Abstract
Rhamdia quelen morphophysiological responses to propofol sedation were examined. The purpose was to investigate whether propofol would be a suitable drug to be used in fish transport procedures. Fish were exposed to 0, 0.4 or 0.8 mg L−1 propofol for 1, 6 or 12 h in 40 L tanks, simulating open transport systems. Propofol was able to prevent the peak of cortisol levels experienced by the group exposed to 0 mg L−1 propofol at 1 h. At 0.4 mg L−1, propofol also preserved the stability of hematological (hematocrit, red blood cell count, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration), morphological (red blood cell area), biochemical (cortisol, glucose, lactate, total protein, ammonia, urea, alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase) and hydromineral (Na+, Cl− and K+ plasma levels) indicators of stress. Such results suggest that sedation with propofol at 0.4 mg L−1 is suitable for R. quelen transport.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The transportation segment of the fish farming system undoubtedly requires operational expertise and knowledge on the physiology of the subjects. Buin et al. (2013) stated that mortality occurred not only during transport but also and most importantly after the fish had been delivered to the recipient location. The addition of sedative/anesthetic substances to the water of transport has been employed in an attempt to reduce fish death arisen from transport-mediated stress (Ross et al. 2007; Becker et al. 2012; Benovit et al. 2012). The purpose is to induce a calming or sedative state during the procedure so that the perception of the stressful event is minimized and its side effects reduced (Iwama et al. 1989).
The stress response in fish is an adaptive mechanism characterized by a cascade of physiological alterations that constitute a three-phase pattern (Wendelaar Bonga 1997). Firstly, upon stress, the neuroendocrine system releases catecholamines and corticosteroids into the blood stream. The presence of these stress hormones in the circulatory system precipitates subsequent responses related to energy requirements, such as increases in blood glucose and lactate levels, and variation in plasma electrolytes concentrations, among others. If the second responses are extreme or sustained, the process culminates with whole-animal changes which compromise metabolism, reproductive output and disease resistance and may ultimately lead to mortality (Barton and Iwama 1991; Wendelaar Bonga 1997; Maricchiolo and Genovese 2011).
Despite the purpose of the use of an anesthetic being to mitigate stress, a common report is that the substance itself may pose as a stressor, thus activating the stress response mechanism (Thomas and Robertson 1991; Sladky et al. 2001; Bolasina 2006). According to Zahl et al. (2012), the unwanted side effects of an anesthetic such as respiratory acidosis and osmotic stress reduce the welfare of the fish and, therefore, caution should be taken when such agents are used. Another point to consider is that the efficacy of a given anesthetic depends on variables such as the intensity of the stressor, the fish species, its developmental stage and the environmental conditions (Rotllant et al. 2001; King et al. 2005).
In view of such considerations, the viability of using propofol as a sedative for juvenile silver catfish Rhamdia quelen transport was investigated. This anesthetic has recently been proven effective for immersion anesthesia of the same fish species (Gressler et al. 2012a). The experiment was performed in a laboratory-controlled setting in order to verify the sole effect of the anesthetic upon the physiology of the species through the analyses of hematological, morphological, biochemical and hydromineral indicators of stress.
Methods
Animals
Juvenile gray silver catfish (n = 90; mean ± SE body mass = 91.44 ± 1.98 g; mean ± SE total length = 20.66 ± 0.15 cm) were acquired from a fish farm in Santa Maria city, southern Brazil, and housed at the Laboratório de Fisiologia de Peixes (LAFIPE) at Universidade Federal de Santa Maria (UFSM). Acclimation lasted 7 days and was performed in 250-L tanks (15 fish/tank) in a semi-static system with constantly aerated dechlorinated well water (200 L/tank) at 21.5 ± 0.08 °C, pH 7.45 ± 0.13 and dissolved oxygen 8.04 ± 0.26 mg L−1 (mean ± SE). The water was renewed every second day and the fish were fed commercial pellets for omnivorous fish (42 % extruded crude protein; 4 % fibrous matter; 14 % mineral matter; 2.5 % calcium) once a day. All procedures were conducted with the approval of the Ethics Committee on Animal Experimentation of the UFSM (registration no. 67/2012).
