Abstract
The ‘Menglina Leddy’ lily cultivar was selected from the Lilium longiflorum Thunb. ‘White Fox’ γ-rays irradiation line. It produces much less pollen than ‘White Fox’ but has similar morphology traits. In order to reveal the effects of gamma irradiations on the chromosomes, mitosis, and meiosis in ‘Menglina Leddy’ cells were investigated by fluorescence in situ hybridization using rDNA and telomeric repeat probes. Although both ‘Menglina Leddy’ and ‘White Fox’ had 24 chromosomes, a considerable amount of chromosomal breaking and rejoining were detected in the former. A super long and two super small chromosomes appeared in all the ‘Menglina Leddy’ cells. Meiotic abnormalities occurred at each separation stage. Chromosomes pairing configuration showed that complex recombination had happened in ‘Menglina Leddy’. The super long chromosome was a Robertsonian translocation product composed of two non-homologous long arms. The chromosome deletions and recombinations did not affect the main ornamental traits, but allowed it to acquire the characteristic of less pollen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As early as the 1920s, it was found that high mutant frequencies can be induced by radiation in Drosophila melanogaster and barley (Stadler 1928; Muller 1927). Over the past century, this technology has been extensively used to analyze gene functioning and improving cereal, fruit, and other crop cultivars (Ma et al. 2021; Khah and Verma 2017; Dou et al. 2003; Lee et al. 2002). Besides causing gross chromosomal variations, radiation also causes point mutations in the individual genes. These mutations increase in genetic variability in the segregating generations, which greatly enhances the scope for selection. Induced mutagenesis, a well-known method that can effectively improve the genetic architecture of ornamental plants, could be used as a supplementary or complementary aid when attempting to impart desirable characteristics into different species of the plant kingdom (Kolar et al. 2013; Basi et al. 2006; Yang 1998; Tiwari et al. 2016), such as lily (Xi et al. 2012; Crouse 1961).
The identification and selection of mutants play important roles in plant mutation breeding. The γ-rays irradiation often causes chromosomal mutagenesis, such as chromosome stickiness, univalent, multivalent, laggards, bridges, micronuclei, and other variations. Chromosomes are the genetic materials carrier, which means that any variations in the chromosomes will inevitably lead to genetic mutation (Han et al. 2017). Elucidating the meiotic and mitotic behavior of chromosomes is critical to check the viability and stability of the mutants (Kiihl et al. 2011). Cytogenetic techniques have been used to identify the chromosome compositions of mutants induced by radiation (Zaman and Rai 1977). However, cytological methods are time-consuming and it is difficult to locate genes in fine detail; therefore they are mainly used for the identification of interesting traits or individual plants (Dai et al. 2020; Zhao et al. 2016; Dou et al. 2003). Cytogenetic techniques including chromosomes banding, FISH and genomic in situ hybridization (GISH) have been extensively used in ploidy level determination (Liu et al. 2017; Stewart 1943; Beal 1942), interspecific hybrids distinguishing and genetic relationships deducing (Khah and Verma 2017; Hwang et al. 2011; Zhou et al. 2008a,b; Barba-Gonzalez et al. 2005a,b; Marasek et al. 2004a,b; Lim et al. 2003, 2001, 2000; Ahn et al. 2017). However, most of these techniques have not been used to analyze lily irradiation mutants. The few relevant studies have only reported simple descriptions of abnormal chromosome behaviors during meiosis (Lu et al. 2002; Crouse 1961). The lack of cytological data hinders the further application of these mutants in breeding.
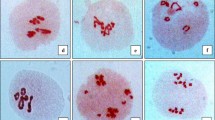
Lily plants generally produce large amounts of oily pollen grains which easily smudge the perianths and the surrounding environment. Therefore, a pollenless lily cultivar is an attractive option for both breeders and consumers and much effort has been expended during the past three decades to develop a pollenless lily (Zhang et al. 2018; Yamagishi 2003; Grassotti and Mercuri 1996). However, most of the ornamental lily varieties on the market are pollen-rich and there are almost no pollenless lily varieties except several double flower lily cultivars. Lilium longiflorum, which originates in Japan and Taiwan, has pure white trumpet-shaped flowers. Its flowers not only have a distinctive fragrance, but also are available all year round. However, L. longiflorum has a major defect: its flowers produce considerable numbers of yellow pollen grains and these grains contain large amounts of pigment, which has strong pigmenting properties and contaminates perianths and clothes (Yamagishi 2003). ‘Menglina Leddy’ was selected from the γ-rays irradiation lines of L. longiflorum Thunb. cv. White fox by our team in 2013. ‘Menglina Leddy’ has similar traits to ‘White Fox’, but produces considerably less pollen and the white perianths are hardly polluted after the anther cracks (Fig. 1A, B) (Xi et al. 2012). None of the ‘Menglina Leddy’ pollens germinated during in-vitro culture and there was a large number of deformed pollen grains (Fig. 1C, D). However, nothing is known about the changes in ‘Menglina Leddy’ at the cytological level.
