 12 h (Fig. 4a), Mn–SOD at 6 h (Fig. 4b), CAT at 24 h and 48 h (Fig. 4c), GPx1a at 12 h and 24 h (Fig. 4e), GPx1b at 12, 24, 48 and 96 h (Fig. 4f), Nrf2 at 6 h and 12 h (Fig. 4g), and Keap1 at 12 h (Fig. 4h). However, mRNA levels of Mn–SOD significantly declined at 24, 48 and 96 h in the 8 mg L−1 Zn group.
Effects of Zn exposure on mRNA levels of Cu/Zn–SOD (a), Mn–SOD (b), CAT (c), GR (d), GPx1a (e), GPx1b (f), Keap1 (g) and Nrf2 (h) in the liver of large yellow croaker. Values are mean ± SEM (n = 4). Different letters represent the statistically significant differences between the control and Zn-exposed groups
Discussion
A large number of studies demonstrate high levels of waterborne Zn exposure induce mortality, Zn accumulation and oxidative damage in fish (Eroglu et al., 2014; Qu et al. 2014; Zheng et al. 2011, 2016), reinforcing the toxicity of this metal on aquatic organisms. This result is agreement with our present data in the 8 mg L−1 Zn group that: 1) the levels of LPO and PC were enhanced during 48–96 h; 2) survival rate was reduced at 96 h; 3) Zn content increased during 24–96 h. On the other hand, Zn functions as an antioxidant by activating antioxidant enzymes (Prasad 2014). Several studies report mild metal exposure can inhibit oxidative stress in fish (Sampaio et al. 2008; Huang et al. 2014). In the present study, the antioxidant role of Zn was demonstrated by a decrease in LPO and PC levels and an increase in activities and mRNA of antioxidant enzyme genes in the 2 mg L−1 Zn group.
One hypothesis can be proposed to explain the positive effect of 2 mg L−1 Zn exposure: development of tolerance and cellular adaptation of fish to low Zn. The adaptation process was observed in the present study. Firstly, there was a lack of changes in ROS level in the 2 mg L−1 Zn group. Secondly, 2 mg L−1 Zn did not induce oxidative damage. Possibly, 2 mg L−1 Zn provoked a general stress response that protected organisms against future severe stress situations (Dolci et al. 2014). The ability of fish to adapt to low metal levels may result from the induction of metallothioneins that will lead to reduction in metal toxicity (Adeyemi and Klerks 2013), which makes it more likely that low-dose Zn-exposed fish are better able to handle the influx of Zn to resist metal challenges (McGeer et al. 2007). This may partially explain why the increased Zn content at 96 h did not induce oxidative damage. In the present study, a coordinated increase was observed in activities and mRNA levels of all tested antioxidant enzyme genes in the 2 mg L−1 Zn group, suggesting a protective role against damage by Zn. But, these changes occurred earlier than 96 h when Zn began to accumulate. That is to say that the up-regulation of antioxidant defenses did not depend on Zn accumulation. Waterborne metal firstly enters the bloodstream by gill absorption and may result in elevations in serum cortisol levels (Fu et al. 1990). A positive relationship between serum cortisol levels and SOD activity was demonstrated in Paralichthys olivaceus exposed to benzo[a]pyrene (An et al. 2008), suggesting the increase in cortisol levels could induce antioxidant defenses.
The negative effect of 8 mg L−1 Zn exposure may be involved in the highly generation of ROS and H2O2 caused by failure to initiate activities of antioxidant enzymes during the late stage of exposure. When the antioxidant system is not able to eliminate or neutralize the excess of ROS and H2O2, there is an increased risk of oxidative damage due to accumulation of lipid peroxidation and protein carbonylation, which may, in turn, decrease enzyme activities or even degrade the enzymes (Zhang et al. 2008). Furthermore, a sharp decrease in CAT activity was observed at 6 h in the 8 mg Zn L−1 group, though activities of the other enzymes were up-regulated during the early stage of exposure. The inhibition of CAT activity may result from the excess production of H2O2, similar to other reports from fishes acutely exposed to Cd, Cr, Mn and Cu (Atli et al. 2006; Hansen et al. 2006; Sampaio et al. 2008; Vieira et al. 2012). Atli et al. (2006) also suggested an inhibition of CAT activity may be related to the accompanied direct binding of metal ions to–SH groups on the enzyme molecule. In the present study, the inhibition of CAT activity could also explained by the down-regulated transcriptional level of CAT, as suggested by Sarkar et al. (2014) and Sun et al. (2014). Although CAT activity was reduced during the early stage of exposure, the increase in GPx activity can eliminate H2O2 more effectively than CAT (Kang et al. 2005). This may explain why 8 mg L−1 Zn did not induce oxidative damage during the early stage of exposure.
