Abstract
Analyzing chromosomal traits is one of the pragmatic ways to establish evolutionary and genetic database of plants that has complicated phylogenetic system. There are some conflicts on the exact phylogeny and evolutionary pathway of Lilium, and section martagon is the most complicated part among them. In this study, chromosomal traits of martagon lily species are described. All martagon lilies were analyzed with FISH (Fluorescence in situ hybridization) technique, followed by detailed karyotyping. Each species showed 2n = 2x = 24 of chromosome complement. Size of chromosomes ranged from 451.04 to 680.06 µm. 5S and 45S ribosomal DNA, general molecular markers in modern evolutionary research were used as probe in this study. Variation in rDNA loci and chromosome translocation were observed in Lilium hansonii; the highest number of 45S rDNA loci was detected in Lilium hansonii, followed by other martagon lilies, in similar locations but with differences, and chromosome translocation was observed from one individual of Lilium hansonii. Additionally, Lilium tsingtauense from Jeju-do Island, Korea was detected with two extra chromosomes. These kind of genetic variations through karyotyping indicate ongoing genetic variations in martagon lilies. In this study, precise analysis of chromosome traits in Lilium species belonging to section martagonperformed to contribute to better comprehension of the evolutionary pathway and establishment of cytogenetic database for further plant breeding research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classification of Lilium is quite difficult due to their great number of variation in phenotypic characteristics, such as flower shape, color, fragrance and genetic characteristics. Over 100 species of the genus Lilium are widely distributed all over the northern hemisphere, including Europe, North America, and East Asia. The genus Lilium is considered to be originated from Korea and its adjacent region; Manchuria of Northeastern China based on their morphological traits (Lighty 1969). Lilium is divided into seven major sections by Comber on the basis of morphological characteristics and germination pattern; Martagon, Pseudolirium, Liriotypus, Archelirion, Sinomartagon, Daurolirion and Leucolirion (Comber 1949). As time went by, several modifications have been suggested by numerous researchers based on the diverse criteria (Haw and Liang 1986; Lin 2000), however, Comber classification is still valid. Lighty (1969) reported patterns of naturally growing lilies by regional groups and stated that lilies with whorled leaves, belonging to the section martagon, are the most primitive species based on morphology analysis. Among the section martagon, L. tsingtauense is majorly growing in Korean Peninsula, L. distichum, limited to Korean Peninsula and Manchuria, while L. hansonii is growing naturally in Ulleung-do Island (Fox 2006). Besides that, L. martagon is naturally growing in Europe, Northern China, Central Asia and Russia, with numerous mutants. The Natural habitat of L. medeoloides, also known as “Wheel lily”, was from Kamchatka Peninsula to Japan (Lee 1989). For the sake of vitalization of breeding industry and research of Lilium, which occupies major part of the ornamental crops market, investigation of genetic and cytogenetic traits of Lilium with various criteria is urgent. Usage of genetic database is crucial in breeding research. Moreover, study of genetic database of wild Lilium will also contribute to complicated existing theories about the origin of Lilium.
Chromosome structure variations among organisms can be decisive factors in discriminating among species or even individual organisms. Distantly related species tends to have morphological variations in chromosome, hence makes cytogenetical research much easier. However, closely related species, for instance, L. tsingtauense, L. distichum and L. medeoloides do not present specific distinction on their chromosomal morphology. Consequently, various chromosome staining methods e.g., C-banding (Holm 1976; Smyth et al. 1989), Q-banding (Holm 1976; Kongsuwan and Smyth 1977), and silver staining (Von Kalm and Smyth 1980) have been performed to understand the precise chromosome morphology in Lilium in the last few decades. These chromosome painting techniques are instrumental for exploration of chromosome dynamics and variations (Husband 2004). Moreover, application of the FISH (Fluorescence in situ hybridization) technique provided an ease in accurate karyotype analysis of plants with larger genome size, such as Lilium (Hwang et al. 2011; Jackson et al. 1998; Jiang and Gill 2006; Younis et al. 2015). The FISH technique enables cytogenetic approach showing where specific markers are located on the chromosome (Heslop-Harrison 1991; Lim et al. 2001; Marasek et al. 2004). Additionally, concrete analysis of the plant genome with detailed karyotyping by FISH technique guides a new way to plant breeding. Noting locations of useful molecular markers on the chromosome leads to construction of chromosome-specific libraries, which may be the first step for economic use of chromosome transportation via fusion techniques and microinjection in plant breeding (Gray and Cram 1990; Harrison and Heslop-Harrison 1995).