Lilium longiflorum Thunb. White Fox and ‘Menglina Leddy’ flowers and their pollen germination tests. A ‘White Fox’ flower; B ‘Menglina Leddy’ flower; C ‘White Fox’ pollen grains were cultured in liquid medium for 6 h and mainly germinated; D ‘Menglina Leddy’ pollen grains were cultured in liquid medium for 24 h. Bars = 200 µm
The objective of this study was to reveal the changes in ‘Menglina Leddy’ at the cytological level. Therefore, we analyzed the karyotype and meiotic chromosome behaviors in ‘Menglina Leddy’. Surprisingly, although extensive chromosome structural changes to the chromosomes were observed in ‘Menglina Leddy’, the total chromosome total number was still 24, and chromosomes 1 and 2, which are the longest and only two metacentric chromosomes in the ‘White fox’ genome, retained their structure in ‘Menglina Leddy’.
Material and methods
Plant materials
‘White Fox’ and ‘Menglina Leddy’ bulblets were planted in the greenhouse and also maintained by tissue culture at Nanjing Forestry University, Nanjing, China.
Mitotic chromosome preparation
The root tips of ‘White Fox’ and ‘Menglina Leddy’ were harvested from hundreds tissue culture bulblets, pretreated with 1.4 mM cycloheximide at room temperature (25 °C) for 8 h, and fixed in Carnoy’s fixative (ethanol: acetic acid = 3:1, v/v). Mitotic chromosomes were prepared using the smearing method according to Lan et al. (2018), the slides were screened under a phase contrast microscope, and the well-spread mitotic chromosome preparations were selected for FISH.
Meiotic chromosome preparation
Young anthers were squashed in a drop of 2% acetocarmine to determine whether they were at the appropriate meiotic stage. Anthers at the appropriate meiotic stage were fixed in freshly prepared Carnoy’s fixative for 12 h at room temperature. The fixed anthers were washed with distilled water and each anther was cut into 6–7 segments. These were then transferred to a 1.5 mL centrifugal tube containing 100 μL 45% acetic acid and mix pipetted about 10 times to release the pollen mother cells (PMC). Then, 8 μL of the PMC mixture was added to a slide, which was covered with a slip, placed on a heater for 10 s at 55 ℃, and the cover slip was squashed. The slide was then frozen in liquid nitrogen for 30 s. Following this, the cover slip was immediately removed using a blade and the sample was dehydrated in absolute ethanol for 5 min, air dried, and stored at -20 ℃ until needed.
Probes preparation
Two oligonucleotide (oligo) probes were used to detect the 5S rDNA and 45S rDNA according to Liu et al. (2022). The 5S and 45S rDNA probes were modified at their both ends with FAM (6-carboxyfluorescein) and TAMRA (6-carboxytetramethylrhodamine), respectively. A plasmid containing (TTTAGGG)n sequences was kindly provided by Professor Jiming Jiang (Michigan State University, East Lansing, MI, USA) and labelled with biotin-16-dUTP using nick translation to mark the chromosome telomeres.
FISH
The FISH procedures were performed according to a published protocol (Jiang et al. 1995) with minor modifications based on Lan et al. (2018). Briefly, a reaction volume of 20 μL per slide contained 40 ng of each probe DNA. The hybridization mixture was denatured at 98 °C for 10 min, immersed immediately in ice for 5 min before use. The chromosomes were denatured in 70% formamide for 5 min at 85 °C, dehydrated in a pre-chilled (− 20 °C) ethanol dilution series (70%, 95%, 100%; 3 min each), and air dried. After hybridization biotin-16-dUTP labeled probe was detected with Fluorescein Anti-Biotin (Fluorescein Anti-Biotin, SP3040, Vector laboratories, Newark, CAL, USA). Hybridization signals were observed using a fluorescence microscope (BX51; Olympus, Tokyo, Japan) and images were acquired using an attached CCD camera. Grayscale images were captured for each color channel and then merged together. The image contrast was processed using Adobe Photoshop 5.0 (Adobe Systems, http://www.adobe.com). A total of 10 well spread metaphase cells were used for karyotyping.
