Abstract
The baroreflex is one of the most important regulators of cardiovascular homeostasis in vertebrates. It begins with the monitoring of arterial pressure by baroreceptors, which constantly provide the central nervous system with afferent information about the status of this variable. Any change in arterial pressure relative to its normal state triggers autonomic responses, which are characterized by an inversely proportional change in heart rate and systemic vascular resistance and which tend to restore pressure normality. Although the baroreceptors have been located in mammals and other terrestrial vertebrates, their location in fish is still not completely clear and remains quite controversial. Thus, the objective of this study was to locate the baroreceptors in a teleost, the Colossoma macropomum. To do so, the occurrence and efficiency of the baroreflex were both analyzed when this mechanism was induced by pressure imbalancements in intact fish (IN), first-gill-denervated fish (G1), and total-gill-denervated fish (G4). The pressure imbalances were initiated through the administration of the α1-adrenergic agonist phenylephrine (100 µg kg−1) and the α1-adrenergic antagonist prazosin (1 mg kg−1). The baroreflex responses were then analyzed using an electrocardiogram that allowed for the measurement of the heart rate, the relationship between pre- and post-pharmacological manipulation heart rates, the time required for maximum chronotropic baroreflex response, and total heart rate variability. The results revealed that the barostatic reflex was attenuated in the G1 group and nonexistent in G4 group, findings which indicate that baroreceptors are exclusively located in the gill arches of C. macropomum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The barostatic reflex is one of the key regulators of cardiovascular homeostasis in vertebrates. It begins with the monitoring of arterial pressure by baroreceptors, which constantly provide the central nervous system with afferent information about the status of this variable. Any increase or decrease in arterial pressure relative to its normal state triggers autonomic responses, which are characterized by an inversely proportional change in heart rate (f H) and systemic vascular resistance and which tend to restore pressure normality (Bagshaw 1985; Van Vliet and West 1994; Mueller et al. 2013; Armelin et al. 2014).
The location of baroreceptors in mammals has long been determined: It most commonly lies in the aortic arch and the carotid sinus of these animals (Bagshaw 1985; Van Vliet and West 1994). However, its location in fish has yet to be confirmed (Bagshaw 1985; Nilsson and Sundin 1998; Sandblom and Axelsson 2005). The first suspicions about the location of baroreceptors in fish were derived from the observation of reflex bradycardia in response to gill stimulation in elasmobranchs and teleosts. The groundbreaking work by McWilliam (1885) demonstrated that electrical, mechanical, chemical, and thermal stimulation of the gills of Anguilla anguilla instantly induced bradycardia of autonomic origin, observations which were later corroborated by Mott (1951). Similarly, Lutz (1930) reported that electrical and mechanical stimulations of the gills of the elasmobranch Scyllium canicula also resulted in immediate transient bradycardia, which was probably caused by an increase in vagal parasympathetic activity.
Subsequently, Lutz and Wyman (1932) demonstrated the occurrence of a reduction in f H, also of parasympathetic origin, that was associated with an abrupt increase in branquial blood pressure in the spiny dogfish (Squalus acanthias). In another study on dogfish (Mustelo canis and S. acanthias), a direct correlation between the firing rate of gill innervation and the arterial blood pressure of the animals was observed (Irving et al. 1935). Decades later, Ristori (1970) and Ristori and Dessaux (1970) artificially increased the branchial artery perfusion pressure of Cyprinus carpio and found that such manipulation resulted in reflex bradycardia, which was successfully eradicated by an administration of the muscarinic cholinergic antagonist atropine or by a denervation of the cardiovisceral branch of the vagus nerve. A similar result was obtained by Burleson and Milsom (1993) and by Sandblom and Axelsson (2005), wherein manipulations of the branchial arterial pressure of Oncorhyncus mykiss produced inversely proportional changes in the animals’ f H.
Although the aforementioned studies point to the gills as the main barosensitive site in fish, their methodologies do not allow us to determine whether the baroreceptors are exclusively located in the gills of these vertebrates. Moreover, as pointed out by Sandblom and Axelsson (2005), such manipulations of branchial arterial pressure may also affect pre- and post-branchial vasculature, which may contain baroreceptors. With regard to the studies that have involved direct gill stimulation, the observed cardiovascular responses cannot be assigned to a barostatic reflex due to the probable existence of gill chemoreceptors, which can induce identical responses when stimulated (Leite et al. 2007; Lopes et al. 2010; Milsom 2012; Zeraik et al. 2013; Belão et al. 2015).
In light of this information, the current study sought to test the hypothesis that the baroreceptors are exclusively located in the gills of the neotropical teleost Colossoma macropomum by analyzing the occurrence of the barostatic reflex in intact fish, first-gill-denervated fish, and total-gill-denervated fish—a methodology that can reveal the existence of extrabranchial baroreceptors and does not promote the above-mentioned ambiguity between baroreflex and chemoreflex responses.