A substantial portion of cellular ROS is generated in mitochondrial electron transport chain (Martínez-Reyes and Cuezva 2014). Mn–SOD is located in the mitochondrial matrix and controls the balance of ROS (Murphy 2009). In the present study, exposure 8 mg Zn L−1 down-regulated mRNA levels of Mn–SOD at 96 h in the liver of large yellow croaker, which could contribute to the increase in ROS level. The decrease in Mn–SOD expression correlated well with low Mn–SOD activity, suggesting Mn–SOD may be regulated by Zn at pre-translational step. However, the mismatch between antioxidant gene expression and enzyme activity was also observed, possibly due to time-lag effect between transcription and translation and/or posttranslational modifications (Nam et al. 2005; Craig et al. 2007). For example, activities of CAT, GR and GPx declined and mRNA levels of them remained unchanged at 96 h.
In the present study, 8 mg Zn L−1 activated expression and activity of antioxidant enzyme genes more quickly than 2 mg Zn L−1, which may be related to transcription factor Nrf2. The time when Nrf2 expression was up-regulated was earlier in the 8 mg Zn L−1 group than that in the 2 mg Zn L−1 group. Several experimental evidences, driven in fish models, demonstrated that antioxidant genes are under the control of Nrf2 activated by ROS through Nrf2–Keap1 signaling pathway (Shi and Zhou 2010; Giuliani and Regoli 2014; Wu et al. 2014b). In particular, Nrf2 is important in protecting the liver, since Nrf2 absence increases hepatic lipid peroxidation (Li et al. 2004). In the present study, 8 mg Zn L−1 might transiently induce a high level of ROS that activate Nrf2–Keap1 signaling pathway in advance compared with 2 mg Zn L−1. The earlier activation of Nrf2 and sequent up-regulation of antioxidant genes may imply the transcription regulation of antioxidant genes. However, the persistent accumulation of Nrf2 in the nucleus may have dangerous effects, like free radical damage, apoptosis and tumorigenesis (Kobayashi et al. 2002; Copple et al. 2008). The enhancement of Keap1 expression would increase Nrf2 degradation, leading to a feedback autoregulatory loop which controls Nrf2 abundance (Lee et al. 2007). Keap1 would thus act as a post-induction repressor to switch off the Nrf2 response (Sun et al. 2007). In the present study, a later increase in Keap1 mRNA levels would support its role in switching of Nrf2 response.
In conclusion, our study clearly demonstrated different effects of low- and high-dose waterborne Zn on survival rate, Zn accumulation, ROS levels, oxidative damage and antioxidant responses. First, oxidative damage was reduced by low-dose Zn, but induced by high-dose Zn. Second, low-dose Zn activated antioxidant defense system at transcriptional and enzymatic levels. However, this phenomenon merely occurred during the early stage of exposure for high-dose Zn. During the late stage of exposure, high-dose Zn inhibited activity and expression of antioxidant enzyme genes. In the process, Nrf2 is required for the protracted induction of antioxidant genes. However, it should be noted that the gene mRNA only provides a portion of the transcriptional information about the de novo syntheses of these factors; detailed mechanisms should be revealed by analyses of their protein levels and posttranslational modifications in future research.