Results
Comparative karyotyping of ‘White Fox’ and ‘Menglina Leddy’
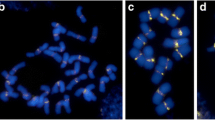
‘White Fox’ is a diploid cultivar with 24 chromosomes. These 24 chromosomes consist of 12 pairs of homologous chromosomes and the chromosomes in each pair are similar in size, arm ratios, and rDNA loci (Fig. 2A1-A3). Among them, chromosomes 1 and 2 are metacentric chromosomes, whereas the others are acrocentric or telocentric chromosomes. Chromosome 4 is a satellite chromosome and the secondary constriction is located on the long arm (Fig. 2A1 and A2). Four of the six 45S rDNA loci are located on the centromeric regions of three pairs of chromosomes: chromosomes 3, 4, and 11 whereas the other two are located in the secondary constriction regions of chromosome 4. The 5S rDNA loci are located on the long arms of chromosome 4 near the 45S rDNA (Fig. 2A1 and A3).
Comparation between the ‘White Fox’ and ‘Menglina Leddy’ karyotypes. Red signals show the 45S rDNA locations; whereas green signals show the 5S rDNA locations and chromosome telomeres. A1-A3: ‘White Fox’ karyotype. A1 and A2 were the same metaphase cell and the white arrows in A1 and A2 indicate the secondary constrictions. B1–B3: ‘Menglina Leddy’ karyotype. B1 and B2 were the same metaphase cell. In B1 and B2, the red arrows indicate the super long chromosome; the white arrows indicate the chromosome with both 5S and 45S rDNA loci; the yellow arrows indicate the two chromosome 1; the green arrows indicate two 45S rDNA bearing chromosomes that are different in size; the two orange arrows indicate the very small chromosomes with distinct telomeric signals. SL: super long chromosome, and S: two small chromosomes. Bars = 10 µm
The karyotype analysis showed that there were also 24 chromosomes in ‘Menglina Leddy’ (Fig. 2B1 and B2). Most of the ‘Menglina Leddy’ chromosomes had considerably changed in size and structure compared to ‘White Fox’ and were difficult to identify (Fig. 2B1–B3). Telomere and rDNA probes were used to analyze the ‘Menglina Leddy’ chromosomes. The 5S rDNA and telomere signals were both green, but the former was much stronger than the latter (Fig. 2B1 and B3). The telomere signals showed that there were 24 chromosomes in ‘Menglina Leddy’ (Fig. 2B1 and B2). However only five of the 24 chromosomes could be identified based on chromosome length, arm ratio, and rDNA locations. These were a chromosome 1 pair, a chromosome 2 pair, and one chromosome 3 (Fig. 2B3). There was a super long chromosome (Red arrow marked in Fig. 2B1 and B2) that was about 1.5 times longer than chromosome 1 (Yellow arrows indicated in Fig. 2B1 and B2). It was metacentric chromosome with a 45S rDNA locus on the centromere. One chromosome with both 5S and 45S rDNA loci (White arrow showed in Fig. 2B1) was detected in ‘Menglina Leddy’. The rDNA loci in ‘Menglina Leddy’ were similar to chromosome 4 in ‘White Fox’, but the arm ratio was much different. In ‘White Fox’, the long arm was about 3–4 times as long as the short arm, but in ‘Menglina Leddy’ the long arm length was similar to that of the short arm. There were another two 45S rDNA bearing chromosomes that were very different in size (Green arrows showed in Fig. 2B1 and B2). Their rDNA locations were similar to chromosome 11 of ‘White Fox’. In addition, two very small chromosomes were found in every cell of ‘Menglina Leddy’ (Orange arrows showed in Fig. 2B1 and B2).
Meiosis analysis of ‘Menglina Leddy’
The homologous relationships among the chromosomes were explored by analyzing the PMC meiosis process in ‘Menglina Leddy’. Meiotic abnormalities occurred at each separation stage and the most frequent chromosome aberrations observed were multivalent, bridge, laggard, and stickiness. Chromosome pairing configuration at the diakinesis stage was recorded in 30 cells (Table 1). All the cells contained five to nine bivalents. There were chromosomes that considerably differed in morphology, but had paired up to form bivalents (Yellow arrows showed in Fig. 3). A total of 26 cells contained one to five univalents (Orange arrows indicated in Figs. 3 and 4) and 23 cells contained one to four trivalents (White arrow indicated in Fig. 4A1 and A2). Half of the cells contained one or two tetravalents (Red arrow marked in Fig. 3A1 and A2). Pentavalent (Green arrow indicated in Fig. 4A1 and A2) was also observed in four cells and hexavalents (White arrow indicated in Fig. 3B1 and B2) in six cells (Table 1). The large number of chromosomes pairing aberrations resulted in chromosome bridges during telophase I (Fig. 4B1 and B2, C1 and C2) and laggard chromosomes in anaphase II (Fig. 4D1 and D2) and telophase II (Fig. 4E1 and E2). These laggard chromosomes eventually formed micronuclei during the tetrad phase (White arrows indicated in Fig. 4F1 and F2).