Materials and methods
Experimental animals
Thirty-five juvenile C. macropomum (Cuvier, 1818) specimens of both sexes and with an average body mass of 229.7 ± 6.8 g (mean ± SEM) were obtained from the Center of Aquaculture of São Paulo State University (CAUNESP—Jaboticabal, São Paulo, Brazil). In the laboratory, fish were kept in a natural photoperiod in four 500-l tanks supplied with dechlorinated and constantly aerated water (\(P_{{{\text{O}}_{2} }}\) ~ 140 mmHg, pH 6.5) at a temperature that varied between 20 and 30 °C. The animals were fed on alternate days with commercial food pellets (32 % protein) and were fasted for at least 72 h prior to experimentation. The water in the tanks was replaced every 72 h. The experiments conducted in the study were approved by São Paulo State University (UNESP/IBILCE) Ethical Committee for Animal Research (No. 078/2013 CEUA) and were in accordance with all the regulations and ethical guidelines in Brazil.
Pre-experimental procedures
Fish were anesthetized in a benzocaine solution (100 mg l−1; pre-dissolved in 3 ml of anhydrous ethanol) and transferred to a surgical table. There, their gills were artificially ventilated with a constantly aerated weaker benzocaine solution (50 mg l−1). They were then fitted with electrocardiogram (ECG) electrodes as described by Glass et al. (1991). The positive electrode was inserted and sutured in a ventral position between the gills and the heart, while the negative electrode was placed in a ventrocaudal position above the pelvic fins. To allow for pharmacological administrations, a cannula (PE-20) filled with saline solution (0.9 %) was inserted into the peritoneal cavity of the fish through a small puncture wound made below the negative ECG electrode with a 21-gauge needle and sutured to the skin at its exit point (Teixeira et al. 2015).
The operculum of the animals was reflected forward, and a small incision was made in the epithelium at the dorsal end of the first and second gill arches where they meet the roof of the opercular cavity, thus allowing access to cranial nerve IX (the glossopharyngeal nerve) and the branchial branches of cranial nerve X (the vagus nerve). For first gill arch denervation (G1 group, N = 8), the cranial nerve IX and the pretrematic branch of cranial nerve X innervating the arch were carefully dissected free of connective tissue and cut with fine ophthalmological scissors. Similarly, for complete branchial denervation (G4 group, N = 8), all gill arch nerves were dissected and sectioned. The cardiac and visceral branches of the vagus nerve were left intact in all cases. In the control group (IN group, N = 8), the opercular cavity was left intact. All denervations were confirmed via autopsy.
After surgery, the animals were ventilated with aerated water until they showed signs of recovering from the anesthesia. They were then placed into 20-l individual experimental chambers (20 cm height × 50 cm length × 20 cm width) to recover for 24 h prior to experimentation. These chambers were continually supplied (100 ml min−1) with temperature-controlled (25.2 ± 0.1 °C; mean ± SEM), normoxic (\(P_{{{\text{O}}_{2} }}\) ~ 140 mmHg) and dechlorinated water with a pH of 6.5. The electrodes implanted in the fish were then plugged into a BIOPAC MP36 data acquisition system (BIOPAC Systems Incorporated, Goleta, CA, USA) to continuously acquire and record the animal’s electrocardiogram signal, from which f H was derived. The ground cable of the data acquisition system was connected to a stainless steel plate inside the experimental chamber. All of the aforementioned proceedings were based on previous studies (Milsom et al. 2002; Reid et al. 2003; Florindo et al. 2006; Boijink et al. 2010) and were performed within 25 min.
Experimental protocol
After the 24-h recovery period, the f H of the animals was monitored until stability was continuous for 30 min. The f H was then recorded for 15 min before any pharmacological treatment. Next, α1-adrenergic agonist phenylephrine hydrochloride (100 µg kg−1; Sigma-Aldrich, USA) was administered via intraperitoneal cannula and was followed by a saline solution flush (0.9 %; 0.4 ml). The f H responses were monitored for another 15 min. Thereafter, the fish underwent another 24-h recovery period. The protocol was repeated the next day, but instead of phenylephrine, the α1-adrenergic antagonist prazosin hydrochloride (1 mg kg−1; Sigma-Aldrich, USA) was administered. This protocol was applied to both the intact group and the denervated groups (IN, G1, and G4) in order to cause pressure variations that would allow for the chronotropic limb of baroreflex to be analyzed. Also, very care was taken for the fish to not perceive the experimenters, and as a consequence, the pharmacological manipulations were performed in ~60 s.
The protocol was also applied to one group of fish with their branchial innervation exposed but not transected (sham-operated group, SH, N = 4) in order to verify whether the externalization of these nerves alters the variables under study. A very similar protocol was applied to another four intact fish (vehicle-treated group, VE, N = 4); the difference was that phenylephrine and prazosin were replaced with isosmotic saline (1 ml kg−1) and alcoholic (5 %) isosmotic saline (2 ml kg−1), respectively, in order to confirm whether the results obtained were induced by the drugs’ vehicle or by the stress of pharmacological manipulation.
