Abstract
In subtropical environments lodging occurs at all stages of the oat plant development, particularly after panicle extrusion, causing severe yield reduction. This study aimed to identify morphological, chemical, and anatomical characteristics of the stem associated with lodging resistance on oats, which may be used to identify and select resistant genotypes. A set of genotypes with diverse response to lodging were grown in nine environments, combining sowing densities, years, and growing seasons within the same year. Were carried out morphological, anatomical, and chemical evaluations for main stems. Shorter stem length and larger stem thickness in the first expanded basal internode are morphological characteristics associated with lodging resistance in Avena sativa. No differences in lignin and cellulose contents were observed, however, there were differences in the distribution pattern and in the intensity of lignin and cellulose staining. Lodging resistant genotypes showed higher number of cell layers in the subdermal parenchyma. The most practical and easily selectable feature for lodging resistance in a breeding program remains plant height together with stem wall thickness at the first basal expanded internode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oats are the fifth-most cultivated cereal, and they are among the top ten annual crops in Brazil. In recent years, world oat production has been reasonably stable (USDA 2020). Currently, Brazil ranks among the leading world oat producers and its production is destined almost exclusively for the Brazilian market, with exports reduced to very low levels in recent years, while there are almost no imports of the grain into the country (Lima 2019). Oats are an alternative to wheat cultivation in winter growing season, mainly due to their higher profitability and easier marketing (Lima 2019), besides its benefits for the no-till system (Fontaneli et al. 2012).
Plant lodging is a grain yield limiting factor in cereals, including oats; therefore, lodging resistance is a main objective for the cereal breeding programs (Berry and Spink 2012). Defined as the permanent displacement of the stems from the vertical position (Pinthus 1967), lodging is caused by stem bending, stem breaking at its base, or root displacement in the soil (Berry and Spink 2012).
In temperate climates, lodging generally occurs after panicle extrusion and is more common during grain filling (White 1995; Tams et al. 2004; Berry and Spink 2012). The yield reduction during the grain filling period is about 0.5% per day (Stapper and Fischer 1990), and grain yield losses can reach up to 80% (Foulkes et al. 2011). These losses result directly from interference on accumulation of grains’ dry matter, resulting in reduced grain filling, and a decrease in number of grains per square meter; indirectly, losses result from difficulties in harvesting, loss of grain quality, and higher drying costs (Zuber et al. 1999; Berry and Spink 2012). In subtropical environments, lodging occurs at all stages of plant development, particularly after tillering, and this is relatively common in oat crops.
Strong winds associated with precipitation are the primary causes of lodging (Tams et al. 2004). Nevertheless, lodging is a complex phenomenon which results from the interaction of weather conditions with several factors intrinsic to the genotype, soil characteristics, and cultivation practices (Tams et al. 2004; Berry et al. 2000).
Lack of sufficient lodging pressure due to weather or growing conditions often makes the visual scoring method ineffective (Nakhforoosh et al. 2020). Norden and Frey (1959) already mentioned in their study that it would be interesting to select for lodging resistance based on some easily identifiable trait in the field, due the complex nature of lodging resistance and the difficulty of establishing a reliable method. This would facilitate the extrapolation of lodging resistance levels for groups of lines in a breeding program, even in years when lodging does not occur naturally.
Selection for shorter plant height is the main strategy used in breeding programs to reduce lodging in cereals. Nevertheless, several factors related to the genotype contribute to lodging resistance, including cellulose, hemicellulose, and lignin contents that make up the stem cell walls, the lengths and numbers of internodes, and the diameter and thickness of the stem (Multamäki 1962; Pinthus 1974; White 1995; Tripathi et al. 2003; Zhu et al. 2004; Kong et al. 2013). Other anatomical traits are also associated with lodging resistance, such as parenchyma and sclerenchyma tissues, cellular organization in these tissues, and numbers of vascular bundles (Kong et al. 2013; Karim and Jahan 2013).
Although there has been extensive research on lodging in cereals, especially on wheat and barley in temperate environments, lodging on oats remains poorly understood, especially for oats grown in sub-tropical environments.
The objective of this study was to identify morphological, chemical, and anatomical characteristics of the stem that are associated with lodging resistance on oats and which may be used to identify and select lodging resistant genotypes.
Materials and methods
Description of the experimental area
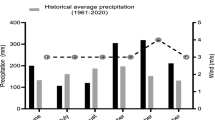
The experiments were conducted at the Agronomic Experimental Station of UFRGS, located in Eldorado do Sul town, Rio Grande do Sul state, Brazil (30°05'52'' S, 51°39'08'' W). The average altitude is 46 m above sea level and the climate of the region is Köppen-Geiger type Cfa (subtropical humid), with an average annual precipitation of approximately 1400 mm. The months with the highest rainfall are from June to October (IRGA, 2019), coinciding with the oat growing season. The soil of the experimental area is characterized as typical Red Dystrophic Argisol (Santos et al. 2018).
