Results are given for a study of the catalytic and physicochemical properties of monometallic Cu/CeO2-Al2O3 and bimetallic Pd-Cu/CeO2-Al2O3 catalysts for the synthesis of methanol from hydrogen and carbon dioxide. The catalytic activity was determined in methanol synthesis in a gradientless reactor at 200°C and 3.5 MPa. The physicochemical properties of these catalytic systems were studied by the BET method (Brunauer−Emmett− Teller method), temperature-programmed reduction of hydrogen (TPR-H2), temperature-programmed desorption of ammonia (TPD-NH3), x-ray diffraction (XRD), and Fourier-transform IR spectroscopy (FTIR). Our results showed high activity for the palladium-promoted catalyst, which is attributed to a synergistic effect between palladium and copper as well as the formation of a PdCu alloy during activation of this bimetallic catalyst in a reducing atmosphere of 5% H2 95% Ar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Methanol is a key component for the production of various chemicals needed for the production of many everyday items [1]. Significant amounts of methanol are used in the production of formaldehyde and acetic acid as well as a methylating agent in the production of such important products as dimethyl terephthalate and methyl methacrylate. These compounds are commonly used for the production of synthetic fibres. Acetic acid, for example, is used in large-scale amounts in the production of vinyl acetate, which is a raw material for the preparation of polymers, and as a solvent in the synthesis of terephthalic acid, which is a starting component in the production of polyethylene terephthalate for bottles and synthetic fibres, Dacron and nylon.
In addition to its use in the manufacture of these products, methanol is also used in the production of alkenes such as ethylene and propylene with sufficient purity for polymerization. The demand for these products worldwide is growing constantly.
In industry, methanol may be obtained in the direct reaction of carbon monoxide with hydrogen [2]:
or by the hydrogenation of carbon dioxide:
For many years, the process [2] was carried out using copper catalysts developed in the 1960’s. Copper supported on the mixed oxide ZnO/Al-2O3 has been usually employed for such purposes [3]. The synthesis of methanol is carried out at relatively high temperature and high pressure (200-300°C, 5-10 MPa) and thus, new catalytic systems are being developed with suitable selectivity and activity at lower temperatures and pressures in order to reduce energy expenditures. The literature data indicate that copper catalysts supported on zirconium oxide display high activity in the hydrogenation of CO2 to give methanol. Copper catalysts supported on zirconium oxide have high thermal and mechanical stability. Modification of the copper catalyst support by cerium oxide also improves the dispersion and reducibility of the copper component [4]. Catalysts used for methanol synthesis are promoted not only by metal oxides but also by noble metals.
The scientific literature concerning methanol synthesis features many studies concerning the action of noble metals (Pd, Pt, Au, and Ag) on the catalytic action of copper catalysts. Palladium is the most suitable promoter of these metals by virtue of its enhancement of the catalytic activity and selectivity of copper catalysts [5,6,7,8,9]. Our previous studies have also confirmed the promoting action of palladium and CeO2 to enhance the activity of copper catalysts in methanol synthesis [10].
Hence, we prepared monometallic Cu/CeO2-Al2O3 and bimetallic Pd-Cu/CeO2-Al2O catalysts by wet impregnation. The physicochemical properties of these catalysts were studied using BET, TPR-H2, TPD-NH3, XRD, and FTIR. The physicochemical properties determined were related to the results found for catalytic activity in the hydrogenation of CO2.
Cerium nitrate and aluminum nitrate were used to prepare the supports. The catalyst systems were obtained by coprecipitation of the corresponding hydroxides using ammonia as the oxidizing agent. Then, the mixture was dried at 100°C for 24 h and roasted for 4 h at 400°C in the air. Corresponding amounts of copper (20 or 40%) and palladium (2%) were deposited on the prepared supports by wet impregnation.
The catalytic activity was studied in a gradientless reaction at 220°C and 3.5 MPa after stabilization of the catalyst over 24 h.
The specific surface of the catalysts was determined by low-temperature nitrogen adsorption using an automatic Sorptomatic 1900 apparatus. The TPR-H2 measurements were carried out using an automated AMI-1 system in the temperature range from 25 to 900°C with linear heating rate of 10 deg/min. The 0.1-g samples were reduced in an atmosphere of 5% H2 and 95% argon with volumetric flow rate 40 cm3/min. The use of hydrogen was recorded using a thermal conduction detector (TCD).