References
Adeyemi JA, Klerks PL (2013) Occurrence of copper acclimation in the least killifish Heterandria formosa, and associated biochemical and physiological mechanisms. Aquat Toxicol 130:51–57
An KW, Shin HS, Choi CY (2008) Physiological responses and expression of metallothionein (MT) and superoxide dismutase (SOD) mRNAs in olive flounder, Paralichthys olivaceus exposed to benzo[a]pyrene.Comp. Biochem Physiol B Biochem Mol Biol 149:534–539
Atli G, Alptekin O, Tukel S, Canli M (2006) Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp Biochem Physiol C Pharmacol Toxicol 143:218–224
Baird L, Dinkova-Kostova AT (2011) The cytoprotective role of the Keap1–Nrf2 pathway. Arch Toxicol 85:241–272
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beutler E (1982) Catalase. Red cell metabolism, a manual of biochemical methods, pp 105–106
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cho YS, Choi BN, Kim KH, Kim SK, Kim DS, Bang IC, Nam YK (2006) Differential expression of Cu/Zn superoxide dismutase mRNA during exposures to heavy metals in rockbream (Oplegnathus fasciatus). Aquaculture 253:667–679
Cho YS, Lee SY, Bang IC, Kim DS, Nam YK (2009) Genomic organization and mRNA expression of manganese superoxide dismutase (Mn–SOD) from Hemibarbus mylodon (Teleostei, Cypriniformes). Fish Shellfish Immunol 27:571–576
Copple IM, Goldring CE, Kitteringham NR, Park BK (2008) The Nrf2–Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology 246:24–33
Craig PM, Wood CM, McClelland GB (2007) Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 293:R1882–R1892
Dolci G, Vey L, Schuster A, Roversi K, Roversi K, Dias V, Pase C, Barcelos R, Antoniazzi C, Golombieski J (2014) Hypoxia acclimation protects against oxidative damage and changes in prolactin and somatolactin expression in silver catfish (Rhamdia quelen) exposed to manganese. Aquat Toxicol 157:175–185
Drotar A, Phelps P, Fall R (1985) Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci 42:35–40
Eroglu A, Dogan Z, Kanak E, Atli G, Canli M (2014) Effects of heavy metals (Cd, Cu, Cr, Pb, Zn) on fish glutathione metabolism. Environ Sci Pollut R 22:3229–3237
Fu H, Steinebach OM, Van den Hamer CJA, Balm PHM, Lock RAC (1990) Involvement of cortisol and metallothionein-like proteins in the physiological responses of tilapia, Oreochromis mossambicus to sub-lethal cadmium stress. Aquat Toxicol 16:257–270
Gallego A, Martin-González A, Ortega R, Gutierrez JC (2007) Flow cytometry assessment of cytotoxicity and reactive oxygen species generation by single and binary mixtures of cadmium, zinc and copper on populations of the ciliated protozoan Tetrahymena thermophila. Chemosphere 68:647–661
Giuliani ME, Regoli F (2014) Identification of the Nrf2–Keap1 pathway in the European eel Anguilla anguilla: role for a transcriptional regulation of antioxidant genes in aquatic organisms. Aquat Toxicol 150:117–123
Halliwell B, Gutteridge JM (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Hansen B, Romma S, Garmo Ø, Olsvik P, Andersen R (2006) Antioxidative stress proteins and their gene expression in brown trout (Salmo trutta) from three rivers with different heavy metal levels. Comp Biochem Physiol C Pharmacol Toxicol 143:263–274
Heath AG (1995) Water pollution and fish physiology. CRC Press, Boca Raton
Huang C, Chen QL, Luo Z, Shi X, Pan YX, Song YF, Zhuo MQ, Wu K (2014) Time-dependent effects of waterborne copper exposure influencing hepatic lipid deposition and metabolism in javelin goby Synechogobius hasta and their mechanism. Aquat Toxicol 155:291–300
Jiang WD, Liu Y, Hu K, Jiang J, Li SH, Feng L, Zhou XQ (2014) Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: protective effects of myo-inositol. Aquat Toxicol 155:301–313
Jiang WD, Liu Y, Jiang J, Wu P, Feng L, Zhou XQ (2015) Copper exposure induces toxicity to the antioxidant system via the destruction of Nrf2/ARE signaling and caspase-3-regulated DNA damage in fish muscle: amelioration by myo-inositol. Aquat Toxicol 159:245–255
Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH (2005) 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med 11:571–578
Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, Takagi Y, Yamamoto M (2002) Identification of the interactive interface and phylogenic conservation of the Nrf2–Keap1 system. Genes Cells 7:807–820
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Lee OH, Jain AK, Papusha V, Jaiswal AK (2007) An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J Biol Chem 282:36412–36420
Li J, Stein TD, Johnson JA (2004) Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics 18:261–272
Liu P, Yu Y, Liu C (1991) Studies on the situation of pollution and countermeasures of control of the oceanic environment in Zhoushan fishing ground—the largest fishing ground in China. Mar Pollut Bull 23:281–288
Livingstone D, Martinez PG, Michel X, Narbonne J, Ohara S, Ribera D, Winston G, (1990) Oxyradical production as a pollution-mediated mechanism of toxicity in the common mussel, Mytilus edulis L., and other molluscs. Funct Ecol 4:415–424