Plant material and experimental design
Five oat (Avena sativa) genotypes, developed by the UFRGS Oat Breeding Program (Table 1), with different average plant heights and lodging resistances were grown in nine environments (seeding rate x year x season) (Fig. 1). Sowing in 2017 occurred on June 23rd. In 2018 sowing occurred on June 21st (full-season) and July 13th (short-season). Each of the three experiments were conducted under a randomized complete block design with four replications, in a factorial scheme of five (genotypes) x three sowing densities (150, 300, and 450 viable seeds per square meter).
Different sowing densities were used to determine if the increase in the number of stems per area, and consequent rise in competition for resources, would influence tillering and cause significant morphological and anatomical changes in the genotypes, increasing or decreasing the risk of lodging. Two growing seasons and two different sowing dates at the second year also allowed us to study their influences on oat morphology and anatomy and their direct influences on lodging.
The experiments were conducted under no-tillage system. Base fertilization consisted of 300 kg ha−1 of the NPK formula 5–30-15. Top dressing nitrogen fertilization consisted of a total of 70 kg ha−1 of N, divided into two applications of 35 kg ha−1 of N each, when the plants reached three and six fully expanded leaves, using urea as the nitrogen source. Fungicide was applied as needed for controlling fungal diseases.
Morphological evaluation
Five random plants from each experimental unit were collected (5 m x 1 m) during flowering in all environments, maintaining the roots and soil moist. From each plant, on the main stem, we assessed the total stem length (TSL), as the distance from the base of the plant to the insertion of the panicle. On the median portion of the first and second basal expanded internodes, of these main stems, the following parameters were measured: length of the first internode (LFI), length of the second internode (LSI); diameter of the first internode (DFI), diameter of the second internode (DSI), stem wall thickness of the first internode (TFI) and stem wall thickness of the second internode (TSI). For these measurements, leaves and leaf sheaths were removed. Stem diameter, stem wall thickness, and internode length were measured using a digital caliper.
Evaluation of lodging occurred weekly according to the angle with the ground and percentage of the experimental unit lodged. However, in 2017, there was a heavy rainstorm after flowering, with very strong winds, making this analysis infeasible. In 2018, lodging did not occur uniformly in the experimental area; therefore, we chose not to use lodging data collected on the experiments; instead, we used prior knowledge about the lodging resistance levels of the genotypes based on records made by the UFRGS Oat Breeding Program.
Plots were harvested mechanically and grain yield calculated at a standard moisture content. After the yield was estimated in kilograms per hectare (kg ha−1).
Anatomical and chemical evaluations
In 2018, we performed anatomical evaluations for the five genotypes conducted in the field under environments 5 and 8 (Fig. 1). We chose these environments because they represent different growing seasons under the sowing density recommended for the oat crop in Southern Brazil (300 seeds per square meter).
On the same plants used to assess the morphological traits, the anatomical characters were determined. The internodes, collected at the flowering stage, were fixed in 90% of 70% ethyl alcohol + 5% of acetic acid + 5% of formaldehyde. For the anatomical assessment of each genotype, ten cross-sections of approximately 20 μm were freehand cut at the middle portion of the first expanded basal internode and examined under a light microscope equipped with a digital camera at 10X magnification. We identified tissue types according to the nature of their composition. Poacea species do not have distinct separation between parenchyma tissues. Nevertless, it was decided to divide the tissues into cortical and medullary parenchyma due to the presence of a gradient in size, thickness, and cell composition. Using a 40X stereomicroscope equipped with a digital camera we counted the number of cell layers of the subdermal parenchyma (SP), the number of cell layers of the sclerenchyma ring (SR), the number of cell layers of the cortical parenchyma (CP), number of cell layers of the medular parenchyma (MP), number of total vascular bundles (VB), and number of layers of total cells (LC).
We used phloroglucinol + hydrochloric acid solution for visualization under a bright-field microscope for the histochemical localization of lignin. The appearance of the red color indicated the presence of lignin. For cellulose detection we used calcofluor solution and evaluated the sections under a fluorescent microscope. The appearance of fluorescent blue staining indicated the presence of cellulose in the cell walls. The analyses were carried out at the Plant Anatomy Laboratory of the UFRGS Biosciences Institute.
To perform chemical analysis, plants of each genotype were collected on a linear meter of a competitive sowing line of each experimental unit at the full-flowering stage, with the leaves and leaf sheaths removed. Subsequently, we dried the stems in a forced-air drying oven at 65 ºC until achieving a constant mass. The stems were ground for further analysis at the Food Research Center at the University of Passo Fundo, RS. We analyzed oat plant stems for lignin, neutral detergent fiber, and acid detergent fiber contents to estimate approximate cellulose concentrations, calculated according to Silva and Queiroz (2009).
Weather data
There were short periods without any rain in 2017, which were in the second half of June and at the ends of July and August. That year, the average monthly temperatures were 14.7, 16.2, and 19.5 for July, August, and September, respectively. The temperature peaks above 30 °C occurred in September, the month of plant flowering. The total rainfall in these months were 26.8, 95.6, and 143.0 mm, respectively.