Chromosome pairing configurations at diakinesis stage in ‘Menglina Leddy’ cells. Red signals showed the 45S rDNA locations, whereas the green signals showed the 5S rDNA locations and chromosome telomeres. A1 and A2 were the same PMC. B1 and B2 were the same PMC. White arrows indicated the hexavalents; red arrows indicated the tetravalents; the yellow arrows indicated the abnormal bivalents and the orange arrows indicated the univalents. Chr 1: bivalent of two homologous chromosomes 1. Chr 2: bivalent of two homologous chromosomes 2. Bars = 10 µm
Abnormalities in ‘Menglina Leddy’ meiotic cells. Red signals showed the 45S rDNA locations, whereas the green signals showed the 5S rDNA locations and chromosome telomeres. A1 and A2, B1 and B2, C1 and C2, D1 and D2, E1 and E2, and F1 and F2 were the same PMC, respectively. A1 and A2: pairing abnormalities at diakinesis stage, univalent (orange arrow indicated), trivalent (white arrow indicated), and pentavalent (green arrow indicated). B1–C2: chromosome bridges in telophase I. D1–D2: laggard chromosomes in anaphase II. E1–E2: laggard chromosomes in telophase II. F1 and F2: micronucleus in the tetrad phase (white arrows). Bars = 10 µm
Chromosomes with special characteristics were tracked at the diakinesis stage, including chromosome 1, chromosome 2, a super long chromosome, one chromosome with both 5S and 45S rDNA sites, and three chromosomes with 45S rDNA sites. The results showed that chromosomes 1 and 2 always formed bivalents in the 30 cells (Fig. 3A1 and A2, Fig. 4A1 and A2). The super long chromosome was the product of a Robertsonian translocation and encompassed the long arms of two non-homologous chromosomes. In some cells, each arm of the super long chromosome paired up with two other chromosomes to form a trivalent (White arrows indicated in Fig. 4A1 and A2, Fig. 5B1 and B2), but in the other cells it only paired with one chromosome to form a bivalent (Fig. 5C1 and C2). None of the chromosomes paired with the super chromosome had a 45S rDNA site. The chromosome containing both 5S and 45S rDNA sites usually paired with another chromosome in its short arm distal region to form a bivalent (Fig. 5A1 and A2). This means the short arm of this chromosome had rejoined with another chromosome segment, which resulted in a significantly change in the arm ratio. There were three other chromosomes with 45S rDNA. One was chromosome 3, which sometimes paired with other chromosomes to form a multivalent (Fig. 5D1 and D2). The other two significantly differed in length, but usually paired up to form a bivalent and the end region of the longer chromosome always folded back (Fig. 5E1 and E2).
Pairing configuration of several chromosomes with special characteristics. Red signals showed the 45S rDNA locations, the green signal with arrow showed the 5S rDNA location and other green signals showed chromosome telomeres. A1 and A2 were the same chromosomes from one cell and showed a bivalent formed by the chromosome containing both 5S and 45S rDNA sites and another chromosome. B1 and B2 were the same chromosomes from one cell and showed a trivalent containing the super long chromosome. C1 and C2 were the same chromosomes from one cell and showed a bivalent containing the super long chromosome. D1 and D2 were the same chromosomes from one cell and showed a tetravalent containing chromosome 3. E1 and E2 were the same chromosomes from one cell and showed a bivalent formed by two chromosomes that contained 45S rDNA and significantly differed in length. The end region of the longer chromosome always folded back. Bars = 10 µm
Discussion
Structure changes to chromosomes caused by γ-rays radiation have been reported in various crops, including Triticum aestivum, Oryza sativa, Zea mays, and Vicia faba(Oney-Birol and Balkan 2019; Morita et al. 2009; Tao and Tang 1992; Kuglik et al. 1990). The γ-rays can directly break up chromosomes and disrupt the natural balance, which provides opportunities to increase crop yield and adaptability (Cortés and López-Hernández 2021). They can also be used to achieve specific breeding goals, such as producing dwarf mutants (Lu et al. 2009) or inducing male sterility (Kravets 2013; Kinoshita 1976). A comparison between ‘Menglina Leddy’ and ‘White Fox’ showed that there were noticeable changes in the chromosomal structures (Fig. 2). Although both cultivars had 24 chromosomes, ‘Menglina Leddy’ showed a significant amount of chromosomal breaking and rejoining. In ‘Menglina Leddy’ cells, one super long and two super small chromosomes appeared after irradiation. These alterations to chromosome structure led to pathologies in the male reproductive system and pollen sterility in ‘Menglina Leddy’. Consequently, ‘Menglina Leddy’ can serve as an experimental material for studying the mechanism underlying lily flower development and male sterility. The telomere structures necessary for chromosomal integrity were present in all the ‘Menglina Leddy’ chromosomes and its chromosome structure and number also remained stable during vegetative propagation. Therefore, it maybe possible to replace ‘White Fox’ with ‘Menglina Leddy’.