Drug
Propofol (Propotil 1 %; BioChimico; www.biochimico.com.br) was commercially acquired. A pilot study based on the literature-derived values (Gressler et al. 2012a) was performed and two low concentration levels of propofol were established to be used in sedative baths: 0.4 and 0.8 mg L−1. These concentrations were able to induce up to stages 2 and 3a, respectively, as described by Schoettger and Julin (1967).
Experimental design
Twenty-four hours after the last feeding the fish were subjected to one of the following concentrations of propofol: 0 (control), 0.4 or 0.8 mg L−1. Each treatment was further divided into an exposure time of 1, 6 and 12 h, reproducing short, medium and long transport procedures, respectively. For every concentration/time combination, 10 fish were tested (two replicates of five fish each).
The trials were performed in 40-L tanks filled to 50 % of their capacity with the same water used in the acclimation tanks. The proper anesthetic concentration was dispersed in the water if that was the case. The experimental setting simulated transportation in tanks. Nonetheless, in order to guarantee that any observed effect would arise from the anesthetic only, care was taken to prevent common transport interferences such as decline in dissolved oxygen, build-up of ammonia, accumulation of carbon dioxide and reduction in pH from happening. Therefore, loading density was low, constant aeration was provided, and the experiment was carried out under controlled environmental conditions at LAFIPE. Water parameters in the experimental tanks were as follows: 21.8 ± 0.12 °C, dissolved oxygen 6.5 ± 0.24 mg L−1 (Yellow Springs Instruments, Yellow Springs, OH, USA; model Y55), pH 7.4 ± 0.06 (Hanna Instruments, Woonsocket, RI, USA; model HI 8424), total ammonia 0.14 ± 0.02 mg L−1 (Verdouw et al. 1978) and un-ionized ammonia 0.002 ± 0.001 mg L−1 (Colt 2002).
Fish were hand-transferred to the trial tanks and kept under the confined experimental conditions for the assigned period. Once exposure time had elapsed, fish were individually removed from the tanks and a 2 mL blood sample was immediately taken from the caudal peduncle with heparinized sterile syringes. Biometrics was also performed and fish were euthanized by sectioning the spinal cord.
Whole blood analyses
Hematocrit was measured in microcapillary tubes centrifuged at 10,000×g for 10 min and reading was performed with the aid of a hematocrit card reader. Total red blood cells (RBC) count was determined with a Neubauer hemocytometer (Tavares-Dias et al. 2002). The concentration of hemoglobin was assayed by the cyanmethemoglobin method using a spectrophotometer (Brow 1976). The indices mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were calculated according to Wintrobe (1934). Blood smears were prepared and then air-dried, fixed in methanol and stained with May-Grünwald (Rosenfeld 1947). Subsequently, with the aid of an image analyzer microscope, ten high-power fields were randomly selected on each blood smear and morphometry of six RBC was observed in each of these fields (Benfey et al. 1984; Dorafshan et al. 2008). All of the morphometric analyzes were performed using the Zeiss Axio Vision System with Remote Capture 4.7 Rel DC—Cannon Power shot G9.
Plasma analyses
The remaining whole blood was placed into microcentrifuge tubes and spun at 3,000×g for 10 min. The obtained plasma was collected in microtubes and stored at −25 °C for further analyses.
EIA kits (EIAgenTM Cortisol, Adaltis Italy S.p.A) were used to measure cortisol in unextracted plasma samples. Test specificity was assessed through comparison of the parallelism between the standard curve and serial dilutions of the samples in PBS (pH 7.4). The standard curve ran parallel to the one achieved with serial dilutions of R. quelen plasma. A high positive correlation (r 2 = 0.9818) was observed between the curves in the linear regression test. Inter- and intra-assay variation coefficients ranged from 9 to 12 % and 6 to 9 %, respectively. Glucose, total protein, urea, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were analyzed using kits by Analisa (www.goldanalisa.com.br). Analyses of lactate and alkaline phosphatase (AP) were determined in Labtest kits (www.labtest.com.br). Ammonia was quantified according to Verdouw et al. (1978). Concentrations of Na+ and K+ were measured in appropriate diluted samples against known standards using flame photometry (Micronal B262). Chloride levels were assessed via the colorimetric method (Zall et al. 1956).
