Abstract
Cowpea (Vigna unguiculata L. Walp) is a legume consumed for its high protein content. It provides nutrient-dense food opportunities for human consumption. Iron deficiency chlorosis (IDC) manifests as yellowing of the leaves and reduced plant growth, resulting in reduced yield potential. Use of IDC tolerant cowpea cultivars is an efficient method to address this problem. The objectives of this study were to conduct a population structure analysis, to carry out an association mapping study, and to identify SNP markers associated with IDC tolerance in cowpea. A total of 353 cowpea accessions were evaluated for tolerance/susceptibly to low soluble iron conditions on higher pH soils. A total of 1006 SNP markers postulated from genotyping-by-sequencing were used after filtering for population structure and association analysis studies. Results revealed that: (1) a substantial variability in degree of tolerance to low soluble iron conditions was found among the cowpea accessions; (2) delta K peak was identified at K equal to 2, indicating two subpopulations within the cowpea accessions tested for adaptation to IDC, a second delta peak corresponding to K equal to 3 was also found; and (3) nine SNP markers, C35081162_3130, Scaffold16136_2033, Scaffold1764_4741, Scaffold18262_4480, Scaffold30165_15499, Scaffold47194_5530, Scaffold73235_6677, Scaffold77932_9959, and Scaffold86559_7193, were significantly associated with IDC tolerance in cowpea. These results can be used as tools to select cowpea genotypes tolerant to IDC under low soluble iron conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cowpea (Vigna unguiculata (L.) Walp), a diploid legume species 2n = 2x = 22, is recommended for human consumption for its high nutritional value. Frota et al. (2008) showed that cowpea cultivars contained, in g per 100-g seed, 24.5 protein, 51.4 carbohydrates, 16.6 insoluble fiber, 2.7 soluble fiber, 2.6 ash. Mineral constituent estimates were, in mg per 100-g seed, 6.8 iron, 4.1 zinc, 1.5 manganese, 510.0 phosphorus, and 1430.0 potassium. Lipid made up 2.2% of cowpea seed, and fatty acid profiling revealed 29.4% unsaturated fatty acids and 70.7% unsaturated. A total of 100-g cowpea provided 323.4 kcal. In addition, Okonya and Maass (2014) showed that iron content in cowpea ranged between 253.2 and 379.4 µg/g. Mduma et al. (2012) reported that cowpea iron bioavailability of 10.04% in traditional Tanzanian cowpea dishes.
Yamato et al. (2006) indicated that high pH soil properties made cowpea perform well through an enhancement of exchangeable cations such as Ca2+, Mg2+, K+, and Na+. However, Li and Lan (2017) stated that neutral or basic aerobic soils limit iron availability to plants. Roles of iron in plants include, but are not limited to, cellular respiration and metabolic processes, cofactor for enzymes, electron transport chain, oxygen transport, DNA stability and repair, and photosynthesis (Zargar et al. 2015). Moreover, iron helps plants withstand toxicity from other elements such as cadmium. Muneer et al. (2014) reported up regulation of Fe2+ transporters (IRT1, IRT2, and IRT3), Fe(III) chelate reductase (FRO1–FRO8), and phytochelatin synthase (PCS1, PCS2, and PCS3) upon addition of iron under cadmium toxicity conditions. Results revealed improved tolerance to cadmium toxicity through an enhancement of iron transport.
In addition to unfavorably affecting crop growth and development and causing important economic losses (Hansen et al. 2004), studies have shown that iron deficiency chlorosis (IDC) was positively correlated with the severity of other diseases and nematode attacks in crops. Research performed by Chen et al. (2007) demonstrated that iron-deficiency scores were higher in soybean (Glycine max L.) genotypes which were susceptible to cyst nematode. Results showed that tolerance to iron-deficiency enhanced plant growth, which led to better resistance to cyst nematode. Unraveling the genetic mechanisms behind tolerance to IDC is critical for developing crops able to cope with low soluble iron conditions. Srinives et al. (2010) investigated the inheritance of IDC in an F2 mungbean (Vigna radiata L.) population consisting of 241 accessions. Phenotyping suggested that IDC is controlled by a major gene (IR) with a dominant effect in mungbean. Srinives et al. (2010) reported two AFLP markers, E-ACT/M-CTA and E-ACC/M-CTG, to be closely linked to the IR gene. Somta et al. (2014) investigated potential QTLs controlling important agronomic traits under low soluble iron conditions in mungbean. A total of 33 QTLs located on linkage group 1A, 2, 3, 4, 5, and 9 were reported using composite interval mapping. Genome-wide studies for tolerance to iron deficiency revealed seven major QTLs in soybean, and 12 previously reported candidate genes associated with iron metabolism were located close to these QTLs (Mamidi et al. 2011). In addition, an RIL mungbean consisting of 122 F8 lines from the cross “NM10-12” (resistant line) and “Kamphaeng Saen 2” (susceptible line) was evaluated for foliar chlorosis in response to iron deficiency (Prathet et al. 2012). Two QTLs, qIDC3.1 and qIDC2.1, accounting for 12.1 and 45.7% of the phenotypic variations, respectively, were reported.
