Abstract
Upland rice production plays an important role in both household consumption and crop rotation. Until now, a blast resistant upland variety has not been released in Thailand. The bimodal pattern of rain distribution in Thailand’s upland rice production areas create a favorable environment for the outbreak of leaf blast when seedling-tillering, and neck blast within the heading stage. The use of genetically resistant cultivars has proven to be an effective way to cope with this problem. In this study, 256 indigenous upland rice plants were screened for blast resistance under greenhouse and field conditions. Ten indigenous upland rice varieties, ULR292, ULR242, ULR219, ULR162, ULR161, ULR134, ULR109, ULR098, ULR081, and ULR066, were identified as resistant to leaf blast disease in both natural infection and artificial inoculation, under greenhouse conditions. Additionally, six of the ten varieties, ULR162, ULR161, ULR134, ULR109, ULR098, and ULR081, were found to be resistant to neck blast under field conditions. These new sources of blast resistance identified from indigenous upland rice varieties proved more resistant than the check varieties, depicting their potential for further use in Thailand’s rice blast resistance improvement program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is a staple food for the world’s population (Maclean et al. 2013). In Thailand, rice is an economic crop grown in both lowland and upland areas. Northeastern Thailand is the largest area of rain-fed rice production, encompassing more than 50% of the country’s total rice production area with 6.4 M ha (2014). Moreover, more than 0.8 M ha in Northeastern Thailand has the potential to grow upland rice (Office of Agriculture Economics 2015). Farmers generally grow sugarcane or cassava as economic crops to rotate with upland rice, for improved soil fertility, increased income, and household consumption. However, upland rice productivity is often limited by various constraints. Rice blast, caused by Pyricularia oryzae, is considered the most devastating disease of rice worldwide, due to its extensive distribution and destructiveness; which limits rice production, since the disease can influence crop growth throughout the crop cycle (Chin 1994). Under favorable conditions, the disease creates yield losses of up to 80% (Joshi et al. 2009), including upland areas (Variar et al. 2009). The disease spreads quickly during Thailand’s rainy season, together with high humidity levels and nitrogen fertilizer (Eamchit and Mew 1982).
The use of genetic cultivar resistance has proven to be an effective way to control rice blast disease (Chen et al. 2006). Currently, more than 100 rice blast resistance genes have been identified (Sharma et al. 2012). However, the heterothallic reproduction and genetic modification of AVR genes have created a genetic variability, and emergence of new virulent races (Dai et al. 2010). These new strains are capable of developing an adaptation to overcome resistant varieties, causing them to become susceptible to blast disease (Sharma and Pandey 2012).
For centuries, indigenous rice was selected and maintained in specific areas, which has allowed it to adapt to a wide range of agro-ecological conditions, such as disease, insects, and environmental stress (Chang 1976). Indigenous upland rice has played important multiple roles in the development of household consumption, sufficiency economy, and cultural activity; and has a high diversity of characteristics, including resistant genes (Samiullah et al. 2015). Several researchers have identified new sources of blast resistance from indigenous upland rice germplasm (Chauhani et al. 2000; Dar et al. 2015; Ghazanfar et al. 2009; Liang et al. 2017; Vasudevan et al. 2014). In Thailand’s northeast, the most widely grown upland rice variety is Sakon Nakhon (SKN), which is susceptible to blast disease. Moreover, the region’s bimodal rain distribution provides favorable conditions for the spread of disease in both seedling tillering and within the heading stage, due to leaf blast and neck or panicle blast, respectively (Jongdee et al. 2004). To date, no resistant cultivar of upland rice has been released.
The objective of the present study was therefore to identify a new source from indigenous upland rice germplasm resistant to blast disease in natural and artificial inoculations, under both greenhouse and field conditions. The results intend to provide a new resistant indigenous upland rice variety for further breeding programs.