Statistical analyses
The experimental variables were three propofol concentrations (0, 0.4 and 0.8 mg L−1) and three exposure durations (1, 6 and 12 h). Data are presented as the mean ± standard error (SE). The Bartlett test was used to evaluate normality, and the Levene test was applied to verify homogeneity of variances. Cortisol analyses were made through the Scheirer–Ray–Hare extension of the Kruskal–Wallis test and the Nemenyi test. The remaining parameters were analyzed through a two-way ANOVA and Tukey’s test. The Statistica software 7.0 (Stat Soft. Inc., wwwstatsoft.com) was used to make the analyses, and differences were considered significant at P < 0.05.
Results
Whole blood analyses
At 0.4 mg L−1 propofol, the hematocrit was lower at 6 compared to 12 h, while at 0.8 mg L−1 propofol, the hematocrit was lower at 1 h than at the remaining times. The level of this blood index was lower at 0.4 than at 0 and 0.8 mg L−1 propofol in fish exposed for 6 h. The concentration of hemoglobin was significantly greater within 6 h of exposure to the highest concentration of the anesthetic. Statistical evidence did not identify any effect on RBC, MCV, MCH or MCHC (Table 1). Propofol exposure reduced the nucleus of the cells (Table 2).
Plasma analyses
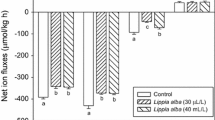
The level of cortisol in control group progressively declined from 1 to 12 h. The hormone concentration increased at 6 and 12 h in comparison with 1 h at 0.4 mg L−1. The level of cortisol was greater at 6 than at 1 h exposure to 0.8 mg L−1 propofol; the group sampled at 12 h showed higher and lower concentrations of the hormone compared to 1 and 6 h, respectively. Cortisol gradually reduced from the highest to the lowest concentration of the anesthetic at 1 h. At 6 h, cortisol was higher at 0.8 than at 0 and 0.4 mg L−1 propofol. The level of the hormone was significantly higher at 0.4 than at 0 and 0.8 mg L−1 propofol in fish exposed for 12 h. Glucose concentration did not vary between groups. The content of lactate was significantly lower at 0.8 mg L−1 propofol after 1 and 6 h of exposure. Protein, ammonia and urea were not significantly affected by the treatments. The activity of AP showed an increase in fish sampled at 12 h after exposure to 0.8 mg L−1 propofol. No statistically significant alterations were observed in ALT or AST (Table 3). At 0 mg L−1 propofol, significantly higher Cl− levels were observed in the group sampled at 12 h compare to 6 h. The level of Na+ increased after exposure to 0.4 mg L−1 propofol for 12 h and decreased at the same sampling time at the highest concentration. There was a significant rise in the level of K+ in fish exposed to 0.8 mg L−1 propofol for 12 h (Fig. 1). Survival during the experiment was 100 %.
Plasma levels of Cl− (a), Na+ (b) and K+(c) in Rhamdia quelen subjected to 0, 0.4 or 0.8 mg L−1 propofol for 1, 6 or 12 h. Values are mean ± SE. Different upper case letters indicate significant difference between times within the same concentration and different lower case letters indicate significant difference between concentrations at a given sampling by two-way ANOVA and Tukey’s test
Discussion
Whole blood analyses
Hematocrit, RBC, hemoglobin, MCV, MCH and MCHC
Propofol has been described as capable of decreasing ventilatory drive as well as cardiac output and contractility in mammals (Grouds et al. 1985; Pagel and Warltier 1993). Nevertheless, the results obtained for the hematological indices show that the anesthetic produced only mild hemodynamic changes in R. quelen. As in the present investigation, Tort et al. (2002), Bressler and Ron (2004) and Filiciotto et al. (2012) noted a decrease in hematocrit percentage as a result of anesthesia. Nevertheless, increased level of this blood index following anesthetic administration is most commonly reported (Thomas and Robertson 1991; Olsen et al. 1995; Gomulka et al. 2008; Sudagara et al. 2009; Maricchiolo and Genovese 2011; Gressler et al. 2012b; Pádua et al. 2012). Elevated hematocrit percentage may occur due to plasma volume reduction, hypoxia and a combination of RBC swelling and/or release by the spleen as a response to acute stress mediated by catecholamines (Davidson et al. 2000; Tort et al. 2002). In this study, in turn, the reduction in hematocrit may have been an adaptive response without major physiological significance especially because RBC number remained unchanged (Franklin et al. 1993).