Iron is an essential element for cowpea. Jokar et al. (2015) conducted a study to evaluate the effect of an increasing iron concentration gradient (0, 0.135, 0.270, and 0.405 mg Fe per kg soil) on cowpea growth and yield. Results revealed that application of Fe fertilizers positively impacted shoot and dry matter, number of pods per plant, pod weight, and seed weight in cowpea. Santos and Boiteux (2015) estimated heritability and number of genes affecting zinc, iron, calcium, phosphorus, potassium, and sodium accumulation in cowpea. Studies involved crosses between IT97K-1042-3 and BRS Tapaihum, and ITK97-K-1042-3 and Canapu, which were contrasting for these aforementioned micronutrients. Narrow sense heritability was high for iron (65–86%), phosphorus (74–77%), and potassium (77–88%) seed accumulation. However, medium narrow sense heritability was recorded for seed accumulation in calcium (41–56%), zinc (51–83%), and sodium (50–55%). Santos and Boiteux (2015) predicted 2–11 genes controlling seed accumulation of these minerals in cowpea.
Investigations on cowpea genetics of tolerance to iron deficiency have long been of interest. Cortinas-Escobar et al. (1995) demonstrated simple inheritance of resistance to iron deficiency and reported that such tolerance was not dependent on maternal inheritance. Hale et al. (2000) investigated the genetics of tolerance to iron-deficiency in cowpea. A cross between a variety susceptible to iron deficiency, Pinkeye Purple Hull (PEPH), and a resistant one, Texas Pinkeye Purple Hull (TXPE), was performed. Chlorophyll was measured using SPAD to assess Iron Deficiency Chlorosis (FeDC = IDC). However, results showed that FeDC was not single-gene dependent as suggested previously (Cortinas-Escobar et al. 1995), and the estimate of narrow sense heritability was 0.3. These studies suggest that tolerance to iron deficiency in cowpea could be a complex trait. Jadhav et al. (1994) showed that siderophore from cowpea Rhizobium GN1 played an important role in iron uptake. Mutant siderophore was unable to grow on a medium with synthetic iron chelators.
Efforts to enhance cowpea tolerance to abiotic stress have been conducted. Providing cultivars with better tolerance to abiotic constraints is one of the most effective ways to overcome effects of abiotic stress. Iron-deficiency-related issues could be addressed by developing iron-deficiency-tolerant cultivars and making them available to producers. Molecular breeding is one way to accomplish this.
The use of SNP markers in breeding programs and genetic-related studies has been shown to be effective (Fang et al. 2014; Wang et al. 2015; Zhou et al. 2015). Developing SNP marker for tolerance to iron deficiency in cowpea will help plant breeders to speed up the development of new cultivars tolerant to low soil iron conditions. This study aimed to conduct an association analysis for tolerance to IDC, and to identify SNP marker for this trait in cowpea.
Materials and methods
Plant materials
A total of 353 United States Department of Agriculture (USDA) Germplasm Resources Information Network (GRIN) cowpea germplasm accessions were evaluated for IDC (Table S1). These cowpea accessions were collected from 53 different countries. The USDA Plant Genetic Resources Conservation Unit at Griffin, GA provided the cowpea seeds, which were increased at the Research and Extension Station of the University of Arkansas at Fayetteville, AR in the summers of 2014 and 2015.
Phenotyping of iron deficiency chlorosis
Evaluation of cowpea accessions for IDC was performed by Dr. Creighton Miller and Douglas Scheuring from Texas A&M University, TX. Experiments were conducted on high pH soils (8.2) at Blackland Research Center near Temple, TX. For each accession, ten seeds were sown in 10-foot rows. The experimental design was a randomized complete block (RCBD) with three replications. A subjective rating system ranging from 1 to 5 and depending on chlorosis was adopted. Scoring was performed as follows: 1 = very susceptible, 2 = susceptible, 3 = moderately susceptible/tolerant, 4 = tolerant, and 5 = very tolerant. Phenotypic data of IDC score in cowpea accessions were available and obtained from the USDA GRIN database (https://npgsweb.ars-grin.gov/gringlobal/descriptors.aspx).
Genotyping
DNA extraction, library preparation, and genotyping-by-sequencing
DNA was extracted from freeze-dried young cowpea leaves using the CTAB (hexadecyltrimethyl ammonium bromide) protocol as described by Kisha et al. (1997). Cowpea leaves were ground. DNA extraction buffer was added to each ground sample, which was centrifuged at 13,000 rpm for 10 min. Addition of chloroform-isoamyl alcohol (24:1) was made to denature protein from each sample. DNA was precipitated using 1 ml of isopropanol for each sample. Upon addition of isopropanol, samples were stored over night at − 20 °C. The use of 70 and 90% allowed DNA pellets to be washed. Use of 3 µl RNAse to each sample removed any RNA contamination. DNA samples was stored in a solution of 200 µl of 0.1 × TE, and quantified using a NanoDrop 200c spectrophotometer (Thermo SCIENTIFIC, Wilmington, DE). DNA quality was checked on a 1%-agarose gel with ethidium bromide stain.
Genotyping-by-sequencing (GBS) libraries were constructed using the ApeKI protocol (Elshire et al. 2011). DNA was sequenced by HiSeq series at the Beijing Genome Institute (BGI) using the GBS method (Elshire et al. 2011; Bastien et al. 2014). GBS Libraries were quality checked. DNA digestion was achieved using the restriction enzyme ApeKI1 to form sticky-ended fragments. To each fragment, two adaptors (P1: barcode and P2: adaptor) were ligated. In situ PCR was done to amplify each fragment. After sequencing, data were filtered. The average GBS data from BGI was 3.26 M. For each cowpea sample, an average of 90 bp-short-read sequence was provided. In this current investigation, cowpea accession having less than 100 Mb of sequence data was not involved in the analysis.