The chromosome breakage caused by radiation is generally random; therefore, the longer the chromosome, the greater the probability of rearrangement. Interestingly, the karyotype analysis in this study showed that the two longest metacentric chromosomes in ‘White Fox’, chromosomes 1 and 2, appeared to retain their integrity in ‘Menglina Leddy’ and they consistently paired to form two normal bivalents in all the diakinesis cells (Fig. 3A1 and A2, Fig. 4A1 and A2). As the two longest chromosomes in ‘White Fox’, the probability of no breakage and recombination after irradiation should be very low. However, no such structural changes were observed in this study, probably because the breakdown or recombination of chromosomes 1 and 2 would considerably affect the individual. Although the chromosomes in Lilium species are mainly telomeric and acrocentric chromosomes, all chromosomes 1 and 2 are metacentric chromosomes (Tang et al. 2020; Liu et al. 2017; Gao et al. 2012; Wang et al. 2012; Kinoshita 1976). The evolutionary trend for the karyotype is from symmetry to asymmetry (Stebbins 1971). However, chromosomes 1 and 2 still retain their symmetry characteristics, whereas the other 10 chromosomes have evolved into telomeric and acrocentric chromosomes. Therefore, it is probable that the structural stability of chromosomes 1 and 2 is crucial for lily species.
The rearrangement of several distinct chromosomes was also predicted based on the rDNA signals and telomeric sequences. First, there was a 45S rDNA distribution at the super long chromosome centromere site and one of the 45S rDNA sites on chromosome 3 had disappeared in ‘Menglina Leddy’. This suggested that one arm of the super long chromosome was from chromosome 3. However, the chromosome 3 bearing 45S rDNA did not pair with the super long one in any of the diakinesis stage cells (Fig. 5B1 and B2, C1 and C2), which suggested that this super long chromosome was not homologous to chromosome 3. Secondly, although chromosome’s behavior in meiosis revealed the short arm of one chromosome 4 rejoined with another chromosome segment, the available information is not enough to reveal the identity of the chromosome segment. Another chromosome 4 could not be identified due deletion of the 5S and 45S rDNA loci. In addition, the two chromosomes 11 with 45S rDNA were always paired to form a bivalent at the diakinesis stage, the long arm of one chromosome 11 had significantly decreased in size, while the other chromosome 11 always folded back to pair itself at the long arm distal end. This suggests that a gross recombination occurred between the two chromosomes 11 and a pair of homologous chromosomes became a duplicate-deletion-heterozygote.
Conclusions
Both ‘Menglina Leddy’ and ‘White Fox’ had 24 chromosomes, but ‘Menglina Leddy’ contained a significant number of chromosomal rearrangements. All ‘Menglina Leddy’ cells had a super long chromosome and two super small chromosomes. An analysis of chromosome behavior during meiosis revealed the compositions of several recombined chromosomes. In conclusion, the chromosomal structural variations induced by γ-rays radiation ultimately led to a significant reduction in pollen quantity and pollen sterility in ‘Menglina Leddy’.