Numerous phylogeny and evolutionary studies in organisms using molecular markers have been conducted so far, among them, 5S and 45S ribosomal DNA are two of the most frequently used markers (Álvarez and Wendel 2003; Baldwin 1992; Chang et al. 2009; Garrido et al. 1994; Grechko 2002; Wolters and Erdmann 1988). Coding regions (18S, 28S, 5.8S and 5S) in ribosomal RNA are suitable for revealing the evolutionary process of living organisms, since they are highly conserved areas. Those highly conserved coding region are surrounded by external transcribed spacer (ETS), and internal transcribed spacer (ITS). Since these non-coding regions evolved at a faster rate, they are an useful tool for analyzing more closely related species. Non-transcribed region (NTS) presents even higher variations, which makes it a more suitable tool for phylogeny analysis among within-population (Grechko 2002). To date, there were several attempts to build up genetic relationships in Lilium; however, results are still debatable.
In this study, a detailed FISH (fluorescence in situ hybridization) analysis using repetitive sequences, 5S and 45S rDNA as probes and precise karyotypes were carried out in Lilium species belonging to the section martagon to observe chromosome-level variations, along with consideration of evolutionary appearance in the interest of establishment of genetic and cytogenetic database for lily breeding.
Materials and methods
Plant materials and slide preparation
Martagon lilies, viz. L. hansonii, L. martagon, L. tsingtauense, L. distichum, L. medeoloides were collected and cultivated in the greenhouse of the Kyungpook National University (Table 1). Five plants of L. hansonii were collected from four different places in Ulleung-do Island (Nari-bunji basin, Nari-ryeong pass, Seongin-bong and Nari-ryeong). L. martagon was given by Wageningen university and research. L. tsingtauense from Daegwanryeong, Kangwondo province and Joekeun-barime volcanic cone, Jeju-do Island, Korea. L. distichum and L. medeoloides were collected from Mt. Ham-baek, Kangwondo province, Korea and Kyoto, Japan, respectively. Geographical distribution of each sample plant is presented in Fig. 1. The young root tips of plants were collected and then treated with 1-bromonaphthalene for 4 h at 20 °C. The roots were then rinsed three times in distilled water thoroughly. After that, roots were soaked in enzyme mixture at 37 °C for 1 h. The root tips were then squashed in a drop of 60% acetic acid directly, air-dried at room temperature and kept in a dry box.
Geographical distribution of the plants are presented. Blue star indicates L. hansonii (Ulleung-do Island, Korea). One red dot on the left side indicates location of L. martagon (Wageningen University and Research, The Netherlands). Red square and red star shape indicate locations of L. tsingtauense (Daegwanryeong, Kangwondo province and Joekeun-barime volcanic cone, Jeju-do Island, Korea). Blue dot and blue square indicate L. distichum (Mt. Ham-baek, Kangwondo province, Korea) and L. medeoloides (Kyoto, Japan), respectively. Blue star indicates location of L. hansonii plants with chromosome variation, and red star indicates location of L. tsingtauense with an extra pair of chromosome
Fluorescence in situ hybridization (FISH)
FISH was carried out as described in Lim et al. (2001) with a slight modification. After drying at 55 °C for 1 h for chromosome fixation, slides were washed in 2 × SSC buffer for 5 min. Then slides were incubated with RNase A (100 µg/µl) in 2 × SSC buffer at 37 °C for 1 h. The slides were washed in 2 × SSC buffer and pose-fixed in 4% paraformaldehyde. Hybridization mixture consist of 50% of deionized formimide, 10% of dextran sulfate, 10% of 2 × SSC, 0.25% of sodium dodecyl sulfate, and 3 ng µl−1 of each probe DNA. Hybridization mixture denatured at 70 °C for 10 min and placed on the slide. Probes were 5S and 45S rDNA labeled with Dig-Nick translation Mix (Roche, Germany) and Biotin-Nick translation Mix (Roche, Germany), respectively. After 10 min of denaturation at 80 °C, the slides were incubated at 37 °C overnight. Slides were detected with FITC-conjugated anti-digoxygenin and Streptavidin Cy3. The chromosomes were counterstained with DAPI (4′, 6-diamdino-2-phenylindole) in Vectashield and observed with a fluorescent microscope (BX 61, Olympus, Japan).