SNP assembly, mapping, discovery, and filtering
SOAP family software (http://soap.genomics.org.cn/) from BGI permitted SNP assembly, mapping, and discovery. SOAPalinger/soap2 (http://soap.genomics.org.cn/) aligned short-reads to the cowpea genome “cowpea_Genome_0.03.fa” (644,126 scaffolds or contigs), which was provided by Dr. Timothy J. Close from the University of California, Riverside. Initial calls were conducted using SOAPsnp v 1.05 (Li et al. 2009).
SNP filtering allowed removal of SNP containing more than 30% missing data. Heterozygous calls greater than 30% were also removed. Minor allele frequency (MAF) threshold was 0.05. After SNP filtering, at total of 1006 SNP remained and were used for further analysis. Cowpea accessions containing more than 30% missing data were also removed from the analysis.
Population structure analysis
Model-based population was inferred using STRUCTURE 2.3.4 (Pritchard et al. 2000). Population structure (K) was assessed using an admixture model with a correlated allele frequency model, which was independent for each run. Such models provided conclusive results in cowpea structure analysis (Shi et al. 2016; Qin et al. 2016, 2017). A total of 10 runs were performed for each estimated K value. Markov Chain Monte Carlo (MCMC) length of the burn-in period was 30,000 and the number of MCMC iterations was to 50,000. Selection of appropriate K-values was achieved by computing delta K and optimal K using STRUCTURE Harvester (Earl and VonHoldt 2011; http://taylor0.biology.ucla.edu/structureHarvester/) and based on the model established by Evanno et al. (2005). Q-matrix along with the K vectors were designed upon selection of optimal K. Q-matrix was used for association analysis in TASSEL 5. Cowpea accessions were assigned to each cluster (Q) depending on the cut-off probability set to 0.55. Population structure was visualized using bar plots of STRUCTURE 2.3.4 with option “Sort by Q” (Ramasamy et al. 2014).
Genetic diversity
A phylogenetic tree was drawn using MEGA 7 (Kumar et al. 2016). The statistical method for genetic diversity analysis was Maximum Likelihood and parameters used in MEGA 7 were set as described by Shi et al. (2016): Analysis: Phylogeny Reconstruction; Statistical method: Maximum Likelihood; Test of phylogeny: None; Substitutions type: Nucleotide; Model/Method: Tamura–Nei Model; Rates among sites: Gamma distributed with Invariant sites (G + I); No of Discrete Gamma Categories: 5; Gaps/Missing Data treatment; ML Heuristic Method: Nearest-Neighbor-Interchange (NNI); Initial Tree for ML: Make initial tree automatically (Default—NJ/BioNJ); Branch Swap Filter: Moderate; Number of threads: 1; Test of Phylogeny: None; No. of Bootstrap Replications: 500; Model/Method: General Time Reversible Model; Rates among Sites: Gamma distributed with invariant sites (G + 1); Number of discrete gamma categories: 5; Gaps/Missing data treatment: use of all sites; ML Heuristic method: Subtree-Pruning-Regrafting-Extensive (SPR level 5); Initial tree for ML: Make initial tree automatically (Neighbor Joining); and Branch swap filter: Moderate.
Population structure outputs and Q clusters were exported from STRUCTURE 2.3.4 and loaded to MEGA 7 for a combined genetic diversity analysis. Such analysis was done while drawing the phylogenetic tree. For the sub-trees corresponding to each cluster (Q), shape of “Node/Subtree Marker” and “Branch Line” color matched to that of the bar plots displayed in structure plots.
Association analysis
Association analysis was performed using TASSEL 5 (Bradbury et al. 2007) and R package (Liu et al. 2016). Models for association analysis consisted of a single marker regression (SMR) using all SNPs regardless of population structure and relatedness, a general linear model (GLM) involving Q matrix (GLM_Q) and PCA (GLM_PCA), a mixed linear model (MLM) using Q matrix and Kinship (MLM_Q + K), PCA and Kinship (MLM_PCA + K), and a fixed and random model Circulating Probability Unification (FarmCPU) using R. PCA was obtained from TASSEL 5, whereas Q matrix was generated using STRUCTURE 2.3.4. LOD (logarithm (base 10) of odds) threshold for significant SNPs was between 2 and 3 as described by Lander and Botsteins (1989).
Favorable haplotype, SNP selection accuracy, and SNP selection efficiency
Significant SNP markers were analyzed further in order to identify the favorable haplotype for tolerance to IDC, and to estimate SNP selection accuracy and efficiency. For each significant SNP identified from association analysis, number of genotype AA, CC, GG, TT, AC, AG, AT, CG, CT, and GT were calculated using VB code developed by Dr. Shi (University of Arkansas, Fayetteville) (unpublished). Dr. Shi’s VB code also allowed determination of percentage of homozygote genotypes AA, CC, GG, TT, major allele frequency, minor allele frequency, heterozygosity percentage, and missing data. SNP selection accuracy and efficiency were computed as described by Shi et al. (2016).