Materials and methods
Greenhouse evaluation (natural infection)
Two-hundred and fifty-six indigenous upland rice varieties together with five resistance checks [Jao Hom Nin (JHN), IR64, P0489, Azucena, and IR62266)] and two susceptibility checks (KDML105 and RD6) were used in this study (Fig. 1). The experimental design, a completely randomized design (CRD) with three replications, was developed at Khon Kaen University (KKU), Khon Kaen, Thailand. The experiments were conducted over 2 year period, in the rainy season of 2015 and 2016. Performed under greenhouse conditions in order to reduce external factors, rice seeds were sown in plastic trays and let to grow two plants/hill. Fertilizer was applied at 14 and 20 days after planting with 28.12 kg/ha of N, P2O5, and K2O. Greenhouse temperature and humidity were adjusted to optimize blast infection via a mist nozzle (disease infection in rainy season by natural infection). Disease severity (leaf blast) of all plant subjects was scored and recorded at 28 days after planting, employing the standards of the International Rice Research Institute (0–9; 0 = no lesion; 1 = small brown specks; 3 = small necrotic brown spots, about 1–2 mm; 5 = infection of 4–10% of the leaf area; 7 = infection of 26–50% of the leaf area; and 9 = infection of greater than 51% of the leaf area, in which many leaves are dead) (IRRI 2002).
Greenhouse evaluation (artificial inoculation)
Experiments were conducted to confirm the resistance of all varieties for both experimental years at the Ubon Ratchathani Rice Research Center (URRC). Forty upland rice varieties which maintained a consistent resistance in both years, and which provided a sufficient number of seeds were selected from natural infection experiment. Consisting of 39 from Thai germplasm and a single variety from Laos, they were subjected to artificial inoculation tests in greenhouse conditions using multiple isolates with four resistance checks (JHN, IR64, P0489 and Azucena), and three susceptibility check varieties (SKN, KDML105 and RD6). In this study, the experimental unit was laid out using CRD with three replications in order to evaluate blast resistance in the seedling stage. Rice varieties were planted in plastic trays, four plants/row for each replication, and kept in the greenhouse. Fertilizer was applied three times daily, on days 9, 11, and 14; with 75 kg N/ha. A total of five isolates of Pyricularia oryzae, which were from different sources, and contained breaking resistant genes were utilized in our study (Supplementary Table 1), including four isolates which were kindly provided by the URRC (UBN71684, NKI11397, KKN191082, and SKN205327). The fifth isolate was identified and isolated from the susceptible rice variety (RD6) from KKU’s natural infection test of 2016 (KKU2016), and was used as an inoculum. The pathogens were cultured on rice polish agar (rice bran 20 g, yeast extract 2 g, agar 20 g, and distilled water 1000 ml) and incubated for 10 days under dark conditions at 25 ± 2 °C. Sporulation was induced using sterilized rice leaves, placed on a petri dish where mycelium grow, then incubated at 25 ± 2 °C under fluorescent lighting for 4 days. Spore suspensions were counted via a haemocytometer, and concentration was subsequently adjusted to 5 × 104 spore/ml with sterile distilled water. Inoculation was accomplished using the spray method at 17 days after planting. Plants were incubated in a plastic chamber to maintain high humidity (95%) for 48 h, and then moved to the greenhouse under 25 ± 2 °C and 90% humidity. Symptoms of all test plants were observed and collected at 7 days after inoculation, following the International Rice Research Institute standard (scoring 0–9) (IRRI 2002).
Field evaluation
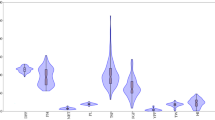
Ten selected Thai indigenous resistant rice varieties (ULR066, ULR081, ULR098, ULR109, ULR134, ULR161, ULR162, ULR219, ULR242, and ULR292) demonstrating high resistance and a sufficient number of seeds were selected from artificial inoculation experiment and ten standard check varieties included Azucena, IR62266, IR64, P0489, JHN, Thanya Sirin, and Phaladum (as resistance checks), and SKN, KDML105, and RD6 (as susceptibility checks) were used to confirm the resistance ability under field conditions. The experiment was randomized complete block design (RCBD) with three replications, developed at KKU. Plants were transplanted at 28 DAS to the field with a plot size of 1 row plot 2 m long. Spacing between row and plant was 25 × 25 cm. Plots were bordered by RD6 as trap plants. Fertilizer (28.12 kg/ha of N, P2O5, K2O) was immediately applied after transplanting, and was reapplied 45 days later. Inoculation was carried out through natural infection. The scoring of leaf blast disease on six test plants was gathered at the tillering stage using the same method employed in natural infection experiment. Moreover, the days-to-flowering and neck blast were recorded at the grain filling stage after disease incidence using the International Rice Research Institute standards (0 = no symptoms; 1 = lesions on pedicels and branches; 3 = lesions on branches and panicle axis; 5 = lesions partially surrounding panicle base; 7 = lesions completely surrounding the panicle base, with fewer than 30% of grain filled; and 9 = lesions completely surrounding the panicle base, with greater than 30% of grain filled) (IRRI 1998). The amount of rain, relative humidity (RH), and temperature during each crop cycle were also recorded (Fig. 2).