Phenylephrine was freshly prepared in a dilution of 100 µg ml−1 of saline solution (0.9 %), and prazosin was freshly prepared in a dilution of 0.5 mg ml−1 of alcoholic (5 %) saline solution (0.9 %). The influence of these drugs on the arterial pressure of C. macropomum was first tested by injecting them intraperitoneally (with a 24-h period between injections) in one fish with a cannula inserted in the third afferent branchial artery for blood pressure acquisition. The test revealed that phenylephrine (100 µg kg−1) successfully augmented arterial pressure and that prazosin (1 mg kg−1) reduced it (Fig. 1a, b). Phenylephrine was also injected into two other fish 24 h after prazosin and did not induce changes in f H. This result reflects the fact that prazosin was still exerting its effects, so the order of pharmacological manipulation could not be reversed.
It is important to note that the vasodepressor agent prazosin was chosen instead of the widely used sodium nitroprusside: While the latter donates nitric oxide, it also releases cyanide when administered into biological systems (Bates et al. 1991). Because it is known that cyanide induces a severe reflex bradycardia in C. macropomum (Sundin et al. 2000), an α1-adrenergic antagonist was used in order to induce systemic vasodilation without triggering a chemoresponse that may have concealed the baroreflex tachycardia.
Data analysis
After deriving the animals’ f H from the electrocardiogram and plotting the data in descriptive graphs, all of the analyses were employed considering the f H acquired during the stabilization period preceding the pharmacological manipulations, and the moment of greatest change in f H reached within the 5 min after the drugs were administered. The data from these periods were also used to calculate the post-injection f H (%) relative to pre-injection f H (%). These values were used to provide an overview of the baroreflex without the influences that gill denervations have on f H. The percentage was converted using the following equation:
The time required for the chronotropic responses induced by phenylephrine and prazosin to reach their maximum values was also analyzed in order to determine the efficiency of the barostatic reflex using another measure. The analysis determined the amount of time between the pharmacological infusions and the greatest change in f H that occurred within the 5 min that followed. In addition, the standard deviation of the RR intervals that occurred in the 5 min before and after pharmacological manipulations was also calculated as a way to obtain total f H variability (T HRV). It should be noted that the data obtained beyond the 5 min following the pharmacological manipulations was not used in any of the analyses in order to minimize the influences of any mechanisms involved in the long-term control of arterial pressure in the variables studied.
Statistics
A Kolmogorov–Smirnov normality test was applied to the data, and the results were considered parametric. Within and between the IN, G1, and G4 groups, changes in absolute f H values as well as the time until the maximum chronotropic response and RR intervals’ standard deviation were identified using a one-way ANOVA followed by a Student–Newman–Keuls multiple comparison test. The post-phenylephrine/prazosin f H values that had been converted into a percentage relative to pre-injection f H were compared among the groups (IN, G1, and G4), as well as to a 100 % baseline via one-way ANOVA, followed by a Student–Newman–Keuls post hoc. A one-way ANOVA followed by a Bonferroni post hoc was conducted in order to determine any divergences between the variables from the IN, SH, and VE groups. The statistical analyses were carried out using the GraphPad InStat 3.0 commercial software (GraphPad Software Inc.). In all comparisons, a significance level of P ≤ 0.05 was adopted. Values are shown as mean ± SEM.
Results
Effects of first and total gill denervation on the tambaqui’s baroreflex response
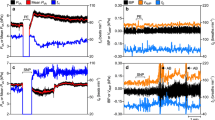
In intact (IN) and first-gill-denervated (G1) fish, the administration of vasoactive drugs instantaneously induced reflex changes in f H, since the vasopressor agent phenylephrine and the vasodepressor agent prazosin caused bradycardia and tachycardia, respectively (Figs. 2a–d, 3a, b). The administration of these drugs did not cause chronotropic baroreflex changes in total-gill-denervated (G4) animals (Figs. 2e, f, 3c); however, prazosin gradually reduced their f H, and this reduction established itself within 15 min (Fig. 2f).
Descriptive graphs showing the heart rate (f H) of tambaqui in the 15 min preceding the pharmacological manipulations with phenylephrine (100 µg kg−1) and prazosin (1 mg kg−1), and in the 15 min following these manipulations. The figure depicts the data of intact fish (IN; N = 8) (a, b), first-gill-denervated fish (G1; N = 8) (c, d), and total-gill-denervated fish (G4; N = 8) (e, f). The time wherein the pharmacological manipulations were performed (~60 s) does not appear in the graphs. Values are mean ± SEM. An asterisk marks a significant difference between the indicated values (two-tailed paired t test; P ≤ 0.05)
Heart rate (f H) of intact fish (IN; N = 8) (a), first-gill-denervated fish (G1; N = 8) (b), and total-gill-denervated tambaqui (G4; N = 8) (c) before and after the administrations of phenylephrine (100 µg kg−1) and prazosin (1 mg kg−1). Values are mean ± SEM. Values that do not share a superscript letter differ significantly (P ≤ 0.05)
Specifically, IN animals were found to have an initial f H of 33.5 ± 1.9 bpm, which decreased to 19.1 ± 1.1 bpm after the phenylephrine infusion and which returned to normal values after 24 h (33.6 ± 2.0 bpm). In addition, f H increased to 45.3 ± 3.1 bpm after the prazosin infusion (Fig. 3a). Likewise, G1 fish expressed an initial f H of 33.8 ± 1.6 bpm, which decreased to 23.8 ± 1.5 bpm in response to phenylephrine and which returned to basal levels after 24 h (31.9 ± 1.9 bpm). G1 f H increased to 45.0 ± 3.9 bpm in response to prazosin (Fig. 3b). The G4 animals, on the other hand, were found to have a higher f H than those of other groups (56.6 ± 2.2 bpm), and the value remained unchanged regardless of phenylephrine administration (57.1 ± 2.3 bpm), a 24-h recovery period (49.8 ± 7.7 bpm), or prazosin administration (49.1 ± 2.6 bpm; Fig. 3c).