In 2018, in the environments 4, 5, and 6 rainfalls until flowering was approximately 80 mm higher than in the environments 7, 8, and 9 (Fig. 1). The accumulated rain fall was 153.6, 121.4, and 183.4 mm for the months of July, August, and September of 2018, respectively. In 2018, the maximum temperatures occurred after flowering (10–18 September), with monthly average temperatures of 13.7, 13.5, and 18.7 °C for July, August, and September, respectively, corresponding to a milder weather compared to 2017.
The average photosynthetically active radiation from emergence to flowering in 2017 was 685 μmol m−2 s−1, while the average photoperiod was approximately 11 h and 20 min. In 2018, the average photosynthetically-active radiation was 584 μmol m−2 s−1; this reduction was primarily due to a significantly higher number of rainy days in 2018. In 2018, from emergence to flowering, the average photoperiod was approximately 11 h and 15 min.
Statistical analysis
Analysis of variance was performed all parameters, comparing genotypes, environments and their interactions, using the ‘PROC GLM’ procedure of the SAS 8.0 statistical package (SAS Institute. Inc., 2000). Means of each assessed trait were compared using the Duncan multiple comparison test when no significant interaction was detected. Both tests were performed at a 5% and 1% probability of error.
Spearman’s rank correlation coefficients among all the evaluated characters were estimated in order to determine the degree of association between the ranking of the recorded values for each pair of assessed traits. For this, we use the procedure ‘PROC CORR,’ option ‘SPEARMAN’ in SAS 8.0 (SAS Institute. Inc., 2000). Linear relationships between resistance to lodging (dependent variable) and the stem morphological characters (independent variables) of the five oat genotypes were estimated through simple and multiple linear regression analysis; avoiding models that included highly colinear independent variables. Principal component analysis was performed using PAST 3.26 software (Hammer et al. 2001).
In addition to these quantitative analyses, we performed qualitative visual analyses on the photographs taken from the anatomical sections to identify differences among genotypes in terms of lignin and cellulose contents.
Results
Morphological characteristics
Stem morphological data for the five oat genotypes are shown in Fig. 2. There was a significant genotype x environment interaction for all morphological parameters, which are dependent on the growing season in which the oat plants were grown (p = 0.01). Due to this interaction, the differences among genotypes were not always significant between the tested environments (Fig. 1a–d). The lodging resistant genotypes, regardless of the growing environment, presented lower stem length (Fig. 2a) and higher stem thickness (Fig. 2d and Supplementary Table 2). More significant differences between resistant and susceptible genotypes concerning the length of internodes were observed in LFI (Fig. 2b), with resistant genotypes having shorter internodes, particularly in environments 1, 2, and 3 during the 2017 season.
Stem characters averages of five oat genotypes under nine different environments (E1–E9). A) Total culm length (TCL) (cm); B) Length of the first expanded basal internode (LFI) (mm); C) Diameter of the first expanded basal internode (DFI) (mm); D) Culm thickness of the first expanded basal internode (TFI) (mm). Bars represent the standard error of the mean. Averages followed by the same letter do not differ by Duncan's multiple range test between genotypes within the same environment. p = 0.05. n = 825
On average, the first expanded basal internode was shorter with smaller diameters and with thicker stem walls than the second internode, on the different oat genotypes. Mean stem wall thicknesses in the lodging resistant genotypes were highest in environments 1, 7, and 9 (Fig. 2d). These same genotypes had shorter culm length and shorter length of the two expanded basal internodes (Supplementary Table 2).
The differences of average grain yield among genotypes were small but significant, which lodging susceptible genotype UFRGS 127013–1 being slightly, but significantly, superior to the other ones (data not show). Differences in grain yield among environments were a little bit higher, but the difference between the highest and the lowest yielding environments did not surpass 15% (Supplementary Fig. 6).
In one hand, lodging resistance scale (LRS) was significantly and positively correlated with diameters of the first (DFI) and the second expanded basal internodes (DSI) (R2 = 0.44 and R2 = 0.74, respectively) and with thickness of the first (TFI) and second expanded basal internodes (TSI) (R2 = 0.61 and R2 = 0.50, respectively). In the other hand, LRS was negatively correlated with length of the total stem and of both expanded basal internodes (Supplementary Table 4). At the same time, total stem length (TSL) was negatively correlated with stem diameter and stem wall thickness of the first two expanded internode. While these two last traits were positively correlated to each other (Supplementary Table 4). These same association were expected to show in the linear regression analyses, which showed that DSI alone explains 52% of the lodging resistance scale variation, while the combined action total stem length (TSL) and diameter of second expanded basal internode (DSI) explained 64% of this variation (Supplementary Table 5). In general, the linear regression analysis reveal that a higher lodging resistant is associated with shorter plants, with thicker stem walls and thicker stem diameters (Supplementary Table 5). In the principal component analysis, the total stem length (TSL) explained 83.3% of the stem morphology data variation; while another 10.12% and 6.51% were explained by the lengths of the first (LFI) and second (LSI) expanded basal internodes, respectively. The remaining variables explained only 0.07% of total variation (Supplementary Fig. 4 and Supplementary Table 3).