Karyotype
On an average, ten cells with somatic chromosomes in metaphase were used for karyotype analysis. The length of each chromosome was measured with CytoVision 3.7 software (Leica Biosystems, Germany). The numbering of chromosomes was based on the short-arm length according to Stewart (1947). Chromosome types were determined based on the reports by Levan et al. (1964). Idiogram of each species and FISH result figures were made with CorelDRAW Graphics Suite X5 (Corel Corporation, Canada).
Results
Chromosome characterization of the section martagon
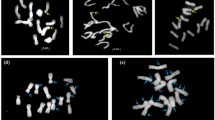
Chromosome morphology of all Martagon lilies was observed under the fluorescence microscope. Karyotype analysis of the five martagon lilies is represented in Table 2. Total length of chromosome ranged from 451.04 to 680.06 µm. The first chromosomes of all martagon lilies were the longest, while tenth chromosomes of all martagon lilies were the shortest. Overall chromosome condensation of L. hansonii and L. distichum was observed rather high than that of the other martagon lilies (Table 2).
Chromosome component of five species belonging to the section martagon was 2n = 2x = 24, consisting of two submetacentric chromosomes (#1 and #2), four subtelocentric chromosomes (#3, #4, #5 and #6), and six telocentric chromosomes (#7, #8, #9, #10, #11 and #12) (Figs. 2, 3). The length of short arm of the chromosomes #1 and #2 is significantly larger than that of the other chromosomes. From chromosome #3, length of the short arm decreases gradually. Since all the martagon lilies, including other sections of Lilium, have similar morphology in chromosomes, it is hard to discriminate each species based on their chromosome morphology itself.
Idiogram of five Lilium species belonging to the section martagon and their generally accepted phylogeny by Lighty (1968). a L. hansonii; b L. martagon; c L. tsingtauense; d L. distichum; e L. medeoloides. Green dots indicate 45 s rDNA loci and red dots indicate 5 s rDNA loci
Detailed FISH analysis with repetitive sequences
Idiogram of five martagon lilies according to FISH results with 5S and 45S rDNA as probes (Fig. 2) and generally accepted phylogeny of the section martagon (Lighty 1968) are presented in Fig. 3. Visualization of rDNA loci and secondary constrictions by FISH analysis enables accurate observation of how these chromosomes differ from each other. All of the secondary constrictions were detected with either 5S or 45S rDNA loci; red or green fluorochromes. For instance, chromosome #1 of L. hansonii possessed 45S rDNA loci on the secondary constriction located on terminal part of the long arm. And chromosome #1 of L. martagon carried 45S rDNA loci on the secondary constriction on the short arm near centromere, while no loci detected on the chromosome #1 in L. tsingtauense, L. distichum and L. medeoloides (Fig. 3). rDNA loci pattern on the chromosome #2 is another chromosomal characteristic. L. hansonii and L. martagon were observed with 45S rDNA loci on the short arm and long arm near centromere, respectively. Similarly, chromosome #2 of L. tsingtauense, L. distichum, L. medeoloides did not possess any rDNA loci (Fig. 3). Chromosome #3, #4, #5 and #10 of Asia-restricted wild martagon lilies including L. hansonii, L. tsingtauense, L. distichum and L. medeoloides possessed 5S and 45S rDNA loci on the long arm in same loci (Figs. 3, 4a), whereas L. martagon was detected possessing a solitary 5S rDNA loci on the long arm near centromere (Figs. 3, 4b). L. hansonii carried excessive 45S rDNA loci on the chromosome #1, #2, #6, #7, and #11 compared to the other martagon lilies (Fig. 3).