-
Selection accuracy (%) = 100 × (number of cowpea accessions tolerant to low soluble Iron condition IDC in the benefit SNP allele)/(number of cowpea accessions tolerant to low soluble Iron condition IDC in the benefit SNP allele + number of cowpea accessions susceptible to low soluble Iron condition IDC in the benefit SNP allele),
-
Selection efficiency (%) = 100 × (number of cowpea accessions tolerant to IDC low soluble Iron condition in the benefit SNP allele/total genotype number).
In this study, we only selected those cowpea accessions with either scored IDC rate 1 or 5 to estimate the selection accuracy and the selection efficiency under low soluble iron conditions.
Results
Phenotyping for iron-deficiency chlorosis in cowpea
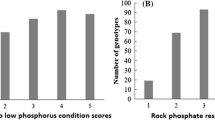
IDC scores varied from 1.0 to 5.0, with an average of 2.9 and a standard deviation of 1.1, suggesting that 35.1% of the cowpea genotypes were moderately susceptible/tolerant to low soluble iron conditions. Highly tolerant accessions to low soluble Iron conditions (PI141355, PI166146, PI170861, PI185647, PI189374, PI194204, PI194208, PI205241, PI208771, PI255774, PI293476, PI300173, PI300175, PI305076, PI339709, PI352936, PI352941, PI354838, PI419102, PI490770, PI527675, PI582565, PI582699, PI582708, PI582738, PI582805, PI582866, and PI583098) made up 8.2% of the total accessions involved in the present investigation; whereas 9.3% of the cowpea accessions were very susceptible to soil iron deficiency (PI147562, PI167024, PI186452, PI186460, PI186466, PI194213, PI202802, PI211754, PI221730, PI225922, PI229796, PI277784, PI292890, PI292892, PI292908, PI292913, PI339591, PI339600, PI354857, PI358716, PI448558, PI448806, PI503326, PI582419, PI582569, PI582571, PI582696, PI582702, PI582707, PI582724, PI582821, PI582941, and PI583195) (Table S1). A near normal distribution of IDC scores was observed among the 353 cowpea genotypes tested (Fig. 1).
IDC phenotypic results suggested that cowpea accessions from Cuba, Thailand, Zaire, Argentina, Brazil, Indonesia, Mozambique, the Philippines, Suriname, Tanzania, and Trinidad and Tobago were tolerant to IDC. Accessions from the aforementioned countries had an IDC score equal or greater than 4.0. However, cowpea accessions from Burkina Faso, Burundi, Israel, Portugal, Sri Lanka, Uganda, Hungary, Peru, Colombia, Ethiopia, and Zambia were iron-deficiency susceptible. Accessions originating from these countries were rated lower or equal to 2.0.
Population structure and genetic diversity
Model-based population structure of the 353 cowpea accessions was inferred using STRUCTURE 2.3.4. Structure Harvester permitted the identification of the optimal delta K. In this investigation, delta K was highest at K equal to 2, indicating that two subpopulations were identified within the cowpea panel (Fig. 2a). Cut-off probability for assigning a cowpea accession to a subgroup (subpopulation) was 0.55. If such probability was greater or equal to 0.55 for Q1 corresponding to an accession, the accession belonged to Q1. In a scenario where Q2-associated probability for an accession was greater or equal to 0.55, the accession was classified as Q2. If none of the two subgroups, Q1 and Q2, had a probability greater or equal to 0.55, the accession fell under admixture Q1Q2. Of the 353 cowpea accessions, 41.9% were Q1; whereas Q2 made up 45.9%. In addition to being clearly structured in two subpopulations, admixture Q1Q2 consisting of 12.2% of the accessions was identified. Subpopulation 1 or Q1 or cluster 1 was shown in red in the structure plot (Fig. 2b); subpopulation 2 or Q2 or cluster 2 was displayed in green (Fig. 2b). With respect to the phylogenetic tree, Q1 was represented in red circles, Q2 in green squares, and admixture Q1Q2 in blue triangles (Fig. 2c). Results from the phylogenetic tree were consistent with that of structure analysis.
Model-based populations of 353 cowpea accessions evaluated for iron-deficiency chlorosis for the first pic K = 2. a Delta K values from Structure Harvester for different number populations inferred (K) from STRUCTURE analysis. b Classification of 353 cowpea accessions into two populations using STRUCTURE 2.3.4 where subgroup is shown on the y-axis, and each accession is represented on the x-axis. Distribution of the 353 cowpea accessions is shown by color coding (Q1 or cluster 1: red, and Q2 or cluster 2: green). c Phylogenetic tree of the 353 cowpea accessions drawn using MEGA 7 and Maximum Likelihood (ML) as statistical method. Color coding Q1 and Q2 in (b) matches that of in (c). Blue triangles reveal the admixture Q1Q2. (Color figure online)
Population structure analysis revealed a second peak of delta K corresponding to K equal to 3 (Fig. 3a). In the structure plot, Q1 was shown in red, Q2 in green, and Q3 in blue (Fig. 3b). Cut-off probability value was similar to that stated earlier. Accessions belonging to Q1 made up 22.9% of the total cowpea accessions involved in this study; Q2 consisted of 26.9%; Q3 comprised 26.1% of the 353 cowpea accessions. Moreover, admixture including Q1Q2, Q1Q3, Q2Q3, and Q1Q2Q3 made up 24.1% of the 353 cowpea accessions. Q1Q2, Q1Q3, Q2Q3, and Q1Q2Q3 represented 8.5, 3.4, 7.4, and 4.8% of the cowpea panel, respectively. In the phylogenetic tree, Q1 was displayed using red circles, Q2 was represented by green squares, Q3 was shown using blue triangles, and the admixture (Q1Q2, Q1Q3, Q2Q3, and Q1Q2Q3) was presented by empty squares (Fig. 3c). Structure analysis outputs were in agreement with that of phylogenetic tree (Table 1).