The values found for hemoglobin concentration at 0.8 mg L−1 propofol after 6 h exposure may indicate that in this group there was a transient requirement for increased blood oxygen-carrying capacity. It was probably achieved by the movement of water from primary to secondary circulation systems, resulting in increased content of hemoglobin (Wells and Weber 1990; Franklin et al. 1993). The capacitance response is a rapid means of preserving oxygen delivery to tissues under hypoxic challenge, which in this case was mostly likely a result of the reduced gill ventilation during sedation or anesthesia, as previously related (Molinero and Gonzalez 1995; Sudagara et al. 2009). Moreover, besides the above-mentioned changes in RBC size or number, which may also be accountable for elevation in hemoglobin, as well as in hematocrit, Speckner et al. (1989) proposed that fish erythrocytes still synthesize hemoglobin while circulating in the peripheral blood. Thus, the increased levels of hemoglobin may reflect enhanced synthesis during low oxygen availability. In line with most of the results observed for hemoglobin in this study, previous works also described no effect of anesthesia on its concentration (Velisek et al. 2005a, b; Pádua et al. 2012).
RBC morphometry
The RBC are the most abundant cells in fish blood and their number and size represent the capacity of oxygen transportation (Fukushima et al. 2012). The RBC area obtained by means of morphometric analyses remained the same throughout the groups, what confirms that the use of propofol did not trigger major changes in the blood oxygen-carrying capacity besides the sole effect seen in the hemoglobin content for one specific experimental group. Morphometry is a valuable and accurate tool for obtaining fish RBC measurements without the interference of other variables, as in the case of MCV. But the literature on it is still scarce; hence, the lack of physiological evidence as to justify the reduction in the RBC nucleus of anaesthetized silver catfish.
Plasma analyses
Cortisol, glucose and lactate
One hour after being subjected to tank transfer, the fish in the control group showed the highest level of cortisol obtained in this investigation, 403.49 ± 48.51 ng mL−1. The 1 h peak is in agreement with responses observed by Wagner et al. (2003), Bolasina (2006), Barcellos et al. (2012) and Koakoski et al. (2012). In comparison with control group, both sedative concentrations of the anesthetic influenced the dynamics of cortisol and prevented the hormone peak. Davis and Griffin (2004), Small (2004) and Gressler et al. (2012b) also observed that administration of anesthetics prevented cortisol increase in fish. In opposition to what was demonstrated by the current results, some authors state that a low concentration may actually act as a stressor because the level of nervous depression does not mitigate certain physiological stress responses (Iwama et al. 1989; Olsen et al. 1995; Maricchiolo and Genovese 2011).
It is well accepted that catecholamines and corticosteroids inhibit glycogen synthesis and stimulate gluconeogenesis in order to fuel homeostatic mechanisms activated during exposure to stressors (Wendelaar Bonga 1997; Sladky et al. 2001). Preceding works observed hyperglycemia along with a rise in cortisol (Davis and Griffin 2004; Maricchiolo and Genovese 2011; Filiciotto et al. 2012), while others did not (Cho and Heath 2000; Iversen et al. 2003; Matsche 2011). Likewise, in the present study, the increased cortisol in control group did not alter carbohydrate metabolism.