Data analysis
Rice varieties were grouped based on leaf blast scores from 2015 and 2016, a cluster analysis program was used for genotype grouping, and the frequency of disease was determined for each year. The highest common varieties sorted by resistance in both experimental years were selected for confirmation with selected isolates. The collected data was analyzed using variance, and the difference of treatment mean was calculated using the Least Significant Difference (LSD) at P < 0.05.
Results
Greenhouse evaluation (natural infection)
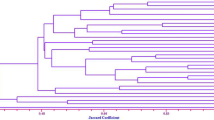
The evaluation of blast resistance in indigenous upland rice germplasm by natural infection showed that the rice varieties were different in disease reaction in both 2015 and 2016 (Supplementary Table 2). In 2015, 114 varieties were classified as resistant (scoring < 5), 111 varieties were susceptible (scoring > 5), and only a single variety was missed (Fig. 3a). In 2016, more resistant varieties (153) were observed than susceptible varieties (103) (Fig. 3b). Disease reaction within the two test years also differed, based on the categories of disease reaction for those varieties (Supplementary Table 2). After clustering, data was formed using the blast reaction of both experimental years, and the rice varieties were separated into five groups following the disease incidence (Fig. 4). Groups 1 and 2, the most resistant to blast disease, were comprised of 81 and 84 rice varieties, with an average blast score of 1.98 and 2.50, in 2015; and 3.71 and 2.30 in 2016, respectively. However, 15 varieties were discarded in each year due to seeds did not geminate.
Greenhouse experiment
The 40 indigenous upland rice varieties, belonging to groups 1 and 2 in both experiments of natural infection, were selected to confirm the resistance of multiple blast isolates. The results determined that four of the five blast isolates (NKI11397, KKN191082, SKN205327, and KKU2016) were highly virulent. Disease scores within the susceptible checks (RD6, KDML105, and SKN) ranged from 7.17 to 9.00 (Table 1). The isolate UBN71684 was not so virulent as to distinguish resistance among varieties. The 40 selected indigenous upland rice varieties were highly resistant against four virulent blast isolates; almost all of which proved greater than the Azucena, JHN, and P0489, the resistant check varieties; with equal resistance to IR64, the resistant check (Table 1). Interestingly, the resistant check varieties Azucena, JHN, and P0489 were susceptible to almost all isolates, and lost their resistance ability throughout the experiment. As a result, all 40 selected varieties were thus classified as broad spectrum resistant varieties.
Field evaluation of blast resistance of indigenous upland rice germplasm
The field experiments were designed to confirm blast disease resistance within indigenous upland rice varieties. The ten selected indigenous upland rice varieties (ULR292, ULR242, ULR219, ULR162, ULR161, ULR134, ULR109, ULR098, ULR081, and ULR066) were resistant to leaf blast as resistance check varieties, whereas susceptible check varieties proved susceptible (Table 2). Moreover, six of the ten varieties (ULR162, ULR161, ULR109, ULR098, ULR081, and ULR066) also showed a significant difference in neck blast resistance from SKN (susceptible check), with the same resistance to the Thanya Sirin, PLD, P0489, JHN, IR64, IR62266, and Azucena. Two susceptibility check varieties (RD6 and KDML105) were not assessed, due to leaf blast death, in which the plant died in the tillering stage (Table 2). The results indicate that the ten selected indigenous upland rice varieties are stable in their resistance to leaf blast and, in some cases, neck blast.
Discussion
The geography of North and Northeast Thailand remains an undulating rainfed area. Upland rice is widely grown in these areas, due to its stability in terms of household consumption (Maclean et al. 2002). Based on the occurrence of bimodal rain pattern in this particular area, rainfall usually begins in May and ends around mid-October (Jongdee et al. 2004). Leaf blast disease favors a humid climate, causing damage in all stages of rice growth. Leaf blast occurs particularly within the seedling and tillering stages; and neck blast, during the heading-grain filling stage. Thailand’s Department of Agriculture (DOA) released the Sakon Nakhon upland rice variety in early 2000. This variety is high yielding, pleasantly aromatic, and well adapted to both lowland and upland conditions. While it has been widely adopted by upland rice growers in Northeast Thailand, Sakon Nakhon is very susceptible to neck blast disease within the grain filling stage, causing significant damage to crop yield. Indigenous upland rice is widely distributed throughout the north and northeast by ethnic groups and field crop growers, making them further susceptible to biotic and abiotic threats in these areas.