Model-based populations of 353 cowpea accessions evaluated for iron-deficiency chlorosis for the second pic K = 3. a Delta K values from Structure Harvester for different number populations assumed (K) from STRUCTURE analysis, b classification of 353 cowpea accessions into three populations using STRUCTURE 2.3.4. Subgroup is shown on the y-axis, and cowpea accession on the x-axis. Distribution of the 353 cowpea accessions is represented using color coding (Q1 or cluster 1: red, Q2 or cluster 2: green, and Q3 or cluster 3: blue). c Phylogenetic tree showing the 353 cowpea accessions, and drawn using MEGA 7 and Maximum Likelihood (ML) as statistical method. Color coding for Q1, Q2, and Q3 in (b) and (c) are in agreement. Admixtures (Q1Q2, Q1Q3, Q2Q3, and Q1Q2Q3) are represented by empty squares. (Color figure online)
Association analysis
Association mapping was conducted using 1006 SNPs obtained from genotyping-by-sequencing (GBS) after filtering. Six models were involved in the analysis. Results revealed nine SNPs, C35081162_3130, Scaffold16136_2033, Scaffold1764_4741, Scaffold18262_4480, Scaffold30165_15499, Scaffold47194_5530, Scaffold73235_6677, Scaffold77932_9959, and Scaffold86559_7193, that were found to be associated with tolerance to low soil iron conditions in cowpea (Table 2). LOD values varied from 1.1 to 5.5 depending upon the statistical models, with a mean of 2.7 and a standard deviation of 1.1. Overall, single marker regression (SMR) offered higher LOD compared to other models. SMR-associated LOD values varied from 2.1 (Scaffold86559_7193) to 5.5 (Scaffold16136_2033 and Scaffold30165_15499) (Table 2), with a mean of 3.7 and a standard deviation of 1.5; however, that of the mixed linear model (MLM_PCA + K) ranged between 1.5 (Scaffold77932_9959) and 3.1 (Scaffold1764_4741), with a mean of 2.1 and a standard deviation of 0.5. In spite of the observed change in LOD values across different models, SNP marker, scaffold16136_2033, displayed consistency with an LOD equal or greater than 2.0. LOD values associated with scaffold16136_2033 were 5.5, 5.5, 4.4, 2.9, 2.5, 2.3, 2.0, and 2.3 using SMR, GLM(Q2), GLM(Q3), GLM(PCA), MLM(Q2 + K), MLM(Q3 + K), MLM (PCA + K), and FarmCPU (Table 2), respectively. LOD consistency across the six models was also identified for scaffold30165_15499 except under mixed linear model (MLM_PCA + K). LOD values associated with that SNP marker were 5.5, 5.3, 4.2, 2.8, 2.2, 2.2, 1.9, and 4.0 using SMR, GLM(Q2), GLM(Q3), GLM(PCA), MLM(Q2 + K), MLM(Q3 + K), MLM (PCA + K), and FarmCPU (Table 2), respectively. Distribution of SNPs was visualized through QQplot. SNP distribution significantly deviated from the straight line at an expected − log10(p) equal to 2 (Fig. 4), indicating the robustness of the SNPs identified in this study.
SNP-associated R-square values for IDC in cowpea were in range of 1.8–7.2%, with a mean of 3.7% and a standard deviation of 1.4%, suggesting that tolerance to low soluble iron conditions in cowpea could be controlled by multiple genes with minor effects. R-square values were highest using SMR. R-square values for scaffold16136_2033 varied from 2.8 to 7.2% depending upon the model. SNP marker, scaffold30165_15499, had R-squares ranging from 2.6 to 7.2% (Table 3).
Structure analysis indicated two subpopulations within the cowpea accession panel. In addition, a second delta K peak was identified at K equal to 3. Both Q2 and Q3 matrices were used for association analysis. Overall results showed that models involving the Q2 matrix engendered higher LOD and R-square values. By using GLM (Q2), the SNP marker scaffold16136_2033, had an LOD value of 5.5 and an R-square 7.2%; whereas the model consisting of GLM (Q3) generated an LOD value of 4.4 and an R-square of 5.7% for the same SNP. Similar results were found for the other significant SNP markers. These results indicated that the SNP markers reported in this investigation were significant regardless of the population structure, hence not significantly affected by population structure and could be suggested as candidate SNP markers in a Marker-Assisted Selection (MAS) breeding program for tolerance to low soluble iron conditions in cowpea.