Histochemical location, anatomical features, and chemical composition
Lignin is primarily present in the sclerenchyma ring, in the bundle sheath sclerenchyma, and in parenchymatous tissues with thicker cell walls, but in a lesser extent in these last tissues (Fig. 3a–d). The lodging resistant genotypes had higher levels of lignin, based on visual inspection of the histological photographs (Supplementary Fig. 7). However, there were no significant differences in lignin content of the whole stems between the resistant and susceptible genotypes in the chemical analysis (Supplementary Fig. 4). In addition, there was significantly higher number of cell layers in the subdermal parenchyma (SP) (Supplementary Table 6), accompanied by significant higher deposition of cellulose in this tissue in the lodging resistant genotypes (Fig. 4e-f and Supplementary Fig. 8). Nevertheless, the chemical analysis of cellulose content did not reveal significant differences between genotypes (data not shown).
A–D: Cross-sections of the first expanded basal internode, phloroglucinol + HCl staining showing anatomical characteristics and deposition of lignin (reddish color) in different oat genotype tissues, under optical microscopy with 10X magnification (100 µm scale); E–H: Cross-sections of the first expanded basal internode, calcofluor staining showing anatomical characteristics and cellulose deposition on the cell wall of various tissues of oat genotypes, under optical microscopy at 10X magnification (100 µm scale). Oat plants cultivated under sowing density of 300 viable seeds for square meter in two growing seasons in 2018. A and E: UFRGS 127013–1 grown in the full-season; B and F: UFRGS 127013–1 grown in the short-season in 2018; C and G: URS Taura grown in the full-season; D and H: URS Taura grown in the short-season. EP: epidermis; SP: subdermal parenchyma; SR: sclerenchymal ring; CP: cortical parenchyma; MP: medullary parenchyma; SVB: small vascular bundles; LVB: large vascular bundles; BSS: bundle sheath sclerenchyma
The number of cell layers in the sclerenchyma ring did not show significant differences among the lodging susceptible genotype UFRGS 127013–1 and other genotypes in the first sowing date (environment 5), but UFRGS 127013–1 had higher number of cell layer for this tissue in the second sowing date (environment 8) (Supplementary Table 6). In one hand, although the sclerenchyma ring provides mechanical support, the number of cell layers in sclerenchyma ring it does not appear to be directly associated with differences in lodging resistance and is influenced by the environment. Once, on average, there were more cell layers in the sclerenchyma ring in environment 5 than in environment 8, suggesting that the character expression depends on the sowing date (Supplementary Table 6). On the other hand, visual analysis of lignin deposition on the extreme genotypes UFRGS 127013–1 (lodging susceptible) and URS Taura (lodging resistant) shows that URS Taura has higher levels of lignin in the sclerenchyma ring (Fig. 3a–d). When visually analyzing the cellulose deposition on the cell of the sclerenchyma ring there is no noticeable difference between these two extreme genotypes, in any of the sowing date (Fig. 3e–h).
The number of cells in the cortical parenchyma (CP) trends to be higher in more lodging resistant genotypes, especially at the second sowing data (Supplementary Table 6). Visual analysis of the cortical (CP) and medullar parenchyma cell layers shows a trend of higher cellulose deposition at second sowing date, among all genotypes, without any perceptible difference among genotypes, within the same growing condition (Supplementary Fig. 8). While no major differences can be seen on these same two parenchyma tissues for lignin deposition for most of genotypes (Supplementary Fig. 7), except for the most lodging resistant genotype, URS Taura, which shows higher lignin staining in the cortical parenchyma (CP) cells (Fig. 3a–d and Supplementary Fig. 7). The number of vascular bundles was not related to lodging resistance; however, it changed under the environment's influence, without any stability in the direction of the changes among genotypes (Supplementary Table 5). The total number of cell layers of the first expanded basal internode of the oat stems was lower in most lodging susceptible genotype (UFRGS 127013–1), while was higher in URS Taura, at both sowing dates; for the other three oat genotypes total number of cell layers was higher in the first sowing date, when compared to UFRGS 127013–1 (Supplementary Table 6).
Discussion
Shorter length (LFI) and larger stem wall thickness (TFI) of the first expanded basal internode were characteristics of the lodging resistant oat genotypes (Fig. 1a–d). This association was also found in lodging resistance genotypes of wheat and rice (Kelbert et al. 2004; Kong et al. 2013; Shah et al. 2017). In general, reduction of plant height decreases the risk of lodging because of the reduction of the bending stress at the base of the culm in unfavorable weather conditions (strong wind and rain) (Tumino et al. 2017). The stem diameter at first internode (DFI) do not always differs among genotypes, but at some environmental conditions, such as environments 4, 5, 6 and 9, the most resistant genotypes show greater stem diameter (Fig. 2c). DFI and stem wall thickness of the first expanded basal internode (TFI) appeared to be important to lodging resistance, as revealed by linear regression analysis to explain the lodging resistance rates (Supplementary Table 5). In most oat varieties the internodes are hollow, thus their flexural rigidity is greatly dependent of both diameter and stem wall thickness combination (Pinthus 1974; Mohammadi et al. 2020).