Comparison of signal patterns among several martagon lilies. a Same rDNA loci patterns in several martagon lilies. a L. hansonii; b L. tsingtauense; c L. distichum; d L. medeoloides. b Different rDNA loci patterns in L. hansonii and L. martagon. a L. hansonii; b L. martagon. White arrows indicate rDNA loci. Size bar = 10 µm
Location and number of 45s rDNA region in the five martagon lilies are represented in Table 3. The highest loci number of 45s rDNA region was detected in L. hansonii; seventeen, followed by the other martagon lilies; eight 45s rDNA (Table 3).
Chromosome variation in L. hansonii
Several plants of L. hansonii were analyzed by FISH (Fluorescence in situ hybridization) in this study. After detailed FISH analysis of each plant, variations were detected in rDNA loci pattern and chromosome characteristics (Fig. 5). L. hansonii showed different numbers of 45 s rDNA loci among themselves randomly. All L. hansonii plants possessed identical 45S rDNA signal patterns on the chromosome #1, #2, #3, #4, #5, #10 and #11, while excessive numbering of the 45 s rDNA loci in L. hansonii was shown in several random L. hansonii plants, mostly on the short arm of the chromosome #6 or #7 (Figs. 5, 6a).
FISH results of several L. hansonii plants from Ulleung-do Island. a L. hansonii from Nari-bunji basin, Ulleung-do Island; b L. hansonii from Nari-ryeong pass, Ulleung-do Island; c L. hansonii from Nari-bunji basin, Ulleung-do Island; d L. hansonii from Seongin-bong, Ulleung-do Island; e L. hansonii from Nari-ryeong, Ulleung-do Island. White arrows indicate rDNA loci. Red arrows indicate chromosomes with morphological variations. Size bar = 10 µm
Ribosomal DNA loci pattern and chromosome variation in L. hansonii. A Excessive 45S rDNA loci in L. hansonii (#7); B chromosome variation in L. hansonii from Nari-ryeong, Ulleung-do Island. a Generally observed 45S rDNA loci in L. hansonii from Ulleung-do Island. b Reciprocal translocation in somatic chromosomes (#4 and #11) of L. hansonii from Ulleung-do Island. Red arrows indicate 45S rDNA signal in morphologically different chromosomes. Size bar = 10 µm
L. hansonii inhabitated in Nari-ryeong, Ulleung-do Island area showed the chromosomal variation among each plant. Two pairs of chromosomes (#4 and #11) can be karyotypically distinguished easily from other chromosomes in relation to chromosomal morphology and 45S rDNA loci (Figs. 5e, 6b).
Appearance of an extra pair of chromosome in L. tsingtauense
An interesting chromosome feature of L. tsingtauense is presented in Fig. 7a, b, which is the appearance of an extra pair of chromosomes in random plants. It is considered that there are no relationships between the plants with these additional chromosome pair so far in any way. These extra pair of chromosome was in submetacentric shape, 9.93 ± 1.4 µm long of short arm, 11.77 ± 0.17 µm long of long arm (Table 4). No nucleolar region was detected on these extra chromosomes.
Karyotype of L. tsingtauense and appearance of an extra chromosome pair in random plants. A a Karyotype of L. tsingtauense with 24 chromosomes. A b Karyotype of L. tsingtauense with an extra pair of chromosome. Red arrow indicates the extra chromosome pair. B Morphology of an extra pair of chromosome. White lines indicates centromere. Size bar = 10 µm
Discussion
Lighty (1969) suggested that the most primitive Lilium species, L. hansonii, has evolved into L. martagon, L. tsingtauense, L. distichum, L. medeoloides as time passed, based on the morphological similarities between martagon lilies and Nomocharis and Fritillaria, which are considered as close species to pre-Lilium. Having bright yellow flowers with more than two whorled leaves, L. hansonii can be considered to be very close to the so-called ‘pre-Lilium’ group with small yellow or orange flowers containing two whorled leaves according to Lighty (1968). Furthermore, shallow nectary furrows and lightly spotted petals also provide similarities even stronger. Considering this similarity, Lighty assumed that Lilium itself might have started in north-eastern Asia, Korea and Manchuria.