Favorable haplotype, SNP selection accuracy, and SNP selection efficiency
Favorable haplotypes for tolerance to low soluble Iron conditions in cowpea consisted of allele A, A, C, C, G, T, C, C, and T for the significant SNP markers C35081162_3130, Scaffold16136_2033, scaffold1764_4741, scaffold18262_4480, scaffold30165_15499, scaffold47194_5530, scaffold73235_6677, scaffold77932_9959, and scaffold86559_7193, respectively. Selection accuracy varied from 30.8 to 88.2%, with a mean of 63.7% and a standard deviation of 20.9%. Selection efficiency ranged between 25.0 and 56.1%, with a mean of 34.5% and a standard deviation of 10.6%. The highest selection accuracy was recorded for scaffold73235_6677 (88.2%) and scaffold16136_2033 (85.7%), which was consistent with the fact that these SNPs had consistent higher LOD scores under most of the models used for association analysis. Selection efficiency values associated with these aforementioned SNP markers were 31.3 and 31.0%, respectively.
Discussion
Iron plays a significant role in plant growth and development. Evidence on the impact of iron toxicity as being lethal to plants has been reported and widely accepted. However, low soluble iron condition leading to leaf chlorosis, plant death, and oxidative stress (Tewari et al. 2005; Li and Lan 2017) concerned cowpea producers. Plants under Iron deficiency stress show chlorotic young leaves (Zhang et al. 1995). In this study, a total of 353 cowpea genotypes were evaluated for tolerance to IDC. Results showed a large variability in tolerance to low soluble iron conditions in cowpea. Distribution of IDC scores was near normal, indicating that tolerance to iron deficiency could be controlled by multiple genes in cowpea.
In this study, we used an association analysis approach to identify significant SNP markers associated with tolerance to IDC in cowpea. This approach has been reported to provide conclusive results for genetic-related studies in cowpea (Shi et al. 2016; Qin et al. 2016; Xiong et al. 2016; Qin et al. 2017). Association analysis showed nine SNP markers, C35081162_3130, Scaffold16136_2033, Scaffold1764_4741, Scaffold18262_4480, Scaffold30165_15499, Scaffold47194_5530, Scaffold73235_6677, Scaffold77932_9959, and Scaffold86559_7193 associated with IDC tolerance in cowpea. The low LOD and low R-square values of associated SNP markers have indicated that IDC tolerance in cowpea is a complex trait as stated by Cortinas-Escobar et al. (1995). Since iron deficiency unfavorably affects crops, complex mechanisms have been adopted for sustainably maintaining iron homeostasis in plant cells. Such mechanisms consist of physiological, morphological, metabolic, and gene expression changes (Jeong and Guerinot 2009; Kobayashi and Nishizawa 2012).
The complexity of the mechanism related to Iron deficiency indicated that this trait could be controlled by more than one gene, and could be significantly affected by environmental factors. In fact, tolerance to Iron deficiency in plants could be attributed to the ability of plants to detect the deficiency and communicate it to different parts of the plants so that soil iron uptake pathway is triggered, hence increased (Hindt and Guerinot 2012). Sensing Iron deficiency and transmitting this information probably involve different proteins encoded by various genes. In Arabidopsis thaliana, the genes ferric-chelate reductase FRO2 and ferrous iron transporter IRT1 promoted tolerance to low Iron conditions (Sivitz et al. 2011). The expression of these genes was further enhanced by a post-transcriptional regulation of a basic helix-loop-helix (bHLH) transcription factor FIT upon Iron deficiency stress. In rice (Oryza sativa L.), tolerance to Iron deficiency was triggered by the transcription factor IDEF1. This transcription factor binds to the cis-acting element of IDE1.
Change in plant proteome was proved to be an important factor contributing towards Iron deficiency tolerance (López-Millán et al. 2013). Plants being able to sufficiently provide regulating-oxidative stress proteins were shown to withstand Iron deficiency stress. Dixon and Edwards (2010) reported that glutathione S transferase (GST) families were key in coffering tolerance to low Iron conditions. The significant increase in enzymes involved in glycolysis and Krebs cycle was associated to tolerance to iron deficiency in plants (Hindt and Guerinot 2012). Doing so helped plants gain energy even though being under stress.
Genes encoding for proteins required for the metabolism of azote are also critical in assisting plants with tolerance to Iron deficiency (Borlotti et al. 2012), which made the study of the mechanism of iron deficiency more complex. Enzymes such as S-adenosylmethionine synthase 1 (SAM 1), glutamine synthetase (GLN 2), urease (UREG), dihydrolipoamide dehydrogenase (LPD 1), omega amidase, alanine aminotransferase, and aspartate aminotransferase were shown to confer tolerance to iron deficiency in plants (Waters et al. 2007).
Breeding for Iron deficiency stress-tolerant crops has been challenging due to the fact that such trait could be highly quantitative. However, efforts towards identifying the most prominent pathway relevant to Iron deficiency tolerance should be undertaken since doing so will have substantial impacts on crop improvement. In this present investigation, we identified SNP markers associated with Iron deficiency in cowpea, which is an economically important legume (Singh et al. 2003). To the best of our knowledge, this is the first report on association analysis of Iron deficiency using SNP markers in cowpea. Since low-R square values were found, we suggest increasing the number of markers for further investigations in order to capture the genomic regions that were missed in this research, which could result in low R-square values. Despite of these constraints, the results from this work could be used in advancing the genetics of IDC in cowpea.
The use of molecular markers such as SSR (Bhattarai and Mehlenbacher 2017) and SNP (Fang et al. 2014) has proven to be effective in a breeding program. The SNP markers identified in this study could be used as a tool to select cowpea genotypes for tolerance and adaptation to iron deficiency. Doing so will speed up the development of new cultivars better adapted to low soluble iron conditions. Since it has been stated that IDC is a complex trait, which is virtually controlled by multiple genes, this study provides insights toward the genetics underlying cowpea tolerance to IDC.