Loro VL, Jorge MB, da Silva KR, Wood CM (2012) Oxidative stress parameters and antioxidant response to sublethal waterborne zinc in a euryhaline teleost Fundulus heteroclitus: protective effects of salinity. Aquat Toxicol 110:187–193
Lushchak VI, Bagnyukova TV, Husak VV, Luzhna LI, Lushchak V, Storey KB (2005) Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. Int J Biochem Cell B 37:1670–1680
Martínez-Reyes I, Cuezva JM (2014) The H+-ATP synthase: a gate to ROS-mediated cell death or cell survival. BBA-Bioenergetics 1837:1099–1112
McGeer JC, Nadella S, Alsop DH, Hollis L, Taylor LN, McDonald DG, Wood CM (2007) Influence of acclimation and cross-acclimation of metals on acute Cd toxicity and Cd uptake and distribution in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 84:190–197
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Nam YK, Cho YS, Choi BN, Kim KH, Kim SK, Kim DS (2005) Alteration of antioxidant enzymes at the mRNA level during short-term starvation of rockbream Oplegnathus fasciatus. Fish Sci 71:1385–1387
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45–e45
Pick E, Keisari Y (1981) Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—induction by multiple nonphagocytic stimuli. Cell Immunol 59:301–318
Prasad AS (2014) Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol 28:364–371
Qu R, Feng M, Wang X, Qin L, Wang C, Wang Z, Wang L (2014) Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat Toxicol 150:9–16
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117
Sampaio FG, de Lima Boijink C, Oba ET, dos Santos LRB, Kalinin AL, Rantin FT (2008) Antioxidant defenses and biochemical changes in pacu (Piaractus mesopotamicus) in response to single and combined copper and hypoxia exposure. Comp Biochem Physiol C Pharmacol Toxicol 147:43–51
Sarkar S, Mukherjee S, Chattopadhyay A, Bhattacharya S (2014) Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: expression of antioxidant genes. Ecotox Environ Safe 107:1–8
Shi X, Zhou B (2010) The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol Sci 115:391–400
Sun S, Ge X, Zhu J, Xuan F, Jiang X (2014) Identification and mRNA expression of antioxidant enzyme genes associated with the oxidative stress response in the Wuchang bream (Megalobrama amblycephala Yih) in response to acute nitrite exposure. Comp Biochem Physiol C Pharmacol Toxicol 159:69–77
Sun Z, Zhang S, Chan JY, Zhang DD (2007) Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol 27:6334–6349
Tanaka K, Sano T, Ishizuka K, Kitta K, Kawamura Y (1994) Comparison of properties of leaf and root glutathione reductases from spinach. Physiol Plant 91:353–358
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F, (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:research0034
Vieira MC, Torronteras R, Córdoba F, Canalejo A (2012) Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotox Environ Safe 78:212–217
Wang L, Gallagher EP (2013) Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol Appl Pharm 266:177–186
Watanabe T, Kiron V (1994) Prospects in larval fish dietetics. Aquaculture 124:223–251
Wu C, Zhang D, Kan M, Lv Z, Zhu A, Su Y, Zhou D, Zhang J, Zhang Z, Xu M (2014a) The draft genome of the large yellow croaker reveals well-developed innate immunity. Nat Commun 5:5227
Wu P, Jiang WD, Liu Y, Chen GF, Jiang J, Li SH, Feng L, Zhou XQ (2014b) Effect of choline on antioxidant defenses and gene expressions of Nrf2 signaling molecule in the spleen and head kidney of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 38:374–382
Zhang XD, Zhu YF, Cai LS, Wu TX (2008) Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 280:136–139
Zheng JL, Luo Z, Chen QL, Liu X, Liu CX, Zhao YH, Gong Y (2011) Effect of waterborne zinc exposure on metal accumulation, enzymatic activities and histology of Synechogobius hasta. Ecotox Environ Safe 74:1864–1873
Zheng JL, Zhu QL, Wu CW, Zhu AY, Shen B, Zeng L (2016) Zinc acclimation mitigated high zinc induced oxidative stress by enhancing antioxidant defenses in large yellow croaker Pseudosciaena crocea. Aquat Toxicol 172:21–29
Acknowledgments
This work was supported by Public Science and Technology Research Funds Projects of Ocean (201505025), Natural Science Foundation of Zhejiang Province (LY15C190009) and Scientific Research Foundation of Zhejiang Ocean University (22115010215). The authors thank the Dahaiyang Fisheries Co. Ltd. for supporting large yellow croaker.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jia-Lang Zheng and Lin Zeng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zheng, JL., Zeng, L., Xu, MY. et al. Different effects of low- and high-dose waterborne zinc on Zn accumulation, ROS levels, oxidative damage and antioxidant responses in the liver of large yellow croaker Pseudosciaena crocea . Fish Physiol Biochem 43, 153–163 (2017). https://doi.org/10.1007/s10695-016-0275-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0275-6