In this study, we screened blast resistance in 256 upland rice accessions under disease outbreak within particular areas. Although the disease reactions were different in each experimental year, due to the pathogens present (Fig. 3), the results demonstrated that more than half of the tested accessions were classified as resistant (Fig. 3). Five groups were created based on the grouping of accessions (Fig. 4). The first group contains 24 accessions, whereas the second group is made up of 16 accessions, belonging to the resistance to blast pathogens found in both experimental years. The results identified several blast resistant accessions from rice germplasm (Castano et al. 1990; Dar et al. 2015; Ghazanfar et al. 2009; Liang et al. 2017; Vasudevan et al. 2014). The different responses of each accession were caused by the presence of diverse resistance genes (Vasudevan et al. 2014). Additionally, the disease incidence results occurring from natural infection may prove to be too insufficient to be conclusive, due to the variation in the conditions, and inadequate inoculum of pathogens (Hoque and Mansfield 2005). We have subsequently found that the number of accessions for future screening, in an attempt to provide more accurate data, may decrease the consistency of resistance in the same accessions by natural infection in both years. We therefore feel that additional field tests, such as multiple isolate inoculation, must be completed.
In the present study, the resistance accessions in Groups 1 and 2 were selected to repeat and confirm their resistance (Fig. 4). Almost all of the accessions tested for blast isolates demonstrated a high accuracy of resistance to blast disease (Table 1). Further field tests are required to confirm the field resistance of these accessions, as their reactions may differ from those of the greenhouse conditions (Aram et al. 2013; Arshad et al. 2008; Shafaullah et al. 2011). We tested ten resistant accessions representing a broad spectrum of upland rice in field conditions, demonstrating that almost every accession was resistant to blast pathogens in the field (Table 2).
The controlled greenhouse conditions interplay environmentally favorable conditions which enhance pathogenicity more so than under the field conditions. Numerous factors affect disease pathogenicity, such as plant age, temperature, and RH and inoculum concentrations. Rice is very susceptible to blast disease at the seedling stage, in which the lesion could cover most of the leaf blades, resulting in holes on dead plants and burns in the field (Faivre-Rampant et al. 2013). This symptom is generally observed in areas which have experienced an over-application of nitrogen (Eamchit and Mew 1982). In this study we over-applied nitrogen to the seedlings in order to induce the favorability of infection, which hastened the generation of both susceptibility and resistance. Moreover, some resistant varieties, such as Azucena, P0489, and JHN; were susceptible, while the selected upland rice accessions were resistant (Table 1). Excessive nitrogen has been found to increase disease susceptibility, and induce susceptibility of rice resistant varieties (Ballini et al. 2013). The IR64 did not respond to the administration of excessive nitrogen, as it possesses the Pi resistance gene; namely Pi31(t) and Pi32(t) on chromosome (chr.) 12, Pi30(t) on chr.11, Pi28(t) on chr. 10, Pi33 and Pi29(t) on chr. 8, Pi27(t) on chr. 6, Pi25(t) on chr. 2, and Pi27(t) on chr. 1 (Tanweer et al. 2015). Furthermore, the 40 indigenous rice varieties also did not respond to excessive nitrogen treatment, demonstrating the broad spectrum and stability of those upland rice accessions and resistance to leaf blast disease.
A correlation between leaf blast and neck blast remain undetermined, as some rice varieties were resistant to leaf blast, but susceptible to neck blast (Faivre-Rampant et al. 2011); some demonstrated susceptibility to leaf blast, while being resistant to neck blast (Titone et al. 2015); and some proved resistant to both leaf blast and neck blast disease (Wang et al. 2016). This evidence corresponds with the previous results of Mei et al. 2006; Miah et al. 2013; Vasudevan et al. 2015; Wang et al. 2016; Xin et al. 2014. Interestingly, the upland rice accessions we selected were not only resistant to leaf blast, but also displayed neck blast resistance (Table 2) at all growth stages, and may be a genetic source for breeding new rice cultivars with durable blast resistance.