The increase in the number of plants per square meter almost did not have an effect on increasing the total stem length (TSL), except for the first sowing date of 2018, environments 5, 6 and 7 (Fig. 2a), but resulted in a decrease of stem wall thickness, environments 3, 6, and 9 (Fig. 2d), possibly due the competition for light. These results suggest that the decrease in sowing density can reduce the lodging rates in oats by reducing culm length and increasing culm diameter. Although some authors recommend this practice (Berry et al. 2007; Shah et al. 2017), it may not be compatible with maximizing grain yield. Our data show that lower seed rating may affect negatively grain yield, while the highest sowing density tends to result in higher grain yield, on the average of the genotypes (Supplementary Fig. 6). Even with an increase in the number of plants per square meter, the stem wall thickness was more stable in the resistant genotypes, which have thicker stem walls (Fig. 2d). Even though utilization of growth regulators is becoming a common practice on the oat crop, it represents an increase in production costs. Therefore, selection of oat genotypes with greater resistance to plant lodging through genetic improvement, remains the best alternative to reduce plant lodging under different cultivation conditions. Our results indicate that selection for shorter plants, as a result of shorter internodes, combining thicker stems and stem walls at the basal expanded internodes (Supplementary Tables 4 and 5).
A recent study conducted by Wu and Ma (2019) indicated that oat leaf architecture appears to contribute to lodging resistance at high densities. In this study, it was observed that with increasing plant density from 200 to 600 plants m−2, lodging resistance increased by 11.5%, and by 3.2%, for cultivars with erect leaves, in relation to genotypes with prostrate leaves, implying that erect leaf posture can promote lodging resistance, especially under conditions of high plant population, because of the light intercept.
Plant growth was higher in environments 1, 2, and 3 (Fig. 2a), which provided higher photosynthetic radiation levels and higher average temperatures, leading to taller plants. The smallest culm length (TSL), accompanied by a shorter length of the first expanded internode (LFI), were observed in environments 7, 8, and 9, which corresponds to the delayed sowing (in July), in comparison to the best sowing window (June). Under these late sowing conditions plants face daily increases in day length, from the beginning of their development, contributing to faster plant growth, entering earlier in the reproductive phase, resulting in reduced plant growth. Late sowing (environments 7, 8, and 9) also contributed to an increase in stem wall thickness of the first expanded basal internode (TFI), especially in the lodging resistant genotypes (Fig. 2d), suggesting that faster growth combined with adequate photoperiod, temperature, and radiation, increases culm thickness. In environments 4, 5, and 6, there were no significant differences between genotypes for TFI (Fig. 2d), which may have resulted due to greater water availability and milder temperatures compared to 2017.
The resistant oat genotypes showed thicker stem walls under environments 1, 7, and 9; the first two were due to the lower sowing densities and due to weather favorable conditions. In environment 9, with late sowing and higher plant density, all genotypes presented thinner stem walls, but the differences between more lodging resistant and less lodging resistant genotypes were very evident (Supplementary Fig. 2d). Taken together, the data for stem wall thickness points out for the higher sensibility of the more lodging susceptible genotypes to changes in the environments, in contrast the higher stability of the more resistant ones.
Lodging resistant oat genotypes showed higher numbers of cell layers in the subdermal parenchyma tissue (Fig. 3a–d). Previous studies on wheat also found increases in number of cell layers in the parenchymatous tissues (Kong et al. 2013; Kelbert et al. 2004). Dunn and Briggs (1989) reported that the stem standability increased with the thickness of the parenchyma layer in barley, and Palombini et al. (2016) found that the parenchyma was resistant tissue in bamboo stems. Thus, lodging resistance in the evaluated oat genotypes may result from the presence of more developed parenchymatous tissues along the culm circumference. In addition, lignin was found in the parenchyma cells as a subdermal and cortical parenchyma (Fig. 3a–d). This structural compound helps reinforce these stem tissues' cell walls, resulting in larger resistance to mechanical damage, especially in the resistant genotypes, which show well developed subdermal fundamental parenchyma, in comparison with the most lodging susceptible oat genotype of this study. In sorghum genotypes, lignin in parenchyma cells was also reported (Wilson et al. 1993). A significant positive relationship between lodging resistance and the proportion of the lignified tissues in the cross section of the basal internodes was found by Multamäki (1962) for oats and for barley.
The resistant genotypes visually presented higher lignin concentrations than the other genotypes (Fig. 3c–d). However, in a previous study on wheat, Kong et al. (2013) found no differences in staining intensity in genotypes with varying levels of resistance. Köhler and Spatz (2002) found that not all lignin types were mechanically important; therefore, the total amount of lignin is not necessarily a direct indication of more resistant culms. This finding corroborates our quantitative results (Supplementary Fig. 4). There was no relationship between lodging resistance levels and quantitative levels of lignin in the two environmental conditions that our set of oat genotypes was evaluated. These results are in line with previous findings in other plants species, such as rice (Kashiwagi et al. 2006) and wheat (Kong et al. 2013). On one hand, Heuschele et al. (2020) also found no differences between lignin levels when evaluating different cereals (oat, wheat, and barley). These authors point out that these factors may independently have only minor effects on lodging resistance. However, collectively these minor effects may be important in creating a model that predicts lodging (Heuschele et al. 2020). On the other hand, adopting more sophisticated evaluation methods, such as biochemical analysis with infrared spectroscopy and plasma optical emission spectrometry, Muszynska et al. (2021) identified high levels of lignin in lodging-resistant rye genotypes.