When only f H modifications were considered, phenylephrine reduced the f H of IN fish to 58.1 ± 4.3 % of the value found before its infusion, while prazosin raised this variable to 135.0 ± 3.5 % of its pre-administration value (Fig. 4a). In G1 animals, pharmacological manipulation with phenylephrine do not decrease f H as much as in the IN group (f H reached 70.4 ± 3.0 % of the pre-injection value). Meanwhile, manipulation with prazosin increased it to 140.6 ± 9.0 % (Fig. 4b). The f H of G4 animals remained unaffected by the administration of the drugs (decreased to 98.2 ± 1.0 % in response to phenylephrine and to 98.9 ± 2.4 % in response to prazosin; Fig. 4c).
Effects of phenylephrine (100 µg kg−1) and prazosin (1 mg kg−1) administrations on the heart rate (percentage change from pre-administration values) of intact tambaqui (IN; N = 8) (a), first-gill-denervated tambaqui (G1; N = 8) (b), and total-gill-denervated tambaqui (G4; N = 8) (c). Values are mean ± SEM. Values that do not share a superscript letter differ significantly (P ≤ 0.05). An asterisk indicates a significant difference from the pre-administration heart rate values (100 % baseline value) (P ≤ 0.05)
The chronotropic baroreflex response to phenylephrine took 110.1 ± 30.4 s to achieve its peak response in IN fish, while in G1 animals, it took twice as long (220.0 ± 26.1 s; Fig. 5). When the prazosin administration was considered, no significant differences were observed between IN and G1 fish, since the former reached its peak f H response in 165.0 ± 30.6 s and the latter reached its peak response in 187.5 ± 21.2 s (Fig. 5).
T HRV was inferred by calculating the standard deviation of the RR intervals that occurred within the 5 min before and after pharmacological manipulations. The results can be found in Fig. 6. Initially, IN animals presented a T HRV of 531.2 ± 61.7 ms. This value almost doubled with the infusion of phenylephrine (807.7 ± 67.9 ms), returned to starting values after 24 h (471.5 ± 38.0 ms), and remained virtually unaltered with the infusion of prazosin (418.5 ± 39.1 ms; Fig. 6a). The T HRV values expressed by the G1 animals before and after phenylephrine administration were significantly lower than those of the IN animals (348.4 ± 31.3 and 563.7 ± 53.9 ms, respectively). They increased to a level similar to the IN animals’ basal level after 24 h (417.5 ± 43.5 ms), and they remained almost completely unchanged with the administration of prazosin (350.4 ± 38.9 ms; Fig. 6b). Finally, the G4 animals exhibited a low T HRV value (64.6 ± 14.4 ms) that did not change significantly under any circumstances (71.9 ± 15.5 ms post-phenylephrine, 105.8 ± 21.5 ms pre-prazosin, and 124.2 ± 45.6 ms post-prazosin; Fig. 6c).
Standard deviation of RR intervals of intact tambaqui (IN; N = 8) (a), first-gill-denervated tambaqui (G1; N = 8) (b), and total-gill-denervated tambaqui (G4; N = 8) (c) before and after the administrations of phenylephrine (100 µg kg−1) and prazosin (1 mg kg−1). Values are mean ± SEM. Values that do not share a superscript letter differ significantly (P ≤ 0.05)
Responses to drug vehicle administration and to exposed innervations
Sham-operated fish (SH) with exposed but not transected gill innervation reacted to phenylephrine and prazosin similarly to IN fish; no significant difference in any variable was detected between these groups (Tables 1, 2, 3, 4). Also, intact animals that were treated with prazosin and phenylephrine excipients (VE) presented no alterations in the variables studied, a finding which confirms that these vehicles do not trigger heart rate responses (Tables 1, 2, 4).
Discussion
Critique of the methods
In the current study, the absence of data on animals’ blood pressure is evident. To measure blood pressure in C. macropomum, a polyethylene cannula typically needs to be inserted into the afferent artery of the third brachial arch or into the animals’ caudal artery (Sundin et al. 2000; Gilmour et al. 2005). In this case, the cannulation of the afferent branchial artery was not performed because the occlusion of such blood vessel is not desirable in a study that hypothesizes that the baroreceptors are exclusively located in the gills of the experimental model. The cannulation of the caudal artery was not a viable alternative because the use of this practice on small animals dramatically increases the time required to complete the pre-experimental procedures—and the prolonged exposure to general anesthesia may jeopardize the autonomic modulation of the heart, thus suppressing the barostatic reflex (Campbell et al. 2004). Opting out of cannulation procedures also prevents the occurrence of hemorrhage, which can significantly alter the blood volume of small-sized individuals.