Although plasma glucose did not show the classic stress-induced catabolic response, the higher lactate observed at 1 and 6 h at 0 mg L−1 propofol in comparison with the highest concentration of the anesthetic showed the provision of a rapid energetic resource following the handling stress when sedation was not present. However, the low levels of the metabolite suggest that there was an initial activation of anaerobic metabolism contributing to ATP supply, but without deficit in oxygen or glycidic resources. Rotllant et al. (2001) and Small (2004) also described stress-related responses of lactate, what happened along with a rise in glucose. Stressful conditions are typically associated with elevated plasma lactate concentrations, for the anaerobic state caused by stress results in muscle glycogen and lactate breakdown, with some of the lactate being released into circulation (Barton and Iwama 1991). In opposition to the present findings, anesthetics have been accountable for lactate rises (Molinero and Gonzalez 1995; Olsen et al. 1995; Iversen et al. 2003; Wagner et al. 2003). As Iwama et al. (1989) explained, lactate increases in blood when insufficient oxygen is available for aerobic cell metabolism, what could be due to reduced ventilation and circulation, common side effects of several anesthetics.
Total protein, ammonia and urea
Depending on the type of stressor imposed to fish, cortisol may have an effect on protein and amino acids metabolism (Conceição et al. 2012). In the current research, the level of total protein was not affected by the experimental conditions. Barcellos et al. (2003), on the other hand, detected an effect of harvesting on total protein, indicating the possible use of such compound as substrate for the gluconeogenesis observed in the study. Laidley and Leatherland (1988) and Matsche (2011), in turn, registered a significant increase in plasma protein in fish subjected to anesthesia comparing to control; the latter authors indicated this shift as a result of RBC destruction.
Ammonia accounts for the greatest fraction of nitrogenous waste in teleost fish, followed by urea (Kajimura et al. 2004). Both waste products were examined but none presented any difference between the groups, corroborating the observed absence of stress-induced protein utilization in this study.
AP, ALT and AST
Propofol has hepatic as well as extra-hepatic clearance routes in mammals (Mather et al. 1989; Matot et al. 1993). In fish, nonetheless, there are no studies assessing propofol metabolism, but the increased AP within 12 h of exposure to 0.8 mg L−1 propofol may be due to hepatic hyperactivity in order to metabolize the drug. In keeping with the present work, some investigations indicated no changes in AST or ALT following anesthesia (Velisek et al. 2005a, 2009). Barcellos et al. (2003) reported increased AP and AST in stressed R. quelen, indicating these elevations as a result of the regulation of hepatic metabolism promoted by the increased cortisol levels, what was not observed in this study.
Cl−, Na+ and K+
Hydromineral disturbance typically arises from stress in fish (Barton and Iwama 1991; Wendelaar Bonga 1997). At 0 mg L−1 propofol, the concentration of Cl− was significantly higher at 12 compared to 6 h, what may have been due to a shift of Cl− from intracellular to extracellular space (McDonald and Robinson 1993).
Following propofol administration, there were slight changes in Na+ and K+ concentrations which were related to both efflux and influx. Some authors state that anesthesia triggered a decline in Cl− (Davis and Griffin 2004), Cl− and Na+ (Gressler et al. 2012b) and K+ (Davidson et al. 2000) levels. Enhanced respiratory demands may arise from anesthesia, and, along with increased oxygen uptake, there is increased diffusive ion and water movements across the gill (Becker et al. 2012).
Conclusion
The findings of this study suggest that the use of propofol at the concentration of 0.4 mg L−1 is suitable for R. quelen transport. No major or irreversible damage was observed through the evaluated indices, what implies that the anesthetic preserved the physiology of the fish during short-, medium- and longtime exposure.