In summary, Thai indigenous upland rice genotypes displayed a variation in their responses to blast disease under greenhouse conditions. The preliminary selection under natural infection within the seedling stage may be useful in screening large accessions. Confirmation by artificial inoculation using various blast isolates and field tests is necessary for accuracy of resistance data. Among the accessions tested in this study were the upland rice accessions ULR292, ULR242, ULR219, ULR162, ULR161, ULR134, ULR109, ULR098, ULR081, and ULR066; which were identified as new sources of blast resistance varieties in Thailand, providing a potential source of blast resistant genes for future rice breeding programs.
References
Aram P, Nadali BJ, Nadali B, Gorbanali N (2013) Leaf blast resistance of rice different genotypes in blast nursery. Int J Agric Crop Sci 5:1307–1313
Arshad HMI, Khan JA, Jamil FF (2008) Screening of rice germplasm against blast and brown spot disease. Pak J Phytopath 20(1):52–57
Ballini E, Nguyen TTT, Morel JB (2013) Diversity and genetics of nitrogen induced susceptibility to the blast fungus in rice and wheat. Rice 6:1–13
Castano JB, Amril B, Syahril D, Zaini Z (1990) Upland rice genotypes resistance to blast (B1) disease in west Sumatra. Int Rice Res Newslet 15:11–12
Chang TT (1976) The origin, evolution, cultivation, dissemination and diversification of Asia and African rice. Euphytica 25:425–441
Chauhani JS, Variar M, Shukla VD, Maiti D, Bhattacharya N, Lodh SB (2000) Screening rice genetic resources for major diseases of uplands and quality. Indian Phytopath 53:80–82
Chen X, Shang J, Chen D, Lei C, Zou Y, Zhai W, Liu G, Xu J, Ling Z, Cao G, Ma B, Wang Y, Zhao X, Li S, Zhu L (2006) A B-lectin receptor kinase gene conferring rice blast resistance. Plant J 46(5):794–804
Chin KM (1994) Collar rot, a new symptom of the rice blast disease. MARDI Res 2:82–84
Dai Y, Jia Y, Correll J, Wang X, Wang Y (2010) Diversification and evolution of the avirulence gene AVR-Pita1 in field isolates of Magnaporthe oryzae. Fungal Genet Biol 47(12):973–980
Dar SH, Rather AG, Najeeb S, Ashraf AM (2015) Screening of rice germplasm against blast disease under temperate conditions. Mol Plant Breed 6:1–4
Eamchit S, Mew TW (1982) Comparison of virulence of Xanthomonas campestris pv. oryzae in Thailand and the Philippines. Plant Dis 66:556–559
Faivre-Rampant O, Bruschi G, Abbruscato P, Cavigiolo S, Picco AM, Borgo L (2011) Assessment of genetic diversity in Italian rice germplasm related to agronomic traits and blast resistance (Magnaporthe oryzae). Mol Breed 27:233–246
Faivre-Rampant O, Geniès L, Piffanelli P, Tharreau D (2013) Transmission of rice blast from seeds to adult plants in a non-systemic way. Plant Pathol 62:879–887
Ghazanfar MU, Habib A, Sahi ST (2009) Screening of rice germplasm against Pyricularia oryzae the cause of rice blast disease. Pak J Phytopath 21:41–44
Hoque ME, Mansfield JW (2005) A simple and reliable method for pathogenicity tests of bacterial disease of rice. Bangladesh J Bot 34:11–16
IRRI (1998) Standard evaluation system for rice, 3rd edn. International Rice Testing Program, International Rice Research Institute, Manila
IRRI (2002) Standard evaluation system for rice (SES). International Rice Research Institute, Los Banos
Jongdee B, Pantuwan G, Fukai S, Fischer K (2004) Improving drought tolerance in rainfed lowland rice: an example from Thailand. New directions for a diverse planet. In: Proceedings of the 4th international crop science congress, 26 Sep–1 Oct 2004, Brisbane, Australia, p 14. www.cropscience.org.au. Accessed 21 Feb 2017
Joshi BK, Hari Bim P, Gopal P, Bedanand C (2009) Molecular tagging, allele mining and marker aided breeding for blast resistance in rice. BSN E-Bull 1:1–23