The baroreflex and the barosensitive sites of the tambaqui
As observed in the descriptive and inferential graphs, both intact (IN) and first-gill-denervated (G1) animals exhibited notable chronotropic responses to pharmacological manipulations. In these two groups, the infusion of the vasoconstrictor agent phenylephrine quickly caused a bradycardia, whereas the administration of the vasodilator agent prazosin elicited a tachycardia (Figs. 2a–d, 3a, b). Such responses indicate the occurrence of an increase in blood pressure caused by the administration of phenylephrine and a decrease in blood pressure triggered by the administration of prazosin. The response to prazosin infusion demonstrates that C. macropomum has a significant basal adrenergic vascular tone. The data also show that the bilateral denervation of the first gill arch did not abolish the barostatic reflex presented by the animals.
The total-gill-denervated fish (G4), on the other hand, did not express reflex chronotropic reactions to the drugs, a finding which demonstrates a lack of barostatic reflex (Figs. 2e, f, 3c). The only f H change involved in the pharmacological manipulations in this experimental group was a gradual onset of bradycardia after prazosin administration. This change cannot be characterized as a baroreflex because it was a very slow f H alteration, which is opposite of that which occurs during hypotension situations (Fig. 2f). There are three possible explanations for this event:
-
1.
Based on the premise that the pacemaker cells of vertebrate hearts are sensitive to stretch, and because there is a direct correlation between this factor and the firing rate of the sinoatrial node, it is conceivable that the systemic vasodilation produced by prazosin decreased the animals’ venous return and cardiac filling pressure, thus reducing the nodal stretch and, as a consequence, the f H—though phenylephrine do not produced the opposite effect, as would be expected (Farrell 1991; Franklin and Axelsson 1994).
-
2.
As observed in the European perch (Perca fluviatilis) by Tirri and Ripatti (1982), the direct effect of the α1-adrenergic antagonist prazosin on heart rate due to cardiac α1-adrenoreceptors blockade is also a possible explanation. However, as in the previous case, a phenylephrine-induced tachycardia would be expected as well.
-
3.
Considering the fact that the myocardium needs a constant blood supply to meet its high demand for oxygen, the decreased venous return and cardiac filling promoted by prazosin may have hindered this supply to the cardiomyocytes, thus decreasing f H and cardiac contraction force due to ischemia or physiological adjustments for myocardial protection (Davie and Farrell 1991; Lillywhite et al. 1999; Farrell 2007). It is important to note that the myocardium of tambaquis is predominantly spongy and exhibits reduced coronary circulation. It is therefore dependent on oxygen diffusion from the luminal blood (Davie and Farrell 1991; Simões et al. 2002). In addition, the fish heart houses only deoxygenated blood in its lumen, which renders the myocardium of tambaquis even more susceptible to ischemia during situations of reduced cardiac filling (Davie and Farrell 1991).
The higher f H presented by the G4 group relative to the IN and G1 groups (Figs. 2a–f, 3a–c) indicates that the baroreceptors, chemoreceptors, and mechanoreceptors located in the gills likely help maintain a basal cardiac cholinergic tone in these animals, and that this tone diminishes after total gill denervation. This possible relationship has been observed in previous studies that performed complete gill denervation in tambaquis and in other species (Reid et al. 2000; Leite et al. 2007; Florindo et al. 2006; Boijink et al. 2010).
The analysis of f H modifications induced by pharmacological interventions (expressed as percentages) also revealed that the infusions of phenylephrine and prazosin in IN and G1 animals reduced and increased f H, respectively (Fig. 4a, b). Once again, these responses were not observed in the G4 group, in which the f H observed after the pharmacological administrations was identical to the 100 % baseline (relative to the f H preceding the administrations; Fig. 4c). Thus, when considered along with the evaluation of raw f H, these data confirm the occurrence of the barostatic reflex in IN and G1 groups, but not in the G4 group.
The data on f H modifications also revealed that the reflex f H change induced by phenylephrine (but not by prazosin) was milder in the G1 group than it was in the IN group (Fig. 4a, b). Furthermore, the analysis of time elapsed until the maximum baroreflex response suggested that the IN group’s reaction to phenylephrine was more immediate than that of the G1 group, while no difference was observed with respect to these groups’ reaction to prazosin (Fig. 5). Based on these results, it can be concluded that the bilateral denervation of the first gill arch impairs the barostatic reflex in C. macropomum, probably due to the presence of baroreceptors in this location.
The similar reflex response to prazosin exhibited by IN and G1 animals can be explained by the 24-h period between the experiments with each vasoactive drug. This interval suggests that the disrupted baroreceptor activity can later be compensated by the remaining baroreceptor populations. This phenomenon has been considered previously by West and Van Vliet (1994), with the argument that the central nervous system may be able to adapt to a new profile of afferent activity in which the information from the remaining baroreceptors is weighed more heavily soon after partial baroreceptor disruptions. Moreover, previous studies have suggested that, in teleosts, baroreceptor sensitivity may be mediated by controlling gill blood flow via vasomotor adjustments of gill arteries that are triggered by non-adrenergic non-cholinergic signaling (generally nitrergic) released from the baroreceptor terminals (Funakoshi et al. 1999; Zaccone et al. 2003, 2006; Hyndman et al. 2006).