Considering the fact that the highly conserved area in rDNA (18S, 28S, 5.8S, 5S) is one of the finest tool for studying ancient stages of evolution (Grechko 2002), FISH results with rDNA (5S, 45S rDNA) in Lilium species belonging to the section martagon could be an intriguing approach towards that theory; L. hansonii, thought to be the most primitive species (Lighty 1968), was observed with the highest number of 45 s rDNA loci. All L. hansonii plants from Ulleung-do Island, Korea showed number variation of 45S rDNA, varied from 12 to 17 loci. To our knowledge, this kind of number variation in one Lilium species from identical geographical traits has not been reported yet. Regardless of where these L. hansonii came from in Ulleung-do Island, they all showed different 45S rDNA numbers from each other. According to Garrido et al. (1994), there are two possible hypotheses about this number variation of rDNA. One possible way is that plants like L. hansonii with a larger amount of rDNA loci gained additional NORs by duplication from other species’ already existing rDNAs, according to reports in Allium by Schubert and Wobus (1985). The opposite hypothesis to look at this phenomenon that also has been made with several reports (Garrido et al. 1994; Maggini and Garbari 1977; Vosa 1977)which is that species evolved as they went through rDNA reduction. If the latter happened in martagon lilies, rDNA reduction in martagon lilies observed in this study could support the previous theory solely based on the morphological traits (Lighty 1968). L. hansonii, considered as the most primitive Lilium species, has 12–17 loci by itself, followed by L. tsingtauense, L. distichum, L. medeoloides with eight 45S rDNA loci in similar locations. L. martagon, which can be assumed as ‘castaway’ from the other martagon lilies long time ago, possessed eight 45S rDNA loci with quite similar patterns to that of L. hansonii, rather than that of the other martagon lilies in East Asia. From this, we can possibly assume that L. martagon has been isolated in Europe by some incident and evolved by itself, derived from the most primitive Lilium, L. hansonii. While in Asia, with numerous crustal movements and biological evolution, L. hansonii evolved into L. tsingtauense, L. distichum, L. medeoloides and all the other lilies in the Northern hemisphere.
Another interesting feature observed in L. hansonii was reciprocal translocation in somatic chromosomes. Several reports about reciprocal translocation caused in meiosis have been made in Allium (Levan 1935), Lathyrus (Ghaffari et al. 2009), even in L. martagon and L. hansonii (Haga 1943; Xie et al. 2013). Reciprocal translocation is more common in meiosis rather than in mitosis, since reciprocal translocation in somatic chromosome would not be inherited, unlike in meiosis. To identify what kind of mechanisms worked in this phenomenon, further chromosome analysis in meiosis should be undertaken. In conclusion, these arbitrary chromosomal traits e.g., duplication or deletion of rDNA loci and reciprocal translocation in somatic chromosome in L. hansonii detected in this study may suggest robust ongoing evolution in L. hansonii (Cerbah et al. 1998).
L. martagon (European Lily), geographically, and morphologically distant from other martagon lilies, presented quite different FISH results as well. While other martagon lilies possessed 45S rDNA signal pairs on the chromosome #3, #4, #5 and #10, 5S rDNA signal pair on the chromosome #3, L. martagon carried 45S rDNA signal pairs on the chromosome #1, #2 and #3, 5S rDNA signal pair on the chromosome #4, showing some similarities in loci pattern to that of L. hansonii (Fig. 3). Judging from this, loci pattern and number of ribosomal DNA could be affected by geographical isolation and evolutionary traits. On the other hand, East Asia restricted species, L. tsingtauense, L. distichum, L. medeoloides, claimed to be closely related to each other than to the rest of the martagon lilies according to their morphological traits, showed the same pattern and number of rDNA loci.