References
Barcellos LJG, Kreutz LC, Rodrigues LB et al (2003) Haematological and biochemical characteristics of male jundiá (Rhamdia quelen Quoy and Gaimard Pimelodidae) and hormonal and biochemical changes after acute stress. Aquac Res 34:1465–1469
Barcellos LJG, Kreutz LC, Koakoski G, Oliveira TA, Rosa JGS, Fagundes M (2012) Fish age, instead of weight and size, as a determining factor for time course differences in cortisol response to stress. Physiol Behav 107:397–400
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 10:3–26
Becker AG, Parodi TV, Heldwein CG, Zeppenfeld CC, Heinzmann BM, Baldisserotto B (2012) Transportation of silver catfish, Rhamdia quelen, in water with eugenol and the essential oil of Lippia alba. Fish Physiol Biochem 38:789–796
Benfey TJ, Sutterlin AM, Thompson RJ (1984) Use of erythrocyte measurements to identify triploid salmonids. Can J Fish Aquat Sci 41:980–984
Benovit SC, Gressler LT, Silva LL, Garcia LO, Okamoto MH, Pedron JS, Sampaio LA, Rodrigues RV (2012) Anesthesia and transport of Brazilian Flounder, Paralichthys orbignyanus, with essential oils of Aloysia gratissima and Ocimum gratissimum. J World Aquacult Soc 43:896–900
Bolasina SN (2006) Cortisol and hematological response in Brazilian codling, Urophycis brasiliensis (Pisces, Phycidae) subjected to anesthetic treatment. Aquacult Int 14:569–575
Bressler K, Ron B (2004) Effect of anesthetics on stress and the innate immune system of gilthead seabream (Sparus aurata). Isr J Aquac 56:5–13
Brow BA (1976) Hematology: principles and procedures. Lea and Febiger, Philadelphia
Buin TM, Phuong NT, Nguyen GH, De Silva SS (2013) Fry and fingerling transportation in the striped catfish, Pangasianodon hypophthalmus, farming sector, Mekong Delta, Vietnam: a pivotal link in the production chain. Aquaculture 388–391:70–75
Cho GK, Heath DD (2000) Comparison of tricaine methanesulphonate (MS-222) and clove oil anaesthesia effects on the physiology of juvenile chinook salmon Oncorhynchus tshawytscha (Walbaum). Aquac Res 31:537–546
Colt J (2002) List of spreadsheets prepared as a complement. In: Wedemeyer GA (ed) Fish hatchery management, 2nd ed. American Fish Society Publication. http://www.fisheries.org/afs/hatchery.html. Accessed 22 July 2013
Conceição LEC, Aragão C, Dias J, Costas B, Terova G, Martins C, Tort L (2012) Dietary nitrogen and fish welfare. Fish Physiol Biochem 38:119–141
Davidson GW, Davie PS, Young G, Fowler RT (2000) Physiological responses of rainbow trout Oncorhynchus mykiss to crowding and anesthesia with Aqui-S. J World Aquacult Soc 31:105–114
Davis KB, Griffin BR (2004) Physiological responses of hybrid striped bass under sedation by several anaesthetics. Aquaculture 233:531–548
Dorafshan S, Kalbassi MR, Pourkazemi M, Amiri B, Karimi S (2008) Effects of triploidy on the Caspian salmon Salmo trutta caspius haematology. Fish Physiol Biochem 34:195–200
Filiciotto F, Buscaino G, Buffa G, Bellante A, Maccarrone V, Mazzola S (2012) Anaesthetic qualities of eugenol and 2-phenoxyethanol and their effect on some haematological parameters in farmed European sea bass (Dicentrarchus labrax L.). J Anim Vet Adv 11:494–502
Franklin CE, Davison W, Mckenzie JC (1993) The role of the spleen during exercise in the Antarctic teleost, Pagothenia borchgrevinki. J Exp Biol 174:381–386
Fukushima H, Bailone RL, Weiss LA, Martins ML, Zaniboni-Filho E (2012) Triploidy in the hematology of jundia juveniles (Siluriformes: Heptapteridae). Braz J Biol 72:147–151
Gomulka P, Wlasow T, Velisek J, Svobodova Z, Chmielinska E (2008) Effects of eugenol and MS-222 anaesthesia on Siberian sturgeon Acipenser baerii Brandt. Acta Vet Brno 77:447–453