The procedure for measurement of the surface acidity of the catalysts involved the following steps: 1) purification of the catalyst surface, 2) ammonia adsorption, and 3) temperature-programmed ammonia desorption. Water was removed from the system by flushing the sample with a stream of pure argon at flow rate 40 cm3/min at 600°C. Then, the sample was cooled to 100°C and saturated with gaseous ammonia over 0.5 h. After ammonia adsorption, the excess of looselybound, physically-adsorbed ammonia on the catalyst surface was removed by flushing the catalyst with a stream of pure argon. The temperature-programmed desorption of ammonia was carried out at from 100 to 600°C with linear heating rate of 25 deg/min. The TPD-NH3 curves were recorded using a thermal conduction detector.
The diffraction curves were recorded using CuKα radiation on a PANalytical X’Pert Pro diffractometer in the 2θ range from 5 to 90° with steps of 0.167° and Δt 10 sec. Since the initial diffraction data may contain interference, the background during the analysis was recorded using the Sonneveld-Visser algorithm. All the calculations were carried out using the X’Pert HighScore Plus computer program.
The IR spectra were taken on a Shimadzu IRTracer-100 equipped with a mercury cadmium telluride detector. Prior to the analysis, the catalysts were reduced at 300°C in an atmosphere of 5% H2 and 95% argon for 2 h. After reduction, the samples were cooled to 220°C and the gas stream was switched over to the reaction mixture of hydrogen and CO2 (3:1 mole ratio).
The study of the catalyst activity in methanol synthesis was carried out at elevated pressure (3.5 MPa) and 220°C. The reaction products were analyzed using a gas chromatograph. Prior to testing the activity, all the catalysts were first reduced for 2 h in a gas mixture of 5% H2 and 95% argon at 300°C and atmospheric pressure. The catalytic activity was measured after initial stabilization for 24 h.
Measurements of the specific surface of these systems showed that an increase in the metal content leads to a decrease in the specific surface from 128 m2/g for 20% Cu/CeO2-Al2O3 to 63 m2/g for 40% Cu/CeO2-Al2O3. Palladium promotion of the catalyst also leads to a slight decrease in the specific surface relative to the monometallic catalyst containing the same amount of cupric oxide. These results of the BET analysis may be attributed to blockage of the pores of the mixed support by CuO and PdO phases.
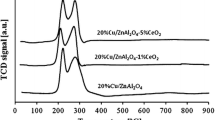
The readiness of these catalysts to undergo reduction was studied by TPR-H2. The reduction curves for these systems show a multistep reduction mechanism in the temperature range studied. Thus, the hydrogen absorption curve for the mixed CeO2-Al2O3 oxide showed two effects: 1) at 400°C related to reduction of surface CeO2 species to give nonstoichiometric CeO2−x oxides (0 < x < 1) and 2) at 850-890°C related to reduction of CeO2 to give Ce2O3 in the crystal bulk. The TPR curves for the copper catalysts show two overlapping reduction peaks at 150-400°C, which may result from the reduction of small and large CuO crystals.
The hydrogen absorption curves for the bimetallic catalysts shown in Fig. 1 display five reduction effects. The first effect is related to the reduction of PdO with maximum hydrogen absorption at 130°C. The next two effects are related to the reduction of small and large cupric oxide crystals. The remaining two effects of the TPR curves are related to the reduction of CeO2 according to the scheme discussed above. We should note that the observed reduction effects of cupric oxide are shifted toward lower temperatures, which confirms the appearance of a spillover effectFootnote 1 between palladium and CuO.
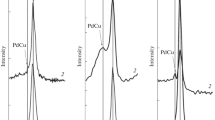
We studied the phase composition of the monometallic (Cu) and bimetallic (Pd-Cu) catalysts supported on the mixed CeO2-Al2O3 oxide in order to elucidate the interaction between the active phase of the catalyst and the support. The XRD results display reflections characteristic for crystalline Cu and CeO2 phases (Fig. 2). Comparison of the diffractograms of the copper and palladium-copper catalysts reveals broadening of the diffraction peak at 2θ = 42.5°, indicating formation of a PdCu alloy. The alloying of palladium and copper was observed for the samples reduced both at 300°C and at 900°C. This finding implies that the high activity and selectivity of the palladium-promoted catalysts in methanol synthesis is due to the formation of an alloy upon activation of the catalyst (reduction at 300°C) prior to the test reactions.