In this study, few differences have been noticed from the previous report on chromosomal rDNA possession in Lilium. Stewart reported L. martagon Hv. album possess nucleolar secondary constrictions in the long arm of chromosome #1, #3, #6 and #11, and in the short arm of chromosome #2 (Stewart 1947), while in this study, rDNA loci on the chromosome #1, #2, #3, #4 in L. martagon. Moreover, rDNA loci were detected on the chromosome #3, #4, #5 and #10 in L. tsingtauense in this study, while Stewart reported rDNA loci detection in the chromosome #3, #4, #6 and #10 (Stewart 1947).
Thus far, an extra pair of chromosomes in Lilium has been reported occasionally; an extra pair of metacentric chromosomes in L. pumilum, L. sargentiae and telocentric chromosomes in L. auratum, L. tsingtauense, L. henryi (Stewart 1947). On the contrary, L. tsingtauense from Jeju-do Island analyzed in this study was observed with an extra pair of metacentric chromosomes.
Shortly, given the fact that reduction of rDNA, chromosome rearrangements and the appearance of extra chromosomes are frequently observed in Lilium, genetic evolution in Lilium is an ongoing process even at this very moment.
References
Álvarez I, Wendel JF (2003) Ribosomal ITS sequences and plant phylogenetic inference. Mol Phylogenet Evol 29:417–434
Baldwin BG (1992) Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: an example from the Compositae. Mol Phylogenet Evol 1:3–16
Cerbah M, Coulaud J, Siljak-Yakovlev S (1998) rDNA organization and evolutionary relationships in the genus Hypochaeris (Asteraceae). J Hered 89:312–318
Chang Y-C, Shii C-T, Chung M-C (2009) Variations in ribosomal RNA gene loci in spider lily (Lycoris spp.). J Am Soc Hortic Sci 134:567–573
Comber HF (1949) A new classification of the lilium. Lily year Book 15:86–105
Fox EE (2006) Martagon lilies: old world, whorled-leaf lilies, EE Fox. https://books.google.co.in/books?id=UsuONAAACAAJ
Garrido M, Jamilena M, Lozano R, Rejon CR, Rejon MR, Parker J (1994) rDNA site number polymorphism and NOR inactivation in natural populations of Allium schoenoprasum. Genetica 94:67–71
Ghaffari SM, Karimzadeh G, Najafi AA (2009) Occurrence of reciprocal translocation in Lathyrus boissieri Sirj (Fabaceae) from Iran. Cytologia 74:195–199
Gray J, Cram L (1990) Flow karyotyping and chromosome sorting. In: Melamed MR, Mendelsohn ML (eds) Flow cytometry and sorting, Wiley-Liss, New York, pp 503–529. http://ci.nii.ac.jp/naid/10016054863/
Grechko V (2002) Molecular DNA markers in phylogeny and systematics. Russ J Genet 38:851–868
Haga T (1943) A reciprocal translocation in Lilium hansonii Leicht. Cytologia 13:19–25
Harrison GE, Heslop-Harrison JS (1995) Centromeric repetitive DNA sequences in the genus Brassica. Theor Appl Genet 90:157–165
Haw SG, Liang S-Y (1986) The lilies of China: the genera Lilium, Cardiocrinum, Nomocharis and Notholirion. Timber Press, Portland
Heslop-Harrison J (1991) The molecular cytogenetics of plants. J Cell Sci 100:5–21
Holm PB (1976) The C and Q banding patterns of the chromosomes of Lilium longiflorum (Thunb.). Carlsberg Res Commun 41:217–224
Husband B (2004) Chromosomal variation in plant evolution. Bot Soc Am 91:621–625. doi:10.3732/ajb.91.4.621
Hwang Y-J, Kim HH, Kim J-B, Lim K-B (2011) Karyotype analysis of Lilium tigrinum by FISH. Hortic Environ Biotechnol 52:292–297
Jackson SA, Wang ML, Goodman HM, Jiang J (1998) Application of fiber-FISH in physical mapping of Arabidopsis thaliana. Genome 41:566–572