Another possible explanation is that baroreceptors from the first gill arch of tambaquis may be more sensitive to an increase in arterial pressure than a decrease in this variable. This has been observed in amphibians, in which distinct baroreceptor populations seem to respond specifically to different ranges of pressure alterations (Bianchi-da-Silva et al. 2000). However, the T HRV results corroborate the hypothesis of disrupted baroreceptor compensation, as will be clarified below.
As the T HRV graphs show, the T HRV value practically doubles relative to its basal magnitude after phenylephrine administration in IN animals. This result indicates that an increased demand for short-term f H regulation was caused by this drug (Fig. 6a). In the G1 group, however, the T HRV was approximately 30 % lower than in the IN group. T HRV values in the G1 group also rose after the administration of phenylephrine but did not reach the levels attained by IN animals (Fig. 6a, b). This lower pre- and post-phenylephrine T HRV values in the G1 group indicate a loss of f H regulatory capacity in these animals, a finding which supports the idea that first gill arch denervation reduces the effectiveness of the barostatic reflex.
Twenty-four hours later, the T HRV of the G1 animals was found to be very similar to that of the IN group prior to the administration of phenylephrine (Fig. 6a, b). This similarity suggests that the initially observed reduction in T HRV values in the G1 group was reversed and thus confirms the hypothesis that the remaining baroreceptors are able to compensate for the disassociated ones over time. Additionally, the infusion of prazosin caused only a slight, nonsignificant decrease in the T HRV value in both the IN and G1 groups (Fig. 6a, b).
The T HRV expressed by the G4 animals was almost nonexistent, a condition which reflects the near lack of short-term f H control in these animals. Both this result and the f H values attest to the absence of a barostatic reflex in this experimental group; consequently, the gills are left as the sole possible baroreceptor location in C. macropomum (Fig. 6c).
It is important to note that the low T HRV presented by the G4 group is not only due to the disassociation of the baroreceptors and the abolition of the baroreflex, since the tambaqui also possesses gill chemoreceptors that can modulate f H (Florindo et al. 2004, 2006). Furthermore, gill denervation may have excised the mechanoreceptors involved in cardiorespiratory coupling (Campbell et al. 2005; Leite et al. 2009). In any case, these factors do not invalidate the conclusion that the low T HRV in the G4 group reaffirms the lack of a baroreflex in these animals, as the infusions of phenylephrine and prazosin did not change this variable in the G4 animals as they did in IN and G1 animals (in which phenylephrine increased T HRV and prazosin tended to reduce it). Additionally, the remaining T HRV values exhibited by the G4 animals likely derives from the activity of other afferences, such as central or orobranchial chemoreceptors (Milsom et al. 2002; Florindo et al. 2006). It is also noteworthy that the activity exerted by the gill baroreceptors, chemoreceptors, and mechanoreceptors seems to be deeply integrated (Funakoshi et al. 1999; Zaccone et al. 2006; Jonz et al. 2015; Porteus et al. 2015), and because of this integration, distinct environmental conditions (e.g., hypoxia) may alter baroreflex function. This possibility has not been considered in this study, which sought only to verify the existence of extrabranchial baroreceptors.
In conclusion, these analyses have provided evidence that the baroreceptors are exclusively located in the gills of the teleost C. macropomum, since no signs of a barostatic reflex were exhibited by total-gill-denervated animals (G4). These analyses also showed that the baroreceptors are probably dispersed among all of the gills, since the first gill denervation (G1) slightly impaired the baroreflex responses of the animals. Therefore, this study confirms the previously documented hypothesis that the gills are the main barosensitive site of fish without fostering the chemoreflex bias and suggests that there are no extrabranchial baroreceptors in this species.