There were no differences between the resistant and susceptible oat genotypes for cellulose deposition in the cells of the oat stems. According to Wang et al. (2006), in wheat, cell wall resistance is related to the amount of cellulose and not lignin. Our finding may be because our quantification method was based on estimates and not absolute values. Histologically, the most significant differences for cellulose were in the presence of a developed subdermal parenchyma (Fig. 3e–h), suggesting that this compound may be important for lodging resistance in oats. Nevertheless, the growing environment appears to influence calcofluor staining intensity and distribution pattern of cellulose in the cells of the oat stems (Fig. 3e–h).
For Zhang et al. (2020), higher non-structural and structural carbohydrate contents of the culm contributed to resistance to lodging, especially the cellulose and sucrose content in the second node at the base of the oat plant. However, Muszynska et al. (2021) when evaluating rye and wheat, reported that other components are important for lodging resistance, not only lignin and cellulose, but also xylan, zinc and silicon. In Rye, the mechanical strength of the stems is probably not associated with lignin, but rather caused by a shift from hemicelluloses to xylan, which adds elasticity and tensile strength to cell walls. These finds suggest further studies on oats may benefit from other evaluation methods and from assessing other cell compounds.
Part of the anatomical features that were evaluate in our study did not show any evidence of being directly related to lodging resistance, probably because they were more influenced by the environment conditions, especially the sowing time. In a study with rye and wheat, Muszynska et al. (2021) showed that lodging resistance lines had higher number of vascular bundles; for rye, the degree of inclination of the vascular bundles was also important. This allows the culm to be more rigid due to an increase in mechanical stabilization structures. In addition, the shape of these fibrils, such as long, compact cellulose, has been found to be associated with lodging resistance.
Although selection for lodging resistance is routine in most oat breeding programs, studies of the causes of lodging susceptibility and lodging resistance are rare in oats. Knowledge on trait associations with lodging resistance is essential to determine whether a given trait may be used as an effective selection criterion for lodging resistance improvement in an oat breeding program. Breeders have long encountered difficulties in identifying practical and reliable methods for determining lodging resistance of oat genotypes due to the environment's effects. In most breeding programs, the basis for comparing lodging resistance levels among oat genotypes is natural lodging occurring in plots on field trials conducted in different locations and years. Unfortunately, usually lodging does not occur uniformly among plots and trials, occurring strong effects in plots adjacent to more susceptible genotypes to lodging.
Selection for short plant height has long been one of the strategies to achieve higher lodging resistance on cereals. Our findings point to the direction that selection of plants stronger/thicker cell walls at basal expanded internodes, associated with thicker stem diameter may be an effective strategy for improving lodging resistance in oat breeding program. Strategy that may be more effective if associated with selection for shorter stem length, given that most of the variation of lodging resistance was explained by total stem length or its associated traits, length of the first and second expanded basal internodes.
Promising genotypes for lodging resistance might benefit from more laborious and tedious evaluations such as lignin deposition through Phloroglucinol + HCl staining and analysis of cell layer composition of stem wall tissues of basal expanded internodes. Our results also suggest the need for further research on the structural components of the cell wall and tissues and the role of the parenchyma in lodging resistance of oats.
Conclusions
Shorter stem length and greater stem wall thickness in the first and second expanded basal internode are morphological characteristics associated with lodging resistance in Avena sativa. While stem wall thickness showed to be a more stable trait among environments, stem diameter is more prone variation due to environmental conditions. Nevertheless, the most lodging resistant oat genotypes showed greater phenotypic stability for stem diameter among environments, besides the fact that these resistant genotypes tend to have thicker diameter of the basal expanded internodes compared to the genotypes which are more susceptible to lodging.
In the evaluated genotypes, there were no differences in lignin and cellulose contents, measured in the bulk of the whole plant stems; however, there were differences among lodging resistant and lodging susceptible genotypes, both for the distribution pattern and for the staining intensity of lignin and cellulose present in the stem cell walls of the first expanded basal internode. Oat genotypes with better resistance to lodging revealed a higher deposition of lignin in the cells of the first expanded basal internode, especially at the schlerenchymal ring.
Except for the number of layers of cells in the subdermal parenchyma, all anatomical characteristics were mostly influenced by the environment and not by the lodging resistance of the oat genotypes. More lodging resistant genotypes showed a higher number of cell layers at the subdermal parenchyma, compared to more susceptible ones.