References
Ahn Y, Hwang Y, Younis A, Sung M, Ramzan F, Kwon M, Kang Y, Kim C, Lim K (2017) Investigation of karyotypic composition and evolution in Lilium species belonging to the section martagon. Plant Biotechnol Rep 11(6):407–416. https://doi.org/10.1007/s11816-017-0462-7
Barba-Gonzalez R, Lim KB, Ramanna MS, Visser RGF, Van Tuyl JM (2005a) Occurrence of 2n gametes in the F1 hybrids of Oriental × Asiatic lilies (Lilium): Relevance to intergenomic recombination and backcrossing. Euphytica 143(1–2):67–73. https://doi.org/10.1007/s10681-005-2657-1
Barba-Gonzalez R, Ramanna MS, Visser RGF, Van Tuyl JM (2005b) Intergenomic recombination in F1 lily hybrids (Lilium) and its significance for genetic variation in the BC1 progenies as revealed by GISH and FISH. Genome 48(5):884–894. https://doi.org/10.1139/g05-057
Basi S, Subedi LP, KC GB, Adhikari NR (2006) Cytogenetic effects of gamma rays on Indica Rice Radha-4. J Inst Agric Anim Sci 27:25–36. https://doi.org/10.3126/jiaas.v27i0.692
Beal JM (1942) Chromosome fragments in Lilium willmottiae and Hybrids between it and L. davidii. Botanical Gazette (chicago, Ill.) 103(3):617–619. https://doi.org/10.1086/335074
Cortés AJ, López-Hernández F (2021) Harnessing crop wild diversity for climate change adaptation. Genes 12(5):783. https://doi.org/10.3390/genes12050783
Crouse HV (1961) Irradiation of condensed meiotic chromosomes in lilium longiflorum. Chromosoma 12(1):190–214. https://doi.org/10.1007/BF00328919
Dai K, Zhao R, Shi M, Xiao J, Yu Z, Jia Q, Wang Z, Yuan C, Sun H, Cao A, Zhang R, Chen P, Li Y, Wang H, Wang X (2020) Dissection and cytological mapping of chromosome arm 4VS by the development of wheat-Haynaldia villosa structural aberration library. Theor Appl Genet 133(1):217–226. https://doi.org/10.1007/s00122-019-03452-8
Dou Q, Chen P, Xie J (2003) Cytological and Molecular Identification of Alien Chromatin in Giant Spike Wheat Germplasm. Acta Botanica Sinica 45(9):1109–1115
Gao Y, Zhou S, He X, Wan J (2012) Chromosome diversity and evolution in tribe Lilieae (Liliaceae) with emphasis on Chinese species. J Plant Res 125(1):55–69. https://doi.org/10.1007/s10265-011-0422-1
Grassotti A, Mercuri A (1996) Lilium elegans: selection of pollenless clones. Acta Hort 414:125–128. https://doi.org/10.17660/ActaHortic.1996.414.12
Han W, Tang F, Li Y, Wang J, Liu J, Liu F, Zhu R (2017) Preliminary study on the effect of aerospace environment mutagenesis and 60Co—γ-ray on agronomic characters and quality of M1 generation of Amaranthus hypochondriacus L. Acta Agriculturae Jiangxi 21:141–144. https://doi.org/10.19386/j.cnki.jxnyxb.2022.03.010
Hwang Y, Kim HH, Kim J, Lim K (2011) Karyotype analysis of Lilium tigrinum by FISH. Hortic Environ Biotechnol 52(3):292–297. https://doi.org/10.1007/s13580-011-0225-2
Jiang J, Gill BS, Wang GL, Ronald PC, Ward DC (1995) Metaphase and interphase fluorescence in situ hybridization mapping of the rice genome with bacterial artificial chromosomes. Proc Natl Acad Sci 92(10):4487–4491. https://doi.org/10.1073/pnas.92.10.4487
Khah MA, Verma RC (2017) Effect of gamma irradiation on seed germination and chromosomal behaviour at meiotic division in bread wheat (Triticum aestivum L.). J Indian Bot Soc 96(3):209–215
Kiihl PRP, Pereira ARA, Godoy SMD, Stenzel NMC, Risso-Pascotto C (2011) Chromosome stickiness during meiotic behavior analysis of Passiflora serrato-digitata L. (PassifloraCEAE). Ciência Rural 41(6):1018–1023. https://doi.org/10.1590/S0103-84782011005000076
Kinoshita T (1976) Genetical studies on cytoplasmic male sterility induced by gamma ray irradiation in sugar beets. Jpn J Breed 26(3):256–265. https://doi.org/10.1270/JSBBS1951.26.256