Abbreviations
- f H :
-
Heart rate
- T HRV :
-
Total heart rate variability
- IN:
-
Intact fish (experimental group)
- G1:
-
First-gill-denervated fish (experimental group)
- G4:
-
Total-gill-denervated fish (experimental group)
- SH:
-
Sham-operated fish (experimental group)
- VE:
-
Vehicle-treated intact fish (experimental group)
References
Armelin VA, Braga VHS, Abe AS, Rantin FT, Florindo LH (2014) Autonomic control of heart rate during orthostasis and the importance of orthostatic-tachycardia in the snake Python molurus. J Comp Physiol B 184:903–912
Bagshaw RJ (1985) Evolution of cardiovascular baroreceptor control. Biol Rev 60:121–162
Bates JN, Baker MT, Guerra R Jr, Harrison DG (1991) Nitric oxide generation from nitroprusside by vascular tissue: evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol 42:S157–S165
Belão TC, Zeraik VM, Florindo LH, Kalinin AL, Leite CAC, Rantin FT (2015) Control of cardiorespiratory function in response to hypoxia in an air-breathing fish, the African sharptooth catfish, Clarias gariepinus. Comp Biochem Physiol A 187:130–140
Bianchi-da-Silva LM, Menescal-de-Oliveira L, Hoffmann A (2000) Baroreceptor control of heart rate in the awake toad: peripheral autonomic effectors and arterial baroreceptor areas. J Auton Nerv Syst 80:31–39
Boijink CL, Florindo LH, Leite CAC, Kalinin AL, Milsom WK, Rantin FT (2010) Hypercarbic cardiorespiratory reflexes in the facultative air-breathing fish jeju (Hoplerythrinus unitaeniatus): the role of branchial CO2 chemoreceptors. J Exp Biol 213:2797–2907
Burleson ML, Milsom WK (1993) Sensory receptors in the first gill arch of rainbow trout. Resp Physiol 93:97–110
Campbell HA, Taylor EW, Egginton S (2004) The use of power spectral analysis to determine cardiorespiratory control in the short-horned sculpin Myoxocephalus scorpius. J Exp Biol 207:1969–1976
Campbell HA, Taylor EW, Egginton S (2005) Does respiratory sinus arrhythmia occur in fishes? Biol Lett 1:484–487
Davie PS, Farrell AP (1991) The coronary and luminal circulations of the myocardium of fishes. Can J Zool 69:1993–2001
Farrell AP (1991) From hagfish to tuna: a perspective on cardiac function in fish. Physiol Zool 64:1137–1164
Farrell AP (2007) Tribute to P. L. Lutz: a message from the heart—why hypoxic bradycardia in fishes? J Exp Biol 210:1715–1725
Florindo LH, Reid SG, Kalinin AL, Milsom WK, Rantin FT (2004) Cardiorespiratory reflexes and aquatic surface respiration in the neotropical fish tambaqui (Colossoma macropomum): acute responses to hypercarbia. J Comp Physiol B 174:319–328
Florindo LH, Leite CAC, Kalinin AL, Reid SG, Milsom WK, Rantin FT (2006) The role of branchial and orobranchial O2 chemoreceptors in the control of aquatic surface respiration in the neotropical fish tambaqui (Colossoma macropomum): progressive responses to prolonged hypoxia. J Exp Biol 209:1709–1715
Franklin CE, Axelsson M (1994) The intrinsic properties of an in situ perfused crocodile heart. J Exp Biol 186:269–288
Funakoshi K, Kadota T, Atobe Y, Nakano M, Goris RC, Kishida R (1999) Nitric oxide synthase in the glossopharyngeal and vagal afferent pathway of a teleost, Takifugu niphobles. The branchial vascular innervation. Cell Tissue Res 298:45–54
Gilmour KM, Milsom WK, Rantin FT, Reid SG, Perry SF (2005) Cardiorespiratory responses to hypercarbia in tambaqui Colossoma macropomum: chemoreceptor orientation and specificity. J Exp Biol 208:1095–1107
Glass ML, Rantin FT, Verzola RMM, Fernandes MN, Kalinin AL (1991) Cardio-respiratory synchronization and myocardial function in hypoxic carp, Cyprinus carpio L. J Fish Biol 39:143–149
Hyndman KA, Choe KP, Havird JC, Rose RE, Piermarini PM, Evans DH (2006) Neuronal nitric oxide synthase in the gill of the killifish, Fundulus heteroclitus. Comp Biochem Physiol B 144:510–519
Irving L, Solandt DY, Solandt OM (1935) Nerve impulses from brachial pressure receptors in the dogfish. J Physiol 84:187–190
Jonz MG, Buck LT, Perry SF, Schwerte T, Zaccone G (2015) Sensing and surviving hypoxia in vertebrates. Ann N Y Acad Sci. doi:10.1111/nyas.12780
Leite CAC, Florindo LH, Kalinin AL, Milsom WK, Rantin FT (2007) Gill chemoreceptors and cardio-respiratory reflexes in the neotropical teleost pacu, Piaractus mesopotamicus. J Comp Physiol A 193:1001–1011
Leite CAC, Taylor EW, Guerra CDR, Florindo LH, Belão T, Rantin FT (2009) The role of the vagus nerve in the generation of cardiorespiratory interactions in a neotropical fish, the pacu, Piaractus mesopotamicus. J Comp Physiol A 195:721–731
Lillywhite HB, Zippel KC, Farrell AP (1999) Resting and maximal heart rates in ectothermic vertebrates. Comp Biochem Physiol A 124:369–382
Lopes JM, Boijink CL, Florindo LH, Leite CAC, Kalinin AL, Milsom WK, Rantin FT (2010) Hypoxic cardiorespiratory reflexes in the facultative air-breathing fish jeju (Hoplerythrinus unitaeniatus): role of branchial O2 chemoreceptors. J Comp Physiol B 180:797–811