Gressler LT, Parodi TV, Riffel APK, da Costa ST, Baldisserotto B (2012a) Immersion anaesthesia with tricaine methanesulfonate or propofol on different sizes and strains of Rhamdia quelen. J Fish Biol 81:1436–1445
Gressler LT, Riffel APK, Parodi TV et al (2012b) Rhamdia quelen immersion anaesthesia with essential oil of Aloysia triphylla (L’Hérit) Britton or tricaine methanesulfonate: effect on stress response and antioxidant status. Aquac Res 45:1061–1072
Grouds RM, Morgan M, Lumley J (1985) Some studies on the properties of the intravenous anaesthetic, propofol (Diprivan): a review. Postgrad Med J 61:90–95
Iversen M, Finstad B, McKinley RS, Eliassen RA (2003) The efficacy of metomidate, clove oil, Aqui-S and Benzoak as anaesthetics in Atlantic salmon (Salmo salar L.) smolts, and their potential stress-reducing capacity. Aquaculture 221:549–566
Iwama GK, McGeer JC, Pawluk MP (1989) The effects of five fish anesthetics on acid-base balance, hematocrit, blood gases, cortisol and adrenaline in rainbow trout. Can J Zool 67:2065–2073
Kajimura M, Croke SJ, Glover CN, Wood CM (2004) Dogmas and controversies in the handling of nitrogenous wastes: the effect of feeding and fasting on the excretion of ammonia, urea and other nitrogenous waste products in rainbow trout. J Exp Biol 207:1993–2002
King WV, Hooper B, Hillsgrove S, Benton C, Berlinsky D (2005) The use of clove oil, metomidate, tricaine, methanesulphonate and 2-phenoxyethanol for inducing anaesthesia and their effect on the cortisol stress response in black sea bass (Centropristis striata L.). Aquac Res 36:1442–1449
Koakoski G, Oliveira TA, Rosa JGS, Fagundes M, Kreutz LC, Barcellos LJG (2012) Divergent time course of cortisol response to stress in fish of different ages. Physiol Behav 106:129–132
Laidley CW, Leatherland JF (1988) Cohort sampling, anaesthesia and stocking-density effects on plasma cortisol, thyroid hormone, metabolite and ion levels in rainbow trout, Salmo gairdneri Richardson. J Fish Biol 33:73–88
Maricchiolo G, Genovese L (2011) Some contributions to knowledge of stress response in innovative species with particular focus on the use of the anaesthetics. Open Mar Biol J 5:24–33
Mather LE, Selby DG, Runciman WB, McLean CF (1989) Propofol: assay and regional mass balance in the sheep. Xenobiotica 19:1337–1347
Matot I, Neely CF, Katz RY, Neufeld GR (1993) Pulmonary uptake of propofol in cats. Anethesiology 78:1157–1165
Matsche MA (2011) Evaluation of tricaine methanesulfonate (MS-222) as a surgical anesthetic for Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus. J Appl Ichthyol 27:600–610
McDonald DG, Robinson JG (1993) Physiological responses of lake trout to stress: effects of water hardness and genotype. Trans Am Fish Soc 122:1146–1155
Molinero A, Gonzalez J (1995) Comparative effects of MS 222 and 2-phenoxyethanol on gilthead sea bream (Sparus aurata L.) during confinement. Comp Biochem Physiol 3:405–414
Olsen YA, Einarsdottir IE, Nilssen KJ (1995) Metomidate anaesthesia in Atlantic salmon, Salmo salar, prevents plasma cortisol increase during stress. Aquaculture 134:155–168
Pádua SB, Ventura AS, Satake F, Ishikawa MM, Hisano H, Rotta MA, Arantes FC (2012) Respostas hematológicas em tuvira após anestesia com diferentes concentrações de óleo de cravo. Bol Inst Pesca 38:181–188
Pagel PS, Warltier DC (1993) Negative inotropic effects of propofol as evaluated by the regional preload recruitable stroke work relationship in chronically instrumented dogs. Anesthesiology 78:100–108
Rosenfeld G (1947) Corante pancrômico para hematologia e citologia clínica. Nova combinação dos componentes do May-Grünwald e do Giemsa num só corante de emprego rápido. Mem Inst Butantan 20:329–334
Ross LG, Blanco JS, Martinez-Palacios C, Racotta IE, Cuevas MT (2007) Anaesthesia, sedation and transportation of juvenile Menidia estor (Jordan) using benzocaine and hypothermia. Aquac Res 38:909–917