Higher temperature, larger photoperiod, and higher radiation lead to increased stem length, which may cause higher lodging rates in the field. In one hand, delayed sowing may be used as a strategy to decrease stem length and plant lodging, in consequence, but it also may result in reduced grain yield. On the other hand, higher sowing rates may be adopted together with the decision for late sowing dates, leading to higher grain yields under less conducive conditions to lodging.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Berry PM, Spink J (2012) Predicting yield losses caused by lodging in wheat. Field Crop Res 137:19–26. https://doi.org/10.1016/j.fcr.2012.07.019
Berry PM, Griffin JM, Sylvester-Bradley R, Scott RK, Spink JH, Baker CJ, Clare RW (2000) Controlling plant form through husbandry to minimize lodging in wheat. Field Crop Res 67:59–81. https://doi.org/10.1016/S0378-4290(00)00084-8
Berry PM, Sylvester-Bradley R, Berry S (2007) Ideotype design for lodging-resistant wheat. Euphytica 154:165–179. https://doi.org/10.1007/s10681-006-9284-3
Dunn GJ, Briggs KG (1989) Variation in culm anatomy among barley cultivars differing in lodging resistance. Can J Bot 67:1838–1843. https://doi.org/10.1139/b89-232
Fontaneli RS, Fontaneli RS, Santos, HP Oliveira JT, Lehmen RI, Dreon G (2012) Gramíneas forrageiras anuais de inverno. In: Fontaneli RS, Fontaneli RS, Santos HP (eds). Forrageiras para integração lavoura-pecuária-floresta na região sul-brasileira. 2ª ed. Embrapa, Brasília. https://www.alice.cnptia.embrapa.br/bitstream/doc/1010247/1/LV2012forrageirasparaintegracaoFontaneli.pdf
Foulkes MJ, Slafer GA, William JD, Berry PM, Sylvester-Bradley R, Martre P, Calderini DF, Griffiths S, Reynolds MP (2011) Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J Exp Bo 62(2):469–486. https://doi.org/10.1093/jxb/erq300
Hammer O, Harper DAT, Ryan PD (2001) Past: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):art. 4:9pp. https://palaeo-electronica.org/2001_1/past/past.pdf
Heuschele DJ, Smith KP, Annor GA (2020) Variation in lignin, cell wall-bound p-coumaric, and ferulic acid in the nodes and internodes of cereals and their impact on lodging. J Agric Food Chem 68(45):12569–12576. https://doi.org/10.1021/acs.jafc.0c04025
IRGA. Instituto Riograndense do Arroz (2019) Médias climatológicas
Karim MH, Jahan MA (2013) Study of lodging resistance and its associated traits in bread wheat. J Agric Biol Sci 8(10):683–687
Kashiwagi T, Madoka Y, Hirotsu N, Ishimaru K (2006) Locus prl5 improves lodging resistance of rice by delaying senescence and increasing carbohydrate reaccumulation. Plant Physiol Biochem 44(2):152–157. https://doi.org/10.1016/j.plaphy.2006.02.004
Kelbert AJ, Spaner D, Briggs KG, King JR (2004) The association of culm anatomy with lodging susceptibility in modern spring wheat genotypes. Euphytica 136:211–221. https://doi.org/10.1023/B:EUPH.0000030670.36730.a4
Köhler L, Spatz HC (2002) Micromechanics of plant tissues beyond the linear-elastic range. Planta 215(1):33–40. https://doi.org/10.1007/s00425-001-0718-9
Kong E, Liu D, Guo X, Yang W, Sun J, Li X, Zhan K, Cui D, Lin J, Zhang A (2013) Anatomical and chemical characteristics associated with lodging resistance in wheat. Crop J 1(1):43–49. https://doi.org/10.1016/j.cj.2013.07.012
Lima UM (2019) Barreiras fitossanitárias sobre as importações no brasil: o caso da aveia. IPEA, Brasília, 61p. http://www.ipea.gov.br/portal/index.php?option=com_content&view=article&id=34457&Itemid=432
Mohammadi M, Finnan J, Sterling M, Baker C (2020) A calibrated oat lodging model compared with agronomic measurements. Field Crops Res 255:107784. https://doi.org/10.1016/j.fcr.2020.107784
Multamäki K (1962) The effect of seed size and depth of seeding on the emergence of grassland plants. Agric Food Sci 34(1):18–25. https://doi.org/10.23986/afsci.71585
Muszynska A, Guendel A, Melzer M, Moya YAT, Röder MS, Rolltschek H, Rutten T, Munz E, Melz G, Ortleb S (2021) A mechanistic view on lodging resistance in rye and wheat: a multiscale comparative study. Plant Biotechnol J. https://doi.org/10.1111/pbi.13689
Nakhforoosh A, Kumar S, Fetch T, Fetch JM (2020) Peduncle breaking resistance: a potential selection criterion to improve lodging tolerance in oat. Can J Plant Sci 100(6):707–719. https://doi.org/10.1139/cjps-2019-0286
Norden AJ, Frey KJ (1959) Factors associated with lodging resistance in oats. Agron J 51(6):335–338. https://doi.org/10.2134/agronj1959.00021962005100060009x
Palombini FL, Kindlein W Jr, Oliveir BF, Mariath JEA (2016) Materials characterization bionics and design: 3D microstructural characterization and numerical analysis of bamboo based on X-ray microtomography. Mater Charact 120:357–368. https://doi.org/10.1016/j.matchar.2016.09.022
Pinthus MJ (1967) Spread of the root system as indicator for evaluating lodging resistance of wheat. Crop Sci 7(2):107–110. https://doi.org/10.2135/cropsci1967.0011183X000700020005x
Pinthus MJ (1974) Lodging in wheat, barley and oats: the phenomenon, its causes and preventative measures. Adv Agron 25:209–263. https://doi.org/10.1016/S0065-2113(08)60782-8
Santos HG, Jacomine PKT, Anjos, LHC, Oliveira, VA, Lumbreras JF, Coelho MR, Almeida JA, Araújo Filho JC, Oliveira JB, Cunha TJF (2018) Sistema brasileiro de classificação de solos. 5ª Edição, revista e ampliada EMBRAPA, Brasília. https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1107206/sistema-brasileiro-de-classificacao-de-solos
SAS Institute (2000) SAS/STAT software: Changes and enhancements through release 8.0. Statistical Analysis System Institute, Cary
Shah AN, Tanveer M, Rehman A, Anjum SA, Iqbal J, Ahmad R (2017) Lodging stress in cereal–effects and management: an overview. Environ Sci Pollut Res 24(6):5222–5237. https://doi.org/10.1007/s11356-016-8237-1
Silva DJ, Queiroz AC (2009) Análise de alimentos: métodos químicos e biológicos. 3ª Ed. UFV, Viçosa.