Jiang J, Gill BS (2006) Current status and the future of fluorescence in situ hybridization (FISH) in plant genome research. Genome 49:1057–1068
Kongsuwan K, Smyth D (1977) Q-bands in Lilium and their relationship to C-banded heterochromatin. Chromosoma 60:169–178
Lee TB (1989) Illustrated flora of Korea, Hyang Mun Sa, Seoul, Korea
Levan A (1935) Cytological studies in allium, VI the chromosome morphology of some diploid species of Allium. Hereditas 20:289–330
Levan A, Fredga K, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220
Lighty R (1968) Evolutionary trends in lilies. Lily Yearbook RHS 31:40–44
Lighty R (1969) The lilies of Korea. Lily Yearbook RHS 31:31–39
Lim K-B, Wennekes J, Jong JHD, Jacobsen E, van Tuyl JM (2001) Karyotype analysis of Lilium longiflorum and Lilium rubellum by chromosome banding and fluorescence in situ hybridisation. Genome 44:911–918
Lin Y (2000). Flora reipublicae popularis sinicae. Tomus
Maggini F, Garbari F (1977) Amounts of ribosomal DNA in Allium (Liliaceae). Plant Syst Evol 128:201–208
Marasek A, Hasterok R, Wiejacha K, Orlikowska T (2004) Determination by GISH and FISH of hybrid status in Lilium. Hereditas 140:1–7
Schubert I, Wobus U (1985) In situ hybridization confirms jumping nucleolus organizing regions in Allium. Chromosoma 92:143–148
Smyth D, Kongsuwan K, Wisudharomn S (1989) A survey of C-band patterns in chromosomes of Lilium (Liliaceae). Plant Syst Evol 163:53–69
Stewart RN (1947) The morphology of somatic chromosomes in Lilium. Am J Bot 34:9–26
von Kalm L, Smyth D (1980) Silver staining test of nucleolar suppression in the Lilium hybrid ‘Black Beauty’. Exp Cell Res 129:481–485
Vosa CG (1977) Heterochromatic patterns and species relationship [UK]. Nucleus 20:33–41
Wolters J, Erdmann VA (1988) Compilation of 5S rRNA and 5S rRNA gene sequences. Nucleic Acids Res 16:r1–r70
Xie S, Ramanna MS, Visser RGF, Arens P, van Tuyl JM (2013) Elucidation of intergenomic recombination and chromosome translocation: meiotic evidence from interspecific hybrids of Lilium through GISH analysis. Euphytica 194:361–370
Younis A, Ramzan F, Hwang Y-J, Lim K-B (2015) FISH and GISH: molecular cytogenetic tools and their applications in ornamental plants. Plant Cell Rep 34:1477–1488
Acknowledgements
This research was supported by Golden Seed Project (Center for Horticultural Seed Development, no. 213003-04-4-WTM11), Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Service (KFS). And this research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Project no. NRF-2016R1D1A1B04932913).
Author information
Authors and Affiliations
Contributions
First author and all the co-authors made contributions to in this manuscript. Conception or design of the work: Y-JA, Y-JH, AY, M-SS, K-BL. Data collection: Y-JA. Cultivation of samples: Y-JA, M-SS, FR, M-JK. Data analysis and interpretation: Y-JA, Y-JH, K-BL. Drafting the article: Y-JA, Y-IK, C-KK, K-BL. Acquisition of funding: Y-IK, C-KK, K-BL. Critical revision of the article: Y-JA, Y-JH, AY, K-BL. Final approval of the version to be published: Y-JA, C-KK, K-BL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahn, YJ., Hwang, YJ., Younis, A. et al. Investigation of karyotypic composition and evolution in Lilium species belonging to the section martagon. Plant Biotechnol Rep 11, 407–416 (2017). https://doi.org/10.1007/s11816-017-0462-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-017-0462-7