Rotllant J, Balm PH, Perez-Sanchez J, Wendelaar-Bonga SE, Tort L (2001) Pituitary and interrenal function in gilthead sea bream (Sparus aurata L., Teleostei) after handling and confinement stress. Gen Comp Endocrinol 121:333–342
Schoettger RA, Julin AM (1967) Efficacy of MS-222 as an anaesthetic on four salmonids. Invest Fish Con US Dept Int 13:1–15
Sladky KK, Swanson CR, Stoskopf MK, Loomis MR, Lewbart GA (2001) Comparative efficacy of tricaine methanesulfonate and clove oil for use as anesthetics in red pacu (Piaractus brachypomus). AJVR 62:337–342
Small BC (2004) Effect of isoeugenol sedation on plasma cortisol, glucose, and lactate dynamics in channel catfish Ictalurus punctatus exposed to three stressors. Aquaculture 238:469–481
Speckner W, Schindlerand JF, Albers C (1989) Age-dependent changes in volume and haemoglobin content of erythrocytes in the carp (Cyprinus Carpio L.). J Exp Biol 141:133–149
Sudagara M, Mohammadizarejabada A, Mazandarania R, Pooralimotlagha S (2009) The efficacy of clove powder as an anesthetic and its effects on hematological parameters on roach (Rutilus rutilus). J Aqua Feed Sci Nutr 1:1–5
Tavares-Dias M, Melo JFB, Moraes G, Moraes FR (2002) Características hematológicas de teleósteos brasileiros: VI. Variáveis do jundiá Rhamdia quelen (Pimelodidae). Ciência Rural 32:693–698
Thomas P, Robertson L (1991) Plasma cortisol and glucose stress responses of red drum (Sciaenops ocellatus) to handling and shallow water stressors and anesthesia with MS-222, quinaldine sulfate and metomidate. Aquaculture (Amsterdam) 1:69–86
Tort L, Puigcerver M, Crespo S, Padrós F (2002) Cortisol and haematological response in sea bream and trout subjected to the anaesthetics clove oil and 2-phenoxyethanol. Aquac Res 33:907–910
Velisek J, Svobodova Z, Piackova V, Groch L, Nepejchalova L (2005a) Effects of clove oil anaesthesia on common carp (Cyprinus carpio L.). Vet Med Czech 50:269–275
Velisek J, Svobodova Z, Piackov V (2005b) Effects of clove oil anaesthesia on rainbow trout (Oncorhynchus mykiss). Acta Vet Brno 74:139–146
Velisek J, Stejskal V, Kouril J, Svobodova A (2009) Comparison of the effects of four anaesthetics on biochemical blood profiles of perch. Aquac Res 40:354–361
Verdouw H, Van Echaematocriteld CJA, Dekkers EMJ (1978) Ammonia determination based on indophenols formation with sodium salicylate. Water Res 12:399–402
Wagner GN, Singer TD, Mckinley RS (2003) The ability of clove oil and MS-222 to minimize handling stress in rainbow trout (Oncorhynchus mykiss Walbaum). Aquac Res 34:1139–1146
Wells RMG, Weber RE (1990) The spleen in hypoxic and exercised rainbow trout. J Exp Biol 150:461–466
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Wintrobe MM (1934) Variations in the size and hemoglobin content of erythrocytes in the blood of various vertebrates. Folia Haematol 51:32–49
Zahl IH, Samuelsen OB, Kiessling A (2012) Anaesthesia of farmed fish: implications for welfare. Fish Physiol Biochem 38:201–218
Zall DM, Fisher MD, Garner QM (1956) Photometric determination of chlorides in water. Anal Chem 28:1665–1678
Acknowledgments
The authors thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for research fellowship to B. Baldisserotto and FAPERGS (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul) for graduate fellowship to L. Gressler.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gressler, L.T., Sutili, F.J., da Costa, S.T. et al. Hematological, morphological, biochemical and hydromineral responses in Rhamdia quelen sedated with propofol. Fish Physiol Biochem 41, 463–472 (2015). https://doi.org/10.1007/s10695-014-9997-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-9997-5