Conclusion
Iron deficiency has been found to be yield-limiting in cowpea. A cowpea accession panel involving 353 accessions was evaluated for IDC on high pH soils. A large variability in tolerance/susceptibility to low soluble iron conditions has been found. Population structure analysis indicated two subpopulations within the cowpea panel. A total of nine SNP markers, C35081162_3130, Scaffold16136_2033, Scaffold1764_4741, Scaffold18262_4480, Scaffold30165_15499, Scaffold47194_5530, Scaffold73235_6677, Scaffold77932_9959, and Scaffold86559_7193, were identified as significantly associated with IDC tolerance in cowpea. These SNP markers can be used as a tool to select cowpea genotypes in a breeding program for t developing cowpea cultivars tolerant to low soluble iron conditions, which are prevalent on high pH soils.
References
Bastien M, Humira S, François B (2014) Genome wide association mapping of Sclerotinia sclerotiorum resistance in soybean with a genotyping-by-sequencing approach. Plant Genome. https://doi.org/10.3835/plantgenome2013.10.0030
Bhattarai G, Mehlenbacher SA (2017) In Silico development and characterization of tri-nucleotide simple sequence repeat markers in hazelnut (Corylus avellana L.). PLoS ONE 12:e0178061
Borlotti A, Vigani G, Zocchi G (2012) Iron deficiency affects nitrogen metabolism in cucumber (Cucumis sativus L.) plants. BMC Plant Biol 12:189
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TE, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23:2633–2635
Chen S, Kurle JE, Stetina SR, Miller DR, Klossner LD, Nelson GA, Hansen NC (2007) Interactions between iron-deficiency chlorosis and soybean cyst nematode in Minnesota soybean fields. Plant Soil 299:131–139
Cortinas-Escobar HM, Scheuring DC, Gerik TJ, Miller JC Jr (1995) Inheritance of resistance to iron deficiency in cowpea. HortScience 30:841
Dixon DP, Edwards R (2010) Glutathione transferases. Arabido Book 8:20131
Earl DA, VonHoldt BM (2011) Structure harvester: a website and program for visualizing structure output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6:e19379
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the Software structure: a simulation study. Mol Ecol 14:2611–2620
Fang WP, Meinhardt LW, Tan HW, Zhou L, Mischke S, Zhang D (2014) Varietal identification of tea (Camellia Sinensis) using nanofluidic array of single nucleotide polymorphism (SNP) markers. Hortic Res 1:14035
Frota KMG, Soares RAM, Arêas JAG (2008) Chemical composition of cowpea (Vigna unguiculata L. Walp), BRS-Milênio Cultivar. Food Sci Technol 28:470–476
Hale AL, Scheuring DC, Gerik TJ, Hart JD, Miller JC Jr (2000) Further investigations on the genetics of resistance to iron deficiency in cowpea. HortScience 35:560
Hansen NC, Jolley VD, Naeve SL, Goos RJ (2004) Iron deficiency of soybean in the North Central U.S. and associated soil properties. Soil Sci Plant Nutr 50:983–987
Hindt MN, Guerinot ML (2012) Getting a sense for signals: regulation of the plant iron deficiency response. Biochem Biophys Acta 1823:1521–1530
Jadhav RS, Desai A, Soong NW, Farmer WJ, Vishwanatha T, Pasternak JJ (1994) Role of siderophore in iron uptake in Cowpea Rhizobium GN1 (peanut isolate): possible involvement of iron repressible outer membrane proteins. FEMS Microbiol Lett 115:185–189
Jeong J, Guerinot ML (2009) Homing in on iron homeostasis in plants. Trends Plant Sci 14:280–285
Jokar L, Ronaghi A, Karimian N (2015) Effects of different Fe levels from Fe-nano-chelate and Fe-EDDHA sources on growth and some nutrients concentrations in cowpea in a calcareous Soil. J Sci Technol Greenh Cult 6:9–18
Kisha TJ, Sneller CH, Diers BW (1997) Relationship between genetic distance among parents and genetic variance in populations of soybean. Crop Sci 37:1317–1325. https://doi.org/10.2135/cropsci1997.0011183X003700040048x
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152. https://doi.org/10.1146/annurev-arplant-042811-105522
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Lander ES, Botstein D (1989) Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–199
Li W, Lan P (2017) The understanding of the plant iron deficiency responses in strategy I plants and the role of ethylene in this process by omic approaches. Front Plant Sci 8:40
Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J (2009) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25:1966–1967
Liu X, Huang M, Fan B, Buckler ES, Zhang Z, Bradbury PJ (2016) Iterative usage of fixed and random effect models for powerful and efficient genome-wide association studies. PLoS Genet 12:e1005767
López-Millán AF, Grusak MA, Abadía A, Abadía J (2013) Iron deficiency in plants: an insight from proteomic approaches. Front Plant Sci 4:254
Mamidi S, Chikara S, Goos RJ, Hyten DL, Annam D, Moghaddam SM, Lee RK, Cregan PB, McClean PE (2011) Genome-wide association analysis identifies candidate genes associated with iron deficiency chlorosis in soybean. Plant Genome 4:154–164