Kolar FR, Pai SR, Dixit GB (2013) EMS, sodium azide and gamma rays induced meiotic anomalies in Delphinium malabaricum (Huth) Munz. Isr J Plant Sci 61(1–4):64–72. https://doi.org/10.1080/07929978.2014.961395
Kravets EA (2013) Cytomixis and its role in the regulation of plant fertility. Ontogenez 44(3):147–165. https://doi.org/10.7868/s0475145013030038
Kuglik P, Slotova J, Dobricka R, Karpfel ZCAV (1990) Vicia faba chromosome damage induced by low doses of gamma radiation. Biol Plant 32(2):113–118. https://doi.org/10.1007/BF02897551
Lan Y, Lianwei Q, Xin H, Gong H, Lei J, Xi M (2018) Physical mapping of rDNA and karyotype analysis in Tulipa sinkiangensis and T. schrenkii. Sci Hortic 240:638–644. https://doi.org/10.1016/j.scienta.2018.06.055
Lee YI, Lee IS, Lim YP (2002) Variations in sweetpotato regenerated from gamma-ray irradiated embryogenic callus. J Plant Biotechnol 4(4):163–170
Lim KB, Chung JD, Van Kronenburg BCE, Ramanna MS, De Jong JH, Van Tuyl JM (2000) Introgeression of Lilium rubellum Baker chromosomes into L. longiflorum Thunb. a genome painting study of the F1 hybrid, BC1 and BC2 progenies. Chromosome Res. https://doi.org/10.1023/a:1009290418889
Lim K, Wennekes J, Jong JHD, Jacobsen E, van Tuyl JM (2001) Karyotype analysis of Lilium longiflorum and Lilium rubellum by chromosome banding and fluorescence in situ hybridisation. Genome 44(5):911–918. https://doi.org/10.1139/gen-44-5-911
Lim KB, Ramanna M, Jacobsen E, van Tuyl J (2003) Evaluation of BC2 progenies derived from 3x–2x and 3x–4x crosses of Lilium hybrids: a GISH analysis. Theor Appl Genet 106(3):568–574. https://doi.org/10.1007/s00122-002-1070-6
Liu G, Zhang X, Lan Y, Xin H, Hu F, Wu Z, Shi J, Xi M (2017) Karyotype and fluorescence in situ hybridization analysis of 15 Lilium species from China. J Am Soc Hortic Sci 142(4):298–305. https://doi.org/10.21273/JASHS04094-17
Liu G, Lan Y, Qu L, Zhao Y, Xin H, Xi M (2022) Analyzing the genetic relationships in Tulipa based on karyotypes and 5S rDNA sequences. Sci Hortic 302:111178. https://doi.org/10.1016/j.scienta.2022.111178
Lu C, Huang S, Liang L, Zhao X, Zhang K (2002) The chromosome aberration of the Asiatic lily (M1) after irradiation on bulbs. Acta Agriculturae Nucleatae Sinica 03:148–151
Lu S, Wang Z, Niu Y, Chen Y, Chen H, Fan Z, Lin J, Yan K, Guo Z, Li H (2009) Gamma-ray radiation induced dwarf mutants of turf-type bermudagrass. Plant Breed 128(2):205–209. https://doi.org/10.1111/j.1439-0523.2008.01544.x
Ma L, Kong F, Sun K, Wang T, Guo T (2021) From classical radiation to modern radiation: past, present, and future of radiation mutation breeding. Front Public Health. https://doi.org/10.3389/fpubh.2021.768071
Marasek A, Hasterok R, Wiejacha K, Orlikowska T (2004a) Determination by GISH and FISH of hybrid status in Lilium. Hereditas 140(1):1–7. https://doi.org/10.1111/j.1601-5223.2004.01721.x
Marasek A, Orlikowska T, Hasterok R (2004b) The use of chromosomal markers linked with nucleoli organisers for F1 hybrid verification in Lilium. Acta Hort 651:77–82. https://doi.org/10.17660/ActaHortic.2004.651.7
Morita R, Kusaba M, Iida S, Yamaguchi H, Nishio T, Nishimura M, Chugoku NARC, National AABR, National IOFS, Institute ORB, Graduate SOS, Tohoku U, Graduate SOAS, National IOAS, Hiroshima U (2009) Molecular characterization of mutations induced by gamma irradiation in rice. Genes Genet Syst 84(5):361–370. https://doi.org/10.1266/ggs.84.361
Muller HJ (1927) Artifificial transmutation of the gene. Science 66:84–87. https://doi.org/10.1126/science.66.1699.84
Oney-Birol S, Balkan A (2019) Detection of cytogenetic and genotoxic effects of gamma radiation on M1 generation of three varieties of Triticum aestivum L. Pak J Bot. https://doi.org/10.30848/PJB2019-3(48)