The acidity of the systems studied was determine by the temperature-programmed desorption of ammonia. Table 1 and Fig. 3 show that all the catalysts have a low content of strongly acidic sites. The total acidity of these catalyst systems decreases with increasing content of copper. Palladium promotion of the catalyst also leads to a slight drop in total acidity.
The surface species formed on separate components of the catalyst during methanol synthesis were identified using IR spectroscopy in a study to explain the differences in catalytic activity between the copper catalysts. The IR spectroscopic study results are given in Fig. 4.
The spectrum for CeO2 shows cerium formates (b-HCO2-Ce: 1335, 1520, and 2958 cm−1 and m-HCO2-Ce: 2867 cm−1) as well as methoxy groups (1065, 2824 cm−1).
The spectrum obtained for CeO2 .Al2O3 shows additional absorption bands at 1570 and 2856 cm−1 related to the corresponding formate groups (b-HCO2-Al). In turn, the addition of copper leads to the appearance of a band at 2928 cm−1 corresponding to the b-HCO2-Cu group.
Furthermore, there is an increase in intensity of the bands in the range extending from 1065 cm−1 belonging to methoxy groups CH3O-Ce, which is evidence for a synergistic effect between the components of the support and the promoting metal.
The catalytic activity given in Table 2 is expressed as the amount of product formed on a mass unit of catalyst in a unit of time (X, g CH3OH/gcat·h) and was calculated using the following formula
where V CO2 is the CO2 flow rate, cm3/min, M met is the molar mass of methanol, g/mole, M cat is the catalyst mass, and MV is the molar gas volume under normal conditions, 22,400 cm3/mole.
This study of the catalytic activity of monometallic catalyst systems showed that the catalyst containing 20% copper is more active than the catalyst containing 40% copper. Furthermore, experiments carried out for monometallic (20% Cu/CeO2 .Al2O3) and bimetallic catalysts (2% Pd-20% Cu/CeO2-Al2O3) confirmed the promoting effect of palladium on the CO2 conversion and the catalytic activity in methanol synthesis. The improved activity of the bimetallic catalyst may be attributed to a spillover effect between palladium and CuO.
This work was carried out in the framework of Project 0305/IP2/2015/73.
Notes
The spillover effect is displacement of the excited hydrogen atom from the metal surface to the catalyst surface.
References
T. Schultz, S. Zhou, and K. Sundmacher, Chem. Eng. and Technol., 24, No. 12, 1223-1233 (2001).
S. Lee, J. G. Speight, and S. K. Loyalka, Handbook of Alternative Fuel Technologies, CRC Press, Boca Raton (2014), p. 331.
C. H. Bartholomew and J. F. Farrauto, Fundamentals of Industrial Catalytic Processes, Wiley Interscience, New York (2006).
C. Li, Y. Sakata, et al., J. Chem. Soc., Faraday Trans., 85, No. 6, 1451-1461 (1989).
J. Kugai, J. T. Miller, et al., J. Catal., 277, 46-53 (2011).
C. C. Chang, C. C. Hsu, et al., Int. J. Hydrogen Energy, 37, No. 37, 11176-11184 (2012).
T. P. Maniecki, P. Mierczynski, et al., Catal. Lett., 130, 481-488 (2009).
P. Mierczynski, T. P. Maniecki, et al., React. Kinet. Mech. Cat., 104, 139-148 (2011).
J. Sloczynski, R. Grabowski, et al., Appl. Catal. A, 278, 11-23 (2004).
P. Mierczynski, W. Maniukiewicz, et al., React. Kinet., Mech. Cat., 114, No. 1, 211-228 (2015).
Author information
Authors and Affiliations
Additional information
Translated from Khimicheskie Volokna, Vol. 48, No. 4, pp. 12−16, July−August, 2016.
Rights and permissions
About this article
Cite this article
Mierczynski, P., Ciesielski, R., Kedziora, A. et al. Methanol Synthesis Using Copper Catalysts Supported on CeO2−Al2O3 Mixed Oxide. Fibre Chem 48, 271–275 (2016). https://doi.org/10.1007/s10692-017-9782-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10692-017-9782-1