Lutz BR (1930) Reflex cardiac and respiratory inhibition in the elasmobranch, Scyllium canicula. Biol Bull 59:170–178
Lutz BR, Wyman LC (1932) Reflex cardiac inhibition of branchio-vascular origin in the elasmobranch, Squalus acanthias. Biol Bull 62:10–16
McWilliam JA (1885) On the structure and rhythm of the heart in fishes, with especial reference to the heart of the eel. J Physiol 6:232–245
Milsom WK (2012) New insights into gill chemoreception: receptor distribution and roles in water and air breathing fish. Resp Physiol Neurobiol 184:326–339
Milsom WK, Reid SG, Rantin FT, Sundin L (2002) Extrabranchial chemoreceptors involved in the respiratory reflexes in the neotropical fish Colossoma macropomum (the tambaqui). J Exp Biol 205:1765–1774
Mott JC (1951) Some factors affecting the blood circulation in the common eel (Anguilla anguilla). J Physiol 114:387–398
Mueller CA, Burggren WW, Crossley DA II (2013) ANG II and baroreflex control of heart rate in embryonic chickens (Gallus gallus domesticus). Am J Physiol 305:R855–R863
Nilsson S, Sundin L (1998) Gill blood flow control. Comp Biochem Physiol A 119:137–147
Porteus CS, Pollack K, Tzaneva V, Kwong RWM, Kumai Y, Abdallah SJ, Zaccone G, Lauriano ER, Milsom WK, Perry SF (2015) A role for nitric oxide in the control of breathing in zebrafish (Danio rerio). J Exp Biol 218:3746–3753
Reid SG, Sundin L, Kalinin AL, Rantin FT, Milsom WK (2000) Cardiovascular and respiratory reflexes in the tropical fish, traira (Hoplias malabaricus): CO2/pH chemoresponses. Resp Physiol 120:47–59
Reid SG, Sundin L, Florindo LH, Rantin FT, Milsom WK (2003) Effects of afferent input on the breathing pattern continuum in the tambaqui (Colossoma macropomum). Resp Physiol Neurobiol 36:39–53
Ristori MT (1970) Réflexe de barosensibilité chez um poisson téléostéen (Cyprinus carpio L.). C R Seances Soc Biol Fil 164:1512–1516
Ristori MT, Dessaux G (1970) Sur l’existence d’un gradient de sensibilité dans les récepteurs branchiaux de Cyprinus carpio L. C R Seances Soc Biol Fil 164:1517–1519
Sandblom E, Axelsson M (2005) Baroreflex mediated control of heart rate and vascular capacitance in trout. J Exp Biol 208:821–829
Simões K, Vicentini CA, Orsi AM, Cruz C (2002) Morphological characteristics of the ventricular myocardium of tambaqui (Colossoma macropomum; Characidae, Cuvier, 1818). Braz J Vet Res Anim Sci 39:74–77
Sundin L, Reid SG, Rantin FT, Milsom WK (2000) Branchial receptors and cardiorespiratory reflexes in a neotropical fish, the tambaqui (Colossoma macropomum). J Exp Biol 203:1225–1239
Teixeira MT, Armelin VA, Abe AS, Rantin FT, Florindo LH (2015) Autonomic control of post-air-breathing tachycardia in Clarias gariepinus (Teleostei: Clariidae). J Comp Physiol B 185:669–676
Tirri R, Ripatti P (1982) Inhibitory adrenergic control of heart of perch (Perca fluviatilis) in vitro. Comp Biochem Physiol C 73:399–401
Van Vliet BN, West NH (1994) Phylogenetic trends in the baroreceptor control of arterial blood pressure. Physiol Zool 67:1284–1304
West NH, Van Vliet BN (1994) The role of arterial baroreceptors in the undivided circulation of anuran amphibians. Physiol Zool 67:1305–1324
Zaccone G, Ainis L, Mauceri A, Lo Cascio P, Lo Giudice F, Fasulo S (2003) NANC nerves in the respiratory air sac and branchial vasculature of the Indian catfish, Heteropneustes fossilis. Acta Histochem 105:151–163
Zaccone G, Mauceri A, Fasulo S (2006) Neuropeptides and nitric oxide synthase in the gill and the air-breathing organs of fishes. J Exp Zool A 305:428–439
Zeraik VM, Belão TC, Florindo LH, Kalinin AL, Rantin FT (2013) Branchial O2 chemoreceptors in Nile tilapia Oreochromis niloticus: control of cardiorespiratory function in response to hypoxia. Comp Biochem Physiol A 166:17–25
Acknowledgments
This study was funded by the Brazilian National Council for Scientific and Technological Development (CNPq) and the São Paulo Research Foundation (FAPESP), through the Brazilian National Institute of Science and Technology in Comparative Physiology (INCT—FisC) [No. 08/57712-4]. This research was also supported by the Coordination for the Improvement of Higher Education Personnel (CAPES) [V.A.A. graduate fellowship] and by the Foundation of Support to Research and Extension of São José do Rio Preto (FAPERP) [No. 001/20143]. We would like to thank the INCT-FisC professors for their comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armelin, V.A., Braga, V.H.S., Teixeira, M.T. et al. Gill denervation eliminates the barostatic reflex in a neotropical teleost, the tambaqui (Colossoma macropomum). Fish Physiol Biochem 42, 1213–1224 (2016). https://doi.org/10.1007/s10695-016-0211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0211-9