Mduma I, Msuya J, Mwanri AW, Yang RY (2012) Carotenoids retention and in Vitro iron bioavailability of traditional cowpea feaf dishes of rural Tanzania. Int J Food Sci Nutr 63:267–272
Muneer S, Jeong BR, Kim TH, Lee JH, Soundararajan P (2014) Transcriptional and physiological changes in relation to Fe uptake under conditions of Fe-deficiency and Cd-toxicity in roots of Vigna Radiata L. J Plant Res 127:731–742
Okonya JS, Maass BL (2014) Protein and iron composition of cowpea leaves: an evaluation of six cowpea varieties grown in eastern Africa. Afr J Food Agric Nutr Dev 14:2129–2140
Prathet P, Somta P, Srinives P (2012) Mapping QTL conferring resistance to iron deficiency chlorosis in mungbean [Vigna radiata (L.) Wilczek]. Field Crops Res 137:230–236
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Qin J, Shi A, Xiong H, Mou B, Motes D, Lu W, Miller JC Jr, Scheuring DC, Nzaramba MN, Weng Y, Yang W (2016) Population structure analysis and association mapping of seed antioxidant content in USDA cowpea (Vigna unguiculata L. Walp.) core collection using SNPs. Can J Plant Sci 96:1026–1036
Qin J, Shi A, Mou B, Bhattarai G, Yang W, Weng Y, Motes D (2017) Association mapping of aphid resistance in USDA cowpea (Vigna unguiculata L. Walp.) core collection using SNPs. Euphytica 213:36
Ramasamy RK, Ramasamy S, Bindroo BB, Naik VG (2014) Structure plot: a program for drawing elegant structure bar plots in user friendly interface. SpringerPlus 3:431
Santos CAF, Boiteux LS (2015) Genetic control and transgressive segregation of zinc, iron, potassium, phosphorus, calcium, and sodium accumulation in cowpea (Vigna unguiculata) seeds. Genet Mol Res 14:259–268
Shi A, Buckley B, Mou B, Motes D, Morris JB, Ma J, Xiong H, Qin J, Yang W, Chitwood J, Weng Y (2016) Association analysis of cowpea bacterial blight resistance in USDA cowpea germplasm. Euphytica 208:143–155
Singh BB, Ajeigbe HA, Tarawali SA, Fernandez-Rivera S, Abubakar M (2003) Improving the production and utilization of cowpea as food and fodder. Field Crops Res 84:169–177
Sivitz A, Grinvalds C, Barberon M, Curie C, Vert G (2011) Proteasome-mediated turnover of the transcriptional activator FIT is required for plant iron-deficiency responses. Plant J 66:1044–1052
Somta P, Prathet P, Kongjaimun A, Srinives P (2014) Dissecting quantitative trait loci for agronomic traits responding to iron deficiency in mungbean [Vigna radiata (L.) Wilczek]. Agrivita 36:101–111
Srinives P, Kitsanachandee R, Chalee T, Sommanas W, Chanprame S (2010) Inheritance of resistance to iron deficiency and identification of AFLP markers associated with the resistance in mungbean (Vigna radiata (L.) Wilczek). Plant Soil 335:423–437
Tewari RK, Kumar P, Sharma PN (2005) Signs of oxidative stress in the chlorotic leaves of iron starved plants. Plant Sci 169:1037–1045
Wang B, Tan HW, Fang W, Meinhardt LW, Mischke S, Matsumoto T, Zhang D (2015) Developing single nucleotide polymorphism (SNP) markers from transcriptome sequences for identification of longan (Dimocarpus longan) germplasm. Hortic Res 2:14065
Waters BM, Lucena C, Romera FJ, Jester GG, Wynn AN, Rojas CL, Alcántara E, Pérez-Vicente R (2007) Ethylene involvement in the regulation of the H+-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiol Biochem 45:293–301
Xiong H, Shi A, Mou B, Qin J, Motes D, Lu W, Ma J, Weng Y, Yang W, Wu D (2016) Genetic diversity and population structure of cowpea (Vigna unguiculata L. Walp). PLoS ONE 11:e0160941
Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci Plant Nutr 52:489–495
Zargar SM, Mahajan R, Farhat S, Nazir M (2015) Understanding the role of iron and zinc in animals and crop plants from genomics perspective. Curr Trends Biotechnol Pharm 9:181–196
Zhang C, Römheld V, Marschner H (1995) Retranslocation of iron from primary leaves of bean plants grown under iron deficiency. J Plant Physiol 146:268–272
Zhou L, Matsumoto T, Tan HW, Meinhardt LW, Mischke S, Wang B, Zhang D (2015) Developing single nucleotide polymorphism markers for the identification of pineapple (Ananas comosus) germplasm. Hortic Res 2:15056
Acknowledgements
This work was supported, in part, by the USDA National Institute of Food and Agriculture Hatch Project Accession No. 1002423.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Cowpea accession, origin of cowpea accessions, tolerance to iron-deficiency, and iron-deficiency chlorosis scores. (XLSX 18 kb)
Rights and permissions
About this article
Cite this article
Ravelombola, W., Qin, J., Shi, A. et al. Population structure analysis and association mapping for iron deficiency chlorosis in worldwide cowpea (Vigna unguiculata (L.) Walp) germplasm. Euphytica 214, 96 (2018). https://doi.org/10.1007/s10681-018-2176-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-018-2176-5