Liang Y, Yan B, Peng Y, Ji Z, Zeng Y, Wu H, Yang C (2017) Molecular screening of blast resistance genes in rice germplasms resistant to Magnaporthe oryzae. Rice Sci 24:41–47
Maclean DC, Dawe DC, Hardy B, Hettel GP (2002) Rice almanac: source book for the most important economic activity on earth, 3rd edn. CABI Publishing, IRRI, Los Baños
Maclean J, Hardy B, Hettel G (2013) Rice almanac: source book for the most important economic activities on earth, 4th edn. GRiSP (Global Rice Science Partnership) IRRI, Los Baños
Mei C, Qi M, Sheng G, Yang Y (2006) Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact 19:1127–1137
Miah G, Rafii MY, Ismail MR, Puteh AB, Rahim HA, Asfaliza R (2013) Blast resistance in rice: a review of conventional breeding to molecular approaches. Mol Biol Rep 40:2369–2388
Office of Agriculture Economics (2015) Agricultural statistics of Thailand 2015. Annual report of 2015. Ministry of Agriculture and Cooperatives, Bangkok, Thailand, p 240
Samiullah RA, Salman M, Sarwar M, Umar A, Hussain A, Habibullah NM, Hussain SM, Ayatullah MN, Akbar I (2015) Evaluation of indigenous rice germplasm for resistance to bacterial blight and yield performance. J Entomol Zool Stud 3:449–453
Shafaullah, Khan MA, Khan NA, Salim-il-Yasin, Mahmood Y (2011) Response of rice germplasm to blast disease under field conditions. Pak J Phytopath 23(1):52–55
Sharma B, Pandey MP (2012) Identification of rice germplasm with resistance to bacterial blight (Xanthomonas oryzae pv. oryzae). Bangladesh J Agric Res 37:349–353
Sharma TR, Rai AK, Gupta SK, Vijayan J, Devenna BN, Ray S (2012) Rice blast management through host-plant resistance: retrospect and prospect. Agric Res 1:37–52
Tanweer FA, Rafii MY, Sijam K, Rahim HA, Ahmed F, Latif MA (2015) Current advance methods for the identification of blast resistance genes in rice. C. R. Biol 338:321–334
Titone P, Mongiano G, Tamborini L (2015) Resistance to neck blast caused by Pyricularia oryzae in Italian rice cultivars. Eur J Plant Pathol 142:49–59
Variar M, Vera CCM, Carrillo MG, Bhatt JC, Sangar RBS (2009) Rice blast in India and strategies to develop durably resistant cultivars. In: Xiaofan W, Valent B (eds) Advances in genetics, genomics and control of rice blast disease. Springer, New York, pp 359–374
Vasudevan K, Vera Cruz CM, Gruissem W, Bhullar NK (2014) Large scale germplasm screening for identification of novel rice blast resistance sources. Front Plant Sci 5:1–9
Vasudevan K, Gruissem W, Bhullar NK (2015) Identification of novel alleles of the rice blast resistance gene Pi54. Sci Rep 5:1–11
Wang R, Fang N, Guan C, He W, Bao Y, Zhang H (2016) Characterization and fine mapping of a blast resistant gene Pi-jnw1 from the japonica rice landrace Jiangnanwan. PLoS ONE 11(12):e0169417
Xin X, Hayashi N, Wang CT, Fukuoka S, Kawasaki S, Takatsuji H, Jiang CJ (2014) Rice blast resistance gene Pikahei-1(t), a member of a resistance gene cluster on chromosome 4, encodes a nucleotide-binding site and leucine-rich repeat protein. Mol Breed 34:691–700
Acknowledgements
This research was supported by the Plant Breeding Research Center for Sustainable Agriculture and Research Center of Agricultural Biotechnology for Sustainable Economy, Khon Kaen University, Khon Kaen, Thailand; and the Ubon Ratchathani Rice Research Center (URRC). Our gratitude is also extended to the Thailand Research Fund (TRF) (Project Code: IRG5780003) and Khon Kaen University’s Faculty of Agriculture for providing financial support for the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chumpol, A., Chankaew, S., Saepaisan, S. et al. New sources of rice blast resistance obtained from Thai indigenous upland rice germplasm. Euphytica 214, 183 (2018). https://doi.org/10.1007/s10681-018-2267-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-018-2267-3