Stapper M, Fischer RA (1990) Genotype, sowing date and plant spacing influence on high-yielding irrigated wheat in southern New South Wales. II. Growth, yield and nitrogen use. Aust J Agric Res 41(6):1021–1041. https://doi.org/10.1071/AR9901021
Tams AR, Mooney SJ, Berry PM (2004) The effect of lodging in cereals on morphological properties of the root-soil complex. In: Proceedings of the SuperSoil 2004: 3rd Australian New Zealand soils conference. University of Sydney, Sidney, 5–9 December 2004. p 1–8. http://www.regional.org.au/au/asssi/supersoil2004/s9/oral/1998_tamsa.htm
Tripathi SC, Sayre KD, Kaul JN, Narang RS (2003) Growth and morphology of spring wheat (Triticum aestivum L.) culms and their association with lodging: effects of genotypes, N levels and ethephon. Field Crops Res 84(3):271–290. https://doi.org/10.1016/S0378-4290(03)00095-9
Tumino G, Voorrips RE, Morcia C, Ghizzoni R, Germeier CU, Paulo MJ, Terzi V, Smulders MJM (2017) Genome-wide association analysis for lodging tolerance and plant height in a diverse European hexaploid oat collection. Euphytica. https://doi.org/10.1007/s10681-017-1939-8
USDA. United States Department of Agriculture Foreign Agricultural Service (2020) Grain: world markets and trade
Wang J, Zhu J, Lin Q, Li X, Teng N, Li Z, Li B, Zhang A, Lin J (2006) Effects of stem structure and cell wall components on bending strength in wheat. Chin Sci Bull 51:815. https://doi.org/10.1007/s11434-006-0815-z
White EM (1995) Structure and development of oats. In: Welch RW (ed) The oat crop: production and utilization. Chapman & Hall, London, pp 88–119
Wilson JR, Mertens DR, Hatfield RD (1993) Isolates of cell types from sorghum stems: digestion, cell wall and anatomical characteristics. J Sci Food Agric 63:407–417. https://doi.org/10.1002/jsfa.2740630406
Wu W, Ma BL (2019) Erect–leaf posture promotes lodging resistance in oat plants under high plant population. Eur J Agron 103:175–187
Zhang R, Jia Z, Ma X, Ma H, Zhao Y (2020) Characterising the morphological characters and carbohydrate metabolism of oat culms and their association with lodging resistance. Plant Biol 22(2):267–276. https://doi.org/10.1111/plb.13058
Zhu L, Shi GX, Li ZS, Kuang TY, Li B, Qk W, Bai KS, Hu YX, Lin JX (2004) Anatomical and chemical features of high-yield wheat cultivar with reference its parents. Acta Botanica Sinica 46(5):565–572
Zuber U, Wnzeler H, Messmer MM, Keller M, Keller B, Schmid JE, Stamp P (1999) Morphological traits associated with lodging resistance of spring wheat (Triticum aestivum L.). J Agronomy Crop Sci 182(1):17–24. https://doi.org/10.1046/j.1439-037x.1999.00251.x
Acknowledgements
The first author was recipient of scholarship from the Coordination Agency for the Improvement of Higher Education Personnel (CNPq).
Funding
Jéssica Argenta has received scholarship to the Coordination Agency for the Improvement of Higher Education Personnel (CNPq). Research Support has received from Foundation of the State of Rio Grande do Sul (FAPERGS) for the research funding (Pronex 16/2551–0000484-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interests.
Consent to participate
All the participants participate in this work.
Consent for publication
All the participants consent to publish this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Argenta, J., Pacheco, M.T., de Araujo Mariath, J.E. et al. Morphological, anatomical, and chemical characteristics associated with lodging resistance in Avena sativa. Euphytica 218, 22 (2022). https://doi.org/10.1007/s10681-022-02971-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-022-02971-8