Stadler LJ (1928) Mutations in barley induced by X-rays and radium. Science 68(1756):186–187. https://doi.org/10.1126/science.68.1756.186
Stebbins GL (1971) Chromosomal evolution in higher plants. Edward Arnold, London
Stewart RN (1943) Occurrence of aneuploids in Lilium. Bot Gaz 104(4):620–626. https://doi.org/10.1086/335175
Tang X, Yu C, Zhang K, Zeng Y, Zhao L, Zhang H, Liu X (2020) Detection the ploidy levels in asiatic lily cross-breeding through karyotype analysis and FISH. Pak J Bot. https://doi.org/10.30848/PJB2020-3(4)
Tao L, Tang S (1992) Analysis of the laws of gamma-radiation-induced M0 chromosomal breakage in corn (Zea mays L.). J Southwest Agricultural Univ 14:348–350
Tiwari VK, Heesacker A, Riera Lizarazu O, Gunn H, Wang S, Wang Y, Gu YQ, Paux E, Koo DH, Kumar A, Luo MC, Lazo G, Zemetra R, Akhunov E, Friebe B, Poland J, Gill BS, Kianian S, Leonard JM (2016) A whole-genome, radiation hybrid mapping resource of hexaploid wheat. Plant J 86(2):195–207. https://doi.org/10.1111/tpj.13153
Wang X, Xie S, Zhang Y, Niu L (2012) Chromosome analysis and mapping of ribosomal genes by fluorescence in situ hybridization (FISH) in four endemic lily species (Lilium) in Qinling Mountians, China. Pak J Bot 44(4):1319–1323
Xi M, Sun L, Qiu S, Liu J, Xu J, Shi J (2012) In vitro mutagenesis and identification of mutants via ISSR in lily (Lilium longiflorum). Plant Cell Rep 31(6):1043–1051. https://doi.org/10.1007/s00299-011-1222-8
Yamagishi M (2003) A genetic model for a pollenless trait in Asiatic hybrid lily and its utilization for breeding. Sci Hortic 98(3):293–297. https://doi.org/10.1016/S0304-4238(02)00230-3
Yang JS (1998) Effects of gamma irradiation on the flavor composition of food commodities. Adv Exp Med Biol 434:277–284. https://doi.org/10.1007/978-1-4899-1925-0_23
Zaman MA, Rai KSND (1977) Identification of chromosomes involved in six radiation-induced translocations in Collinsia heterophylla: a search for the “Pseudo-supernumerary” chromosome. Cytologia 42(1):49–51. https://doi.org/10.1508/cytologia.42.49
Zhang W, Wang C, Xue L, Zheng Y, Lei J (2018) Production of pollenless triploid lily hybrids from Lilium pumilum DC. × ‘Brunello’. Euphytica. https://doi.org/10.1007/s10681-018-2248-6
Zhao L, Ning S, Yu J, Hao M, Zhang L, Yuan Z, Zheng Y, Liu D (2016) Cytological identification of an Aegilops variabilis chromosome carrying stripe rust resistance in wheat. Breed Sci 66(4):522–529. https://doi.org/10.1270/jsbbs.16011
Zhou S, Ramanna MS, Visser RG, van Tuyl JM (2008a) Analysis of the meiosis in the F1 hybrids of Longiflorum x Asiatic (LA) of lilies (Lilium) using genomic in situ hybridization. J Genet Genomics 35(11):687–695. https://doi.org/10.1016/S1673-8527(08)60091-0
Zhou S, Ramanna MS, Visser RGF, van Tuyl JM (2008b) Genome composition of triploid lily cultivars derived from sexual polyploidization of Longiflorum × Asiatic hybrids (Lilium). Euphytica 160(2):207–215. https://doi.org/10.1007/s10681-007-9538-8
Acknowledgements
The authors wish to thank Professor Shujun Zhou (Jiangxi Agricultural University, China) for his valuable assistance in meiotic chromosome preparation. Financial support from the National Natural Science Foundation of China (31670603). We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 31670603.
Author information
Authors and Affiliations
Contributions
M.X. and G.L. designed the research; R.N.,Y.N. and Z.W. performed experiments; Y.Z., G.L. and M.X. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ni, R., Liu, G., Ning, Y. et al. Extensive chromosome rearrangements induced by γ-rays irradiation in lily mutant ‘Menglina Leddy’. Euphytica 220, 133 (2024). https://doi.org/10.1007/s10681-024-03392-